2. The Components of PRESAGE
The main material of PRESAGE is polyurethane, which consists of 61% carbon, 20% oxygen, 10% nitrogen, and 9% hydrogen. It has an effective atomic number of 6.6 and a density of 1.05 g/cm
3 [77,78][10][11]. Polyurethane has a clear solid form and can polymerise at low temperatures, which is crucial to ensuring the reduction in the unwanted thermal oxidation reactions that amplify the background radiochromic reaction
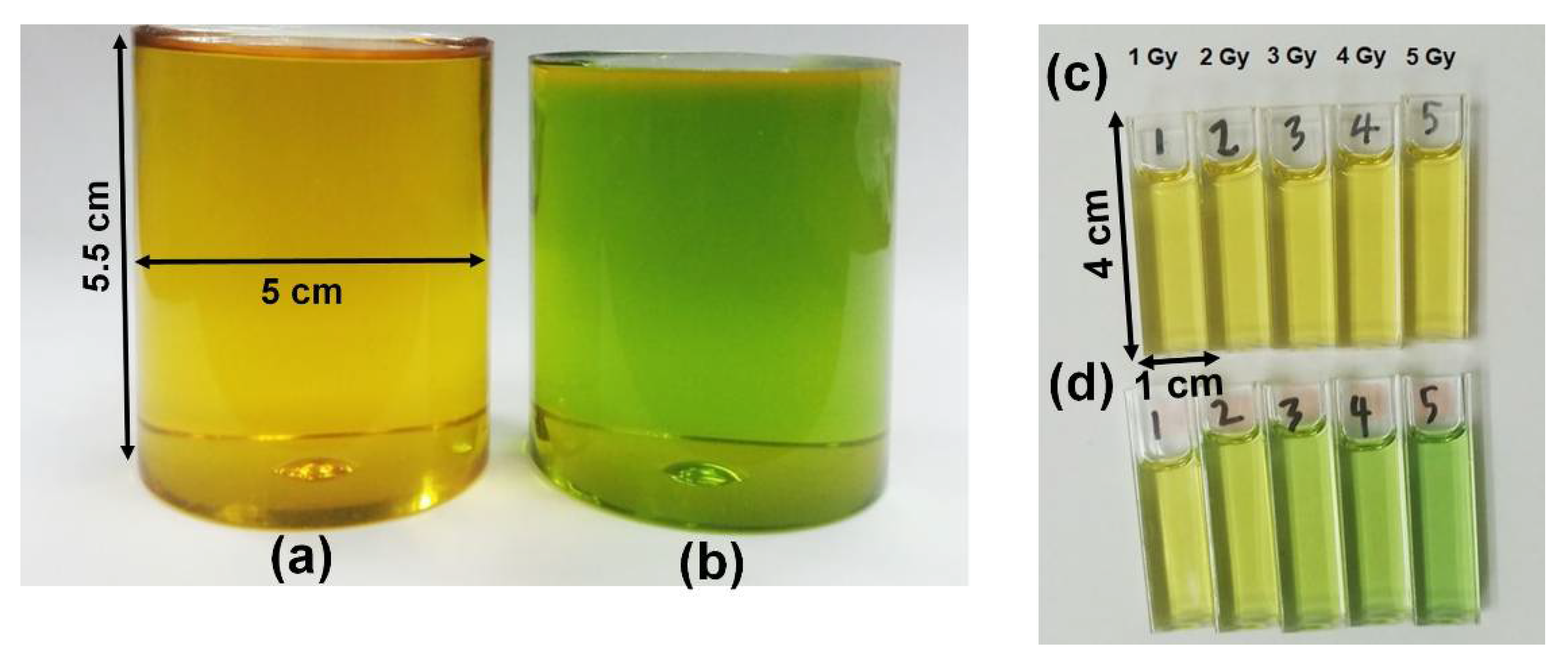
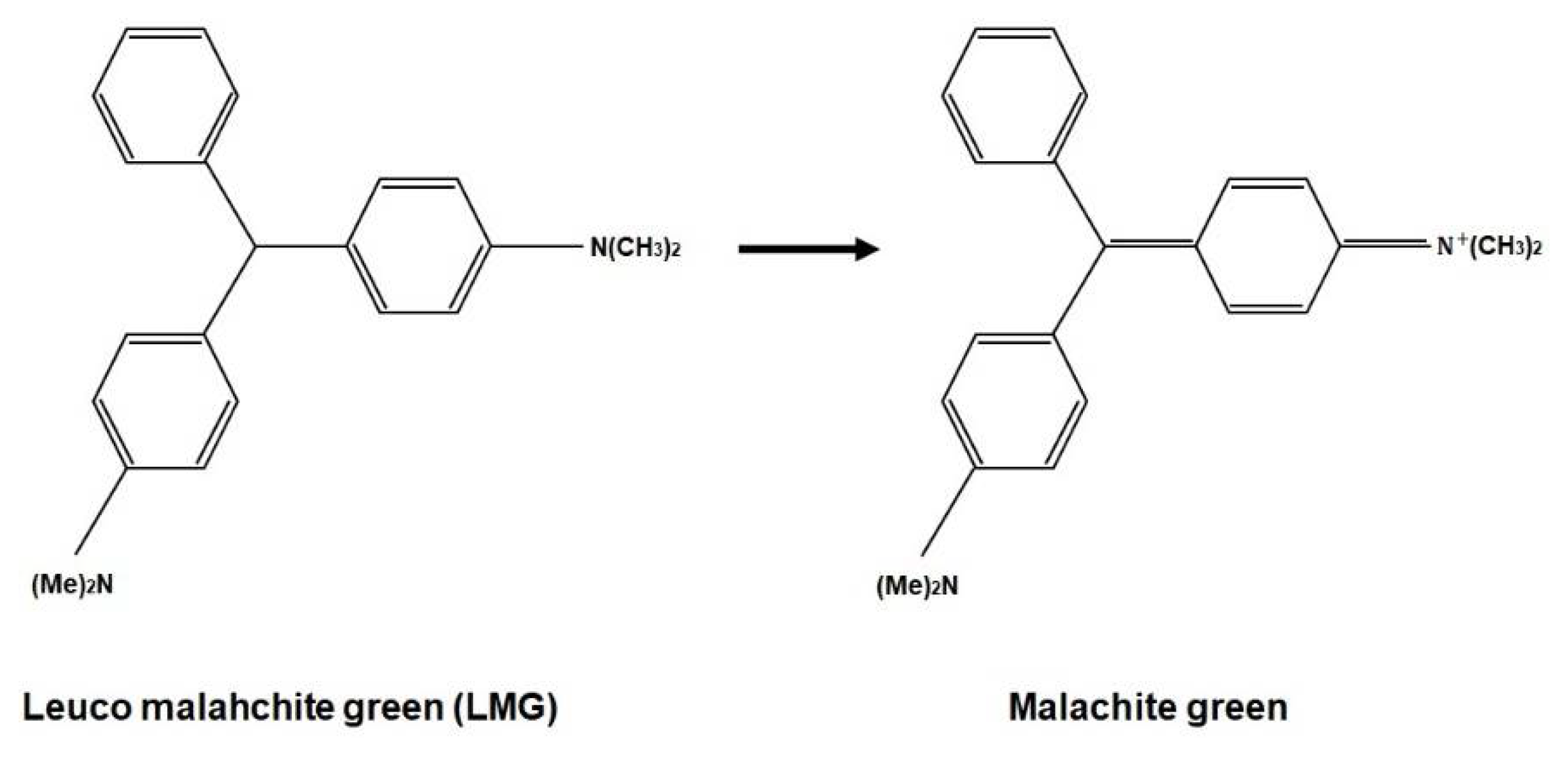
[71][1]. The radiochromic part of PRESAGE is made up of leucomalachite green (LMG) dye and a halocarbon free radical initiator. Notably, LMG shows maximum absorbance at 633 nm. The free radicals created from halocarbon radiolysis and oxidisation during radiation interaction change the LMG into malachite green (MG)
[61,79][12][13]. The change of optical density has developed the dosimeter into a colour agent that can be read out.
PRESAGE fabrication comprises two steps, which are the fabrication of the polymer and the addition of the leuco-dye
[20,77,80][3][10][14]. The first step requires the formation of prepolymer, which involves a reaction of a molar equivalent of polyol with two molar equivalents of diisocyanate. Polyol is an organic compound carrying multiple hydroxyl groups (OH), whereas the diisocyanate is an organic compound of two isocyanate groups. A non-reactive prepolymer that can be stored at room temperature is created due to the reaction of these compounds. The chemical reaction of the first step can be described as follows:
The second step is where the leuco-dye, free radical initiator, polyol, and a catalyst are combined. The product from this combination is integrated with the prepolymer created from the first step to obtain a homogenous mixture. The mixture is placed in a suitable mould and maintained under the pressure of 60 psi at the optimum temperature to reduce outgassing. The chemical response of the second phase can be described as follows:
Besides polyurethane, other base materials have been utilised to fabricate PRESAGE, such as epoxies, polyesters, acrylics, polycarbonates, polystyrene, and polyvinyl chloride (PVC). However, polyurethane shows several advantages compared to other materials. For instance, the effective atomic number of PVC does not hold the same value as the tissue. Additionally, the heat produced during the polymerisation of polycarbonates, polystyrene, acrylics, and polyesters at >100 °C can degrade the leuco-dye in PRESAGE. Epoxy, on the other hand, has low radiation sensitivity
[77][10].
Figure 2 presents the chemical formula of the radiochromic response due to the irradiation of PRESAGE.
Figure 2. The chemical formula of radiochromic response.
A number of other organic compounds can be used as a leuco-dye; these include crystal violet lactone, green diaminofluoran, orange diaminofluoran, black fluoran, and leucomalachite green (LMG). The wide use of LMG as a leuco-dye in the fabrication of PRESAGE is due to its higher sensitivity and high reactivity to high-energy radiation as compared with the other organic compounds. Furthermore, LMG shows the highest visible absorbance at 633 nm, while the green diaminofluoran, orange aminofluoran, and black fluoran show the lowest sensitivity and response
[77][10]. As for the free radical initiator, several materials can be employed, such as organic peroxides, carbon tetrachloride, halogenated carbons, halogenated hydrocarbons, azo compounds, sulphur components, and carbonyl
[71,78,79,80,81][1][11][13][14][15]. Halogenated carbons (or halocarbons), such as methylene chloride and chloroform, can trigger oxidation of the leuco-dye in the water system
[78,82][11][16]. Halocarbons are the organic compounds that have halogens such as iodine (I), chlorine (Cl), or bromine (Br) covalently bonded with one or more carbons.
Among the closest formulations of PRESAGE to water are those that employ methoxy-LMG as a new LMG derivative. The PRESAGE has an effective atomic number of 7.46, which holds a 0.54% difference from the effective atomic number of water (7.42) in the kilovoltage energy range
[83][17]. In the megavoltage energy range, the PRESAGE has an effective atomic number of 7.69, with 3.57% difference from the effective atomic number of water
[84][18].
3. Radiological Properties of PRESAGE
Extensive research has been conducted on the ionising radiation interaction probability in materials of diverse effective atomic number and density. Ionising radiation interacts with a material and deposit energy as it crosses along its path. High-energy radiation such as X-rays and gamma rays transfers most of its energy to secondary electrons that are generated by Compton, a photoelectric and pair production effect
[85,86][19][20]. The interaction probability is heavily dependent on mass density (
ρ), atomic number, and electron density (
ρe). In addition, Compton scattering does not depend on the atomic number of the absorbing material to occur because the Compton scattering process only requires free electrons. Thus, it relies on electrons per gram of the material (
ρe/ρ)
[87][21].
In PRESAGE, the carbon consisted of over 60% elemental components. Compared to the oxygen that amounted to over 80% in water in low photon energies, carbon shows a lower attenuation coefficient
[88][22]. In the kilovoltage energy range (10 kV–100 kV), where the photoelectric effect was prevalent, the higher energy absorption and the mass attenuation coefficient of PRESAGE were higher than water. However, due to the significant proportion of carbon, which has a low atomic number, the stopping power of PRESAGE is weaker than water, which has a high proportion of oxygen, which has a high atomic number.
3.1. The Role of Effective Atomic Number of Elements
Despite the major formulation of PRESAGE, which has a higher effective atomic number than water, it can be considered to have water equivalency in a good approximation at a higher energy range as long as the ratio of the electron density per the density of the material (
ρe/ρ) remains closed to the water
[72][5]. PRESAGE that has
ρe/ρ of 3.28 e/kg shows almost same photon probability interactions as water that has
ρe/ρ of 3.34 e/kg, which is only a less than 5% difference at an energy range above 300 keV to 30 MeV. However, due to the high effective atomic number, the photon interaction probability of the PRESAGE is not same as that of the water at the lower energy range, with the large difference of 81%. This indicates that the PRESAGE in the study is not suitable for low-dose dosimetry
[72,89][5][23]. For the usage of higher radiation energy, the electron density and the mass density should be taken into account because of the Compton scattering dominancy at that range of energies. PRESAGE has made an improvement known as Formulation A to reduce the effective atomic number by adding a small percentage of sulphur and reducing the percentage of bromine (Br). The reduction by even a small percentage of Br decreased the effective atomic number of PRESAGE significantly (Z
eff = 7.56), due to the high atomic number of the Br.
According to a study, the prominent difference in the photoelectric absorption between the PRESAGE and water is 40%, due to the reduction of Br, which is a reduction by 41% when compared with the original PRESAGE
[84,89][18][23]. Furthermore, the Compton scattering at the energy range of 2 MeV to 20 MeV of PRESAGE has a difference of 3% compared to water. For a pair production cross-section, PRESAGE has a maximum difference of 9% when compared with the water
[84][18]. A study shows that PRESAGE has a percentage difference within 2% in photon absorption as compared with water over an energy range of 10 keV to 10 MeV, due to the addition of DBTDL, which was able to modify the effective atomic number of PRESAGE
[90][24].
3.2. The Effect of Metal Compounds
The addition of metal compounds also has an effect on the photon interaction probability in PRESAGE. The increased percentage of metal compound in the PRESAGE would increase the effective atomic number. The increment is due to the high atomic number of metal atoms in the compound. A study shows that the PRESAGE that is incorporated with 3 mM of bismuth neodecanoate (Bi Neo) has a higher effective atomic number when compared with the PRESAGE that is incorporated with 3 mM of zinc octoate (Zn Oct), due to the high atomic number of Bi in the Bi Neo. Therefore, the photoelectric interaction probability of PRESAGE + Bi Neo is higher than PRESAGE + Zn Oct. Nevertheless, all the PRESAGE compositions used in the study do not have water equivalency at low energy ranges due to the higher effective atomic number than water. However, at high energy ranges, all the PRESAGEs have water equivalency due to the insignificant change of material density and electron density, especially at small concentration of the metal compounds
[91][25]. This indicates that the delicate incorporation of metal compounds, even at low concentration, has to be considered for low energy ranges, which is very important for low-dose radiotherapy. In addition, the study also shows a negligible difference for predominant Compton scattering energy ranges when low concentrations of metal compounds are included in the PRESAGE composition.
A PRESAGE that is known as a metal optimised dosimeter (MOD), fabricated by Alqathami et al., illustrates the closest water equivalency for both the low- and the high-energy ranges
[92][26]. It has an effective atomic number of 7.416, which is only a 0.013% difference from water. In addition, the photoelectric absorption cross-section for the PRESAGE (MOD) shows a less than 18% variation when compared with water. As compared with the previous study, the PRESAGE (MOD) has reduced the deviation from water by 22%. This improvement was attributed to an extremely small concentration of metal compounds in the formulation (
~0.01 wt.%). Following that, the Br and Cl were reduced, while S was removed from the composition. The Compton scattering cross-section demonstrated an extremely small deviation due to the low physical density. The available photon cross-section of PRESAGE is illustrated in
Table 1.
Table 1.
The low-energy and high-energy cross-section of PRESAGE.
83][17]. The study shows that different types and concentrations of halocarbons lead to different sensitivities. A higher effective atomic number possesses higher sensitivity. The enhancement is due to the carbon-halogen bond dissociation energy
[93][27]. Therefore, the closest effective atomic number to water does not necessarily have optimal dosimetric properties. Thus, there is a delicate balance between the effective atomic number to retain tissue-like radiological properties and the sensitivity of the dosimeter.
A high concentration of radical initiator led to a higher effective atomic number of the PRESAGE, which was undesirable as it caused deviation from the water equivalency
[80,91][14][25]. Nevertheless, the post-irradiation stability was constant for all the formulations in the study. A high radical initiator can increase the sensitivity. However, a high percentage of radical initiators can reduce the stability
[75,80][8][14]. Therefore, a small concentration of radical initiator was sufficient to obtain a high sensitivity of PRESAGE with a high stability that can be maintained. The sensitivity of PRESAGE can also be enhanced by increasing the concentration of carbon tetrachloride, another radical initiator for PRESAGE, up to 30%, and the sensitivity remains the same beyond this percentage
[80][14]. Another study reported that the ideal composition for the high sensitivity of PRESAGE is 4% LMG and 32% carbon tetrachloride
[95][29]. The concentration of radical initiator can impact the magnitude of dose quenching in proton therapy. A study found that the concentration of radical initiator below 12% or above 18% demonstrated a rapid rise of dose quenching compared to the intermediate concentration
[96][30]. An addition of 0.7% of dibutyltin dilaurate (DBTDL), a catalyst, also increases the sensitivity
[75][8]. Another factor that affects the sensitivity is the volume of the dosimeter. A study shows that a large dosimeter has lower sensitivity; it is less than half the sensitivity of a small dosimeter. This is seen to be formulation-dependent and related to different hardenings of PRESAGE cured in different volumes
[76][9]. A recent study demonstrated that dose rate influences the sensitivity of PRESAGE. The sensitivity of LMG elastomer-based PRESAGE was reduced as the dose rate increased
[97][31]. However, the study suggests there is no dose-rate dependency if the sensitivity is observed in large number of samples.
4.2. The Effect of Metal Compounds on the Sensitivity
The sensitivity of PRESAGE can be enhanced further through the incorporation of metal compounds, including those which are zinc-, bismuth-, and tin-based, at a very low concentration (0.2 wt.%), without altering the radiological properties. Among the three metal-based compounds, the bismuth-based exhibited higher sensitivity
[91][25]. These metal compounds offered advantages in accelerating polymerisation, increasing post-irradiation stability, improving post-response absorption value retention, and maintaining the dosimeter sensitivity
[75,91][8][25]. Moreover, the high percentage of an organometallic catalyst can also increase the sensitivity of PRESAGE due to the bonding between the halocarbons and the metal component. A high atomic number among the organometallic compounds increases the production probability of the secondary electrons and causes the increase in radical initiator production in the halocarbons, which in turn increases the oxidation of LMG. PRESAGE (MOD) holds the closest effective atomic number to water, which is 7.416. The formulation in PRESAGE (MOD) improved post-response photostability
[92][26]. A recent study demonstrated that PRESAGE with low Shore hardness presents lower sensitivity than PRESAGE with high Shore hardness. The study shows that the sensitivity of PRESAGE with high Shore hardness further increased by 36.6% upon incorporation with tartrazine
[98][32]. However, PRESAGE exhibits a high effective atomic number, which compromised its water equivalency. One study incorporated zinc oxide nanoparticles and revealed a PRESAGE that has a sensitivity of 0.0105 Gy
−1. A bromine-based RI PRESAGE was introduced recently and has improved the sensitivity of PRESAGE greatly, to 0.1109 Gy
−1—the highest sensitivity of PRESAGE ever attained. This might be due to the addition of solvent dimethyl sulfoxide (DMSO) in the composition of PRESAGE. DMSO has a stabilising effect on the LMG that contributes to the dose response. However, its water equivalency has to be a trade-off with the effective atomic number of 9.657
[94][28].
A different source of ionising radiation provides a different optical absorption for PRESAGE. PRESAGE has greater sensitivity or absorption on photons when compared with the carbon ions. A study shows that the carbon ions were observed to have a lower value of the dose-response slope when compared to photons
[99][33]. This indicates that PRESAGE has a higher sensitivity to photons than carbon ions.
Table 2 summarises the sensitivity of PRESAGE based on the value of the slope or gradient. The higher the value of the slope, the higher the sensitivity.
Table 2.
The sensitivity of various PRESAGEs with their corresponding density and effective atomic number.
5. Dose Rate and Energy Dependency
5.1. The Dose Rate Dependency
A range of studies demonstrated that the dose response of PRESAGE was neither significantly influenced by the photon energy nor the dose rate
[71,105,106][1][39][40]. An earlier study reported that the original PRESAGE
TM retained almost the same optical density (dose response) with an extremely small deviation over a different dose rate, particularly at lower doses. At 5 Gy, a difference of 2% was observed from the dose response of PRESAGE over six different dose rates. However, a notable deviation of the dose response between the different dose rates was observed at high doses of approximately 30 Gy to 50 Gy, with a difference ranging from 3% to 12%
[71][1]. A study shows that the reusable PRESAGE, known as PRESAGE
REU, also exhibits no dependence on the dose rate at 400 MU/min and 2400 MU/min. The study indicates that PRESAGE is capable of showing no dose rate dependency at a low dose rate and a high dose rate
[107][41]. However, another study demonstrated that the dose response of PRESAGE was unstable at an extremely low dose rate, with an over-response rise of 16% from 0.018 Gy/min to 1.0 Gy/min. In addition, the low dose rate also led to a higher dose response
[108,109][42][43]. This could be associated with the long-time exposure to the ionising radiation due to the extremely small dose rate. Thus, the chemical reaction occurred at a longer period in the PRESAGE, which yielded a high dose response. A recent study demonstrated that the incorporation of tartrazine did not influence the dose-rate dependency of PRESAGE
[98][32]. The silicone-based radiochromic dosimeter has been developed and shows insignificant dose-rate dependency
[110][44].
5.2. The Energy Dependency
The photon and electron energy showed a negligible effect on the dose response of PRESAGE
[111,112][45][46]. A study found that the 5 Gy dose response of 6 MV, 10 MV, 18 MV, and 1.25 MeV showed a difference within 4%
[71][1]. While another study reported that PRESAGE had a difference of less than 2.5% for a 5 Gy dose response from 6 MV and 18 MV
[113][47]. Moreover, PRESAGE was also capable of producing almost the same beam profile at the different energy levels of 6 MV and 18 MV
[114][48]. The dose response of PRESAGE showed no discrepancy between photon energy, proton energy, and electron energy
[112,115][46][49]. In general, no considerable difference in terms of dose response was present within the wide range of dose rates and energy. However, the difference of dose response was clear only at the extremely low dose rates and high doses delivered to the PRESAGE. It was suggested that more research work on the energy dependence of PRESAGE be conducted to investigate the energy dependence at a high dose (>30 Gy) and an extremely low dose (<1 Gy).
6. Stability of PRESAGE
The stability of the dosimeter refers to the ability of the dosimeter to maintain the same dose after irradiation over time. Notably, the most stable dosimeter is the one that can maintain same dose and resistance to the fading or change of optical density for a long period of time. The original PRESAGE has a colour bleaching rate of approximately 4% per 24 h over the week. The effect of heating prior to irradiation has an insignificant effect upon the stability of PRESAGE
[71][1].
6.1. The Effect of Radical Initiator
The radical initiator plays an important role in the stability of PRESAGE. A study shows that increasing the radical initiator more than 20 wt.% resulted in unstable PRESAGE over 2 days after irradiation. The colour bleaching increased to nearly 35%
[80][14]. The higher concentration of radical initiator led to continuation of LMG oxidisation after irradiation. The advantage of a high content of radical initiator was that it increased the sensitivity of PRESAGE. However, as the radical initiator increased, the PRESAGE became more unstable. A study demonstrated that a less sensitive formulation of PRESAGE has higher stability and fading is reduced
[99][33]. The stability of PRESAGE can be maintained by putting in a very low concentration of radical initiator. One study utilised different types of halocarbons as radical initiators and demonstrated a stable PRESAGE over a one week period after irradiation due to the small concentration of the radical initiator, which was less than 4 wt.%
[93][27].
6.2. The Effect of Metal Compound
The incorporation of a small amount of metal can improve the stabilisation of PRESAGE. The incorporation of around 5% of Bi, Sn, and Zn in the formulation shows the stability of PRESAGE after more than 12 days. This is due to the metal compounds working as a singlet oxygen quencher that reduces photofading. Singlet oxygen is the main cause of the photofading when combined with leuco-dye
[91,116][25][50]. In addition to the stability, the metal compounds also increase the sensitivity of PRESAGE by around 40%. However, the increase in the metal compound was limited because the high atomic numbers of the metal elements influenced the effective atomic number of PRESAGE which led to water inequivalence. One study claimed that the excessive use of an organometallic catalyst concentration increased the stability of PRESAGE. However, the high concentration of metal catalyst led to a sensitivity reduction in the PRESAGE. A small amount of tin-based catalyst has been observed to increase the stability of PRESAGE over 5 days after irradiation. Due to the small concentration of the catalyst, the sensitivity of PRESAGE remained unchanged
[75][8]. The most recent PRESAGE, known as water-equivalence PRESAGE, has been shown to have stability up to 7 days after irradiation when stored at a low temperature (4 °C). The low temperature halted the post-processing of PRESAGE up to 100 Gy. The water-equivalence PRESAGE was unstable at a dose of 200 Gy for up to 30 min post-irradiation. However, it remained stable after that for 21 days. Despite its long stability, the water-equivalence PRESAGE is considered to have low sensitivity
[103][37].
It can be concluded that a high concentration of radical initiator increases the sensitivity and decreases the stability of PRESAGE. Then, a high concentration of metal catalyst increases the stability and decreases the sensitivity of PRESAGE. The incorporation of metal compounds increases the stability and sensitivity of PRESAGE. However, to maintain the water equivalency of PRESAGE, there is a limitation to the amount of the metal compound. There is delicate balance between the amount of radical initiator, metal compound, and catalyst in the formulation of PRESAGE to obtain acceptable sensitivity, stability, and water equivalency.
7. Reusability and Reproducibility
Most PRESAGEs can typically be used once because the optical density gradually increases over time after irradiation. Following that, the absorbed dose is not reliable to read and analyse. There are a few PRESAGE dosimeters that have proven to have the potential for reusability. Instead of increased optical density, the PRESAGE gradually reduces optical density over time. After initial irradiation, the PRESAGE has the ability to return to its original optical density when exposed to room temperature.
The first reusable PRESAGE, known as PRESAGE
REU, is capable of going through four re-irradiations. However, the PRESAGE
REU is only capable of reproducing a consistent absorbed dose and sensitivity during the second, third, and fourth irradiation. During the first irradiation, a lower sensitivity is present by a factor of ~2 in comparison to the consecutive re-irradiation sensitivity. The PRESAGE
REU requires over 12 days for optical clearing. It is believed that the newly synthesised LMG derivative plays an important role in the reusability of the PRESAGE
REU [117][51]. Another study reported that the PRESAGE-RU was effectively cleared after exposure to room temperature for 5 to 7 days in a dark room. The PRESAGE-RU shows a slight decrease in sensitivity between irradiations from the first irradiation to the fifth irradiation. However, the PRESAGE-RU only reproduced the same absorbed dose for 0 Gy to 2.5 Gy. Higher than that, the PRESAGE-RU absorbed an inconsistent dose; specifically, this occurred on the first irradiation and the fifth irradiation
[118][52].
The most recent study demonstrated that PRESAGE could return to its original state in 2 days after irradiation. However, due to the exposure to room temperature following irradiation, the PRESAGE can only be used twice. In contrast, the PRESAGE can be reused up to four times when stored at low temperature after the irradiation. Subsequently, the results also were more stable and reliable. The study indicated that the PRESAGE used has high reproducibility and high reusability when stored at low temperature and only exposed to room temperature if necessary
[107][41]. The reusability of the PRESAGE is summarised in
Table 4. The percentage of reproducibility is estimated by the ratio of the same optical density linearity over the total reusability of the PRESAGE.
Table 4.
Reusability and reproducibility of PRESAGE.
80][14]. Another study reported that PRESAGE showed excellent linearity (R
2 > 0.99), from 0 Gy to 30 Gy with different halocarbon radical initiators, such as iodoform, bromoform, and chloroform. The increment of bromoform and chloroform concentration increased the linearity of PRESAGE by 0.07% and 0.04%, respectively. However, the increase in the iodoform concentration reduced the linearity by 0.1%
[93][27]. It was suggested that further research had to be conducted to look into the significant effect of halocarbon radical initiators on the linearity of PRESAGE.
The investigation of the oxygen influence on PRESAGE demonstrated that PRESAGE was capable of maintaining good linearity (R
2 > 0.99), with or without the presence of oxygen
[73,104][6][38]. Although good linearity was obtained by PRESAGE with the presence of a catalyst (DBTDL), the catalyst reduced the linearity by 2%
[75][8]. The increment in the concentration of the catalyst could reduce the linearity. A recent study shows that the PRESAGE also retained a good linearity with the change of LMG dyes, such as MeO-LMG, Cl-LMG, and Br-LMG
[83][17]. The various change of metal compound compositions also does not change the linearity of PRESAGE
[91][25]. In general, over the course of the PRESAGE reformulation to obtain an optimal sensitivity, stability, and water equivalency, the linearity of PRESAGE barely deteriorated.
Table 3 summarises the linearity of PRESAGE based on the correlation coefficient.
Table 3.
The correlation coefficient that represents degree of linearity for various PRESAGEs.
3.3. The Effect of Radical Initiator
Among the halocarbons, iodoform demonstrated higher density (4.01 g/cm
3) in comparison to the bromoform and chloroform, with the densities of 2.89 g/cm
3 and 1.49 g/cm
3, respectively. Nevertheless, PRESAGE that uses iodoform at 100 mM as a radical initiator shows a minor difference (<2.5%) in the Compton scattering cross-section, in comparison to water at the range of energy from 1 MeV to 20 MeV. Similarly, PRESAGE that employs iodoform shows a minor variation (<4%) for the pair production cross-section in comparison to water. In addition, due to the high atomic number of iodine (Z = 53), PRESAGE (iodoform 100 mM) has an effective atomic number of 16.03
[93][27]. This strongly indicates that the effective atomic number plays an insignificant role in water equivalency at megavoltage energies. However, PRESAGE (iodoform 100 mM) possesses a high deviation of more than 96% for photoelectric cross-section when compared with water. The most recent study, which used bromine-based RI PRESAGE, shows the small difference of 8% in the photoelectric absorption between the PRESAGE and water. This is due to the small fractional weight of Br in the composition. Increasing the proportion of Br has increased the percentage difference by more than 50%, due to the increment of the effective atomic number
[94][28].
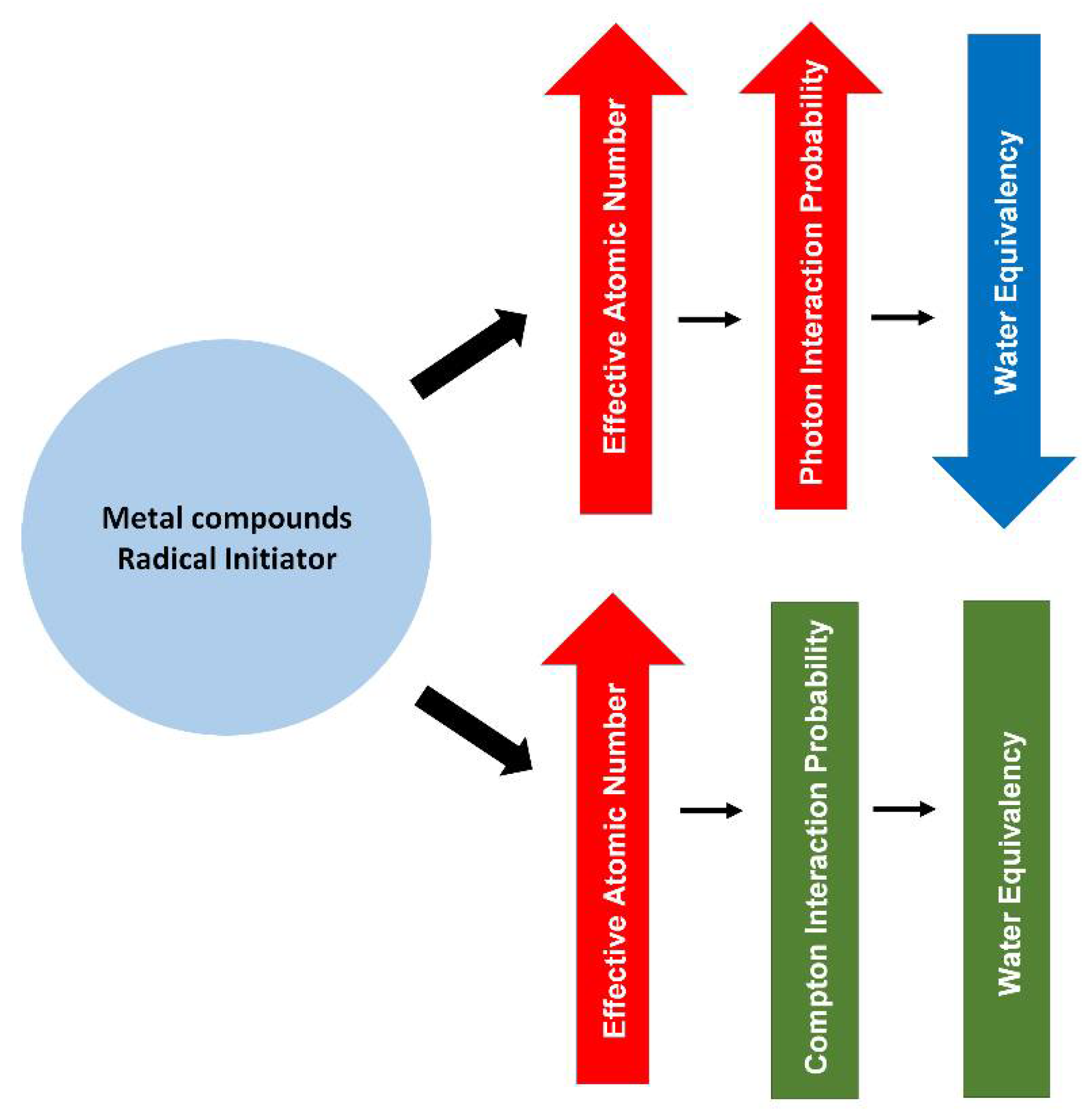
Figure 3 shows a simple diagram that summarises the effects of the metal compounds and the radical initiator on the radiological properties of PRESAGE.
Figure 3.
A diagram summarising the effect of metal compounds and radical initiator on the radiological properties of PRESAGE.
4. Sensitivity and Linearity of PRESAGE
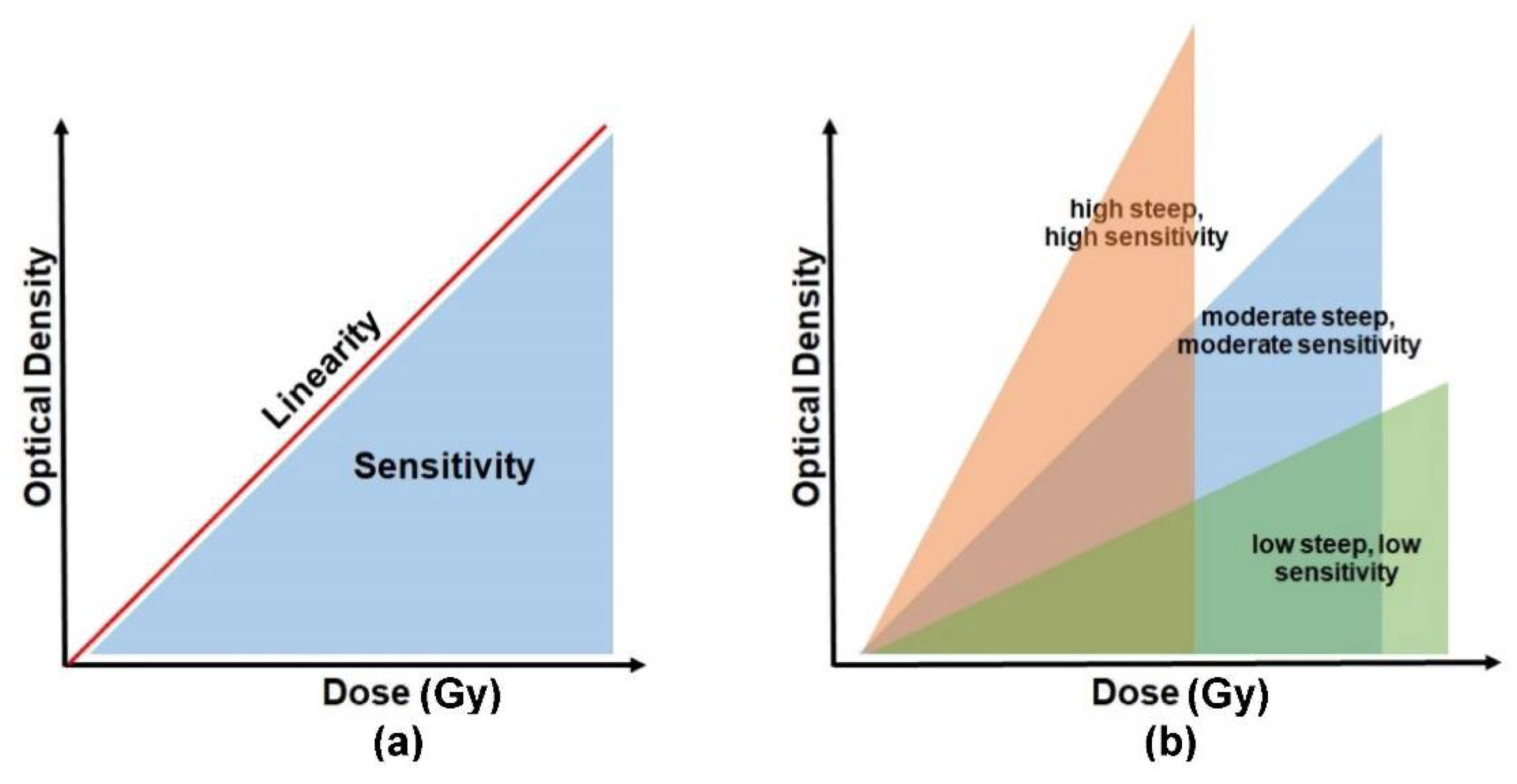
The absorbed radiation dose causes the irradiated PRESAGE to change its colour. The dosimeters become darker as the absorbed dose increases. The intensity of the changes is read out in terms of optical density. The absorbed dose is directly proportional to the optical density, and the relationship between the two parameters can be indicated as dose linearity, as shown in
Figure 4a. A good dosimeter should be capable of demonstrating dose linearity. The dose sensitivity of PRESAGE, on the other hand, can be illustrated by the slope of the optical density vs. the dose absorbed, as shown in
Figure 4b. The steeper slope of the curve indicates a higher sensitivity of the detector to radiation. PRESAGE will exhibit different sensitivity characteristics based on the effective atomic number and the weight fraction of the leuco-dye, the free radical initiator (halocarbons), and the catalyst.
Figure 4. The basic concept to interpret the sensitivity and linearity of PRESAGE. Diagram (a) represents a relationship between the sensitivity and the linearity. (b) The degree of sensitivity is illustrated by the slope of the optical density vs. the absorbed dose.
4.1. The Effect of Radical Initiator Concentration on the Sensitivity
The sensitivity of PRESAGE can vary upon the use of different radical initiators. The halocarbons that are often employed as radical initiators comprise various types of compounds which include iodoform, bromoform, and chloroform. Chloro-LMG and bromo-LMG have the effective atomic numbers of 7.5 and 8.14, respectively, but bromo-LMG has a higher sensitivity than chloro-LMG, which in turn has higher sensitivity than methoxy-LMG
[
4.3. Linearity of PRESAGE
The PRESAGE demonstrated a linear relationship between dose and optical response from 0 to 80 Gy, with a very good correlation coefficient of 0.9986
[101][35]. A study reported that the PRESAGE was capable of attaining a linearity of up to 100 Gy, with a correlation of more than 0.98. Another study found that the percentage of LMG in PRESAGE influenced its linearity. At the absorbed dose higher than 100 Gy, the PRESAGE was observed to be saturated with a lower response for another addition of doses and the relationship become non-linear. This result implied a consistent sensitivity of PRESAGE from 0 Gy to 100 Gy. In addition, the absorbance error increased for doses greater than 100 Gy
[99,102][33][36]. A later investigation showed that the PRESAGE was capable of providing linearity up to 200 Gy. This result could be associated with the inclusion of DBTDL as a catalyst in the PRESAGE formulation
[103][37].
A study demonstrated that the percentage of LMG in PRESAGE influenced the linearity. The linearity of PRESAGE was reduced (R
2 > 0.90) upon the presence 3 wt.% of LMG. Excellent linearity (R
2 > 0.99) was observed in the PRESAGE that had 1 wt.% and 2 wt.% LMG
[
8. Readout Modalities
Magnetic resonance imaging (MRI), ultrasound, optical computed tomography (OCT), and X-ray computed tomography (X-ray CT) are the common types of systems that are employed as the readout method of the irradiated PRESAGE dosimeter. The PRESAGE exhibited an absorption peak at the wavelength of 633 nm, which is the typical absorption of oxidised leucomalachite green (LMG)
[71,93,99][1][27][33]. Therefore, the appropriate source for optical scanning is red LED or laser of helium-neon monochromatic
[93,99][27][33]. In comparison to the polymer gel and ferric gel, the strong points of PRESAGE include its significant proportion of light absorption compared to light scattering
[121,122,123][55][56][57]. The light scattering and light refraction are able to produce artefacts in optical dosimetry. Notably, the elimination of the light-scattering impact is important in designing an ideal optical computed tomography (OCT) scanner which minimises the scattering artefact
[124][58].
8.1. Magnetic Resonance Imaging (MRI)
MRI is heavily implemented as a quantification system for the Fricke gel and the polymer gel dosimeter. MRI evaluates the change of magnetic resonance which occurs in the irradiated Fricke gel dosimeter as a result of the transformation of ferrous ion (Fe
2+) into the ferric ion (Fe
3+). It can also assess the change of transverse and lateral relaxation time that occurs due to the polymerisation of the irradiated polymer gel. Despite being highly accurate with precise evaluation, the MRI suffers from a low signal-to-noise ratio (SNR), long scanning time, and susceptibility to imaging artefact and limited spatial resolution
[18,47,125,126][59][60][61][62]. The cost of imaging a dosimeter using an MRI can be expensive
[18,127][59][63].
8.2. Ultrasound
Ultrasound is dependent on the ultrasonic properties of the gel dosimeters, which are its acoustic velocity, ultrasound attenuation, and ultrasound flight time; these are associated with the polymerisation to read the dose absorbed by the dosimeter
[60,128,129][64][65][66]. The ultrasound has the advantage of being low-cost and dynamic, and it can produce high-resolution images. However, the sensitivity of the readout is low, and it takes a certain period to achieve the ideal reading after irradiation
[60,128][64][65]. In addition, the ultrasound is also incapable of reading the linear dose response at the lower doses (<10 Gy)
[130][67]. Furthermore, the dose response is also observed to be saturated at a dose higher than 30 Gy, which implies its low sensitivity to reading lower doses and higher doses
[60,128][64][65]. To date, no attempt at reading the dose response of PRESAGE through ultrasound has been made.
8.3. X-ray Computed Tomography (X-ray CT)
The X-ray CT scan, on the other hand, depends on the changes in the attenuation coefficient of the dosimeter that are characterised as the changes in electron density. The X-ray CT has the potential to become an important readout tool due to its high SNR and short scanning time. However, the major drawback of the X-ray CT is the low image contrast (thus, low-dose resolution) and low dose sensitivity
[18,35,127][4][59][63]. In addition, the practicality of its utilisation still demands to be proven
[18][59].
8.4. Optical Computed Tomography (OCT)
OCT is the most common type of system that has been used as a readout technique for the radiochromic dosimeter. The readout devices are responsible for evaluating and quantifying the dosage distribution of the dosimeter. OCT has high spatial resolution and a short scanning time and has a small physical size, which makes it portable and easy to mobilise
[18,35,40][4][59][68]. Furthermore, it also provides high SNR due to the laser beam high intensity and the capability to scan large samples without the necessity of high-cost optical components
[35,81][4][15]. The first generation of OCT, commercialised as OCTOPUS, has been the only commercial OCT for a number of years. The first generation of OCT capable of producing high quality images was pointed out as a “gold standard”
[131][69]. Even more, it has the ability to remove light contamination
[40][68]. A slow scanning speed is the major drawback of the laser-scanned OCT; the speed is 12 min per slice with 128 × 128 pixels
[132][70]. OCTOPUS has improved the scanning time to 5 min per slice. However, full 3D imaging still requires up to 16 h
[133][71].
The second generation of OCT was developed as an alternative for a faster scanner that is based on a coupled-charged detector (CCD) and a broad cone light beam that is commercially known as Vista
[132,134][70][72]. The CCD-based OCT provides an advantage over its first generation in terms of scanning speed due to the CCD chip. It delivers complete two-dimensional (2D) projection in an instant instead of forming 2D distribution from collected one-dimensional projection, which significantly consumes time. The CCD-based OCT is capable of obtaining a complete 2D image as fast as the laser-scanned OCT obtains a 1D image
[132][70]. The broad scanned light gives advantages when scanning a big sample, without the necessity of purchasing expensive optical component. It is cheap and easily scalable
[135][73]. However, it suffers from a scattered radiation issue that needs various methods of correction to reduce the scattered light artefacts
[136][74]. The parallel beam scanner with a telecentric lens is another type of CCD-based OCT that has the advantage of reducing the scattered radiation effect
[137][75].
Another OCT system was developed based on the Complementary Metal Oxide Semiconductor (CMOS) active pixel image sensor. A CMOS image sensor has higher signal-to-noise ratio (SNR), higher spatial resolution, and a faster frame rate when compared to the CCD chip. The active pixel has the ability to integrate signal processing at the pixel level. The CMOS has gained increasing attention as a competitive technology to CCD and has been used in high-end consumer products and scientific instruments
[35,138][4][76]. A CMOS-OCT system has been developed as a measurement in radiotherapy
[35,138][4][76]. A study demonstrated that a CMOS-OCT was able to produce a multidimensional dose analysis with consistent results
[139][77]. The CMOS-OCT system was also capable of visualising stereotactic radiosurgery (SRS) treatment dose distribution in a PRESAGE dosimeter. The system can measure dosimetric and geometric information during radiotherapy delivery accurately. In addition, the CMOS-OCT system also provides a shorter scanning time than the CCD-based OCT, with a higher dynamic range
[140,141][78][79].
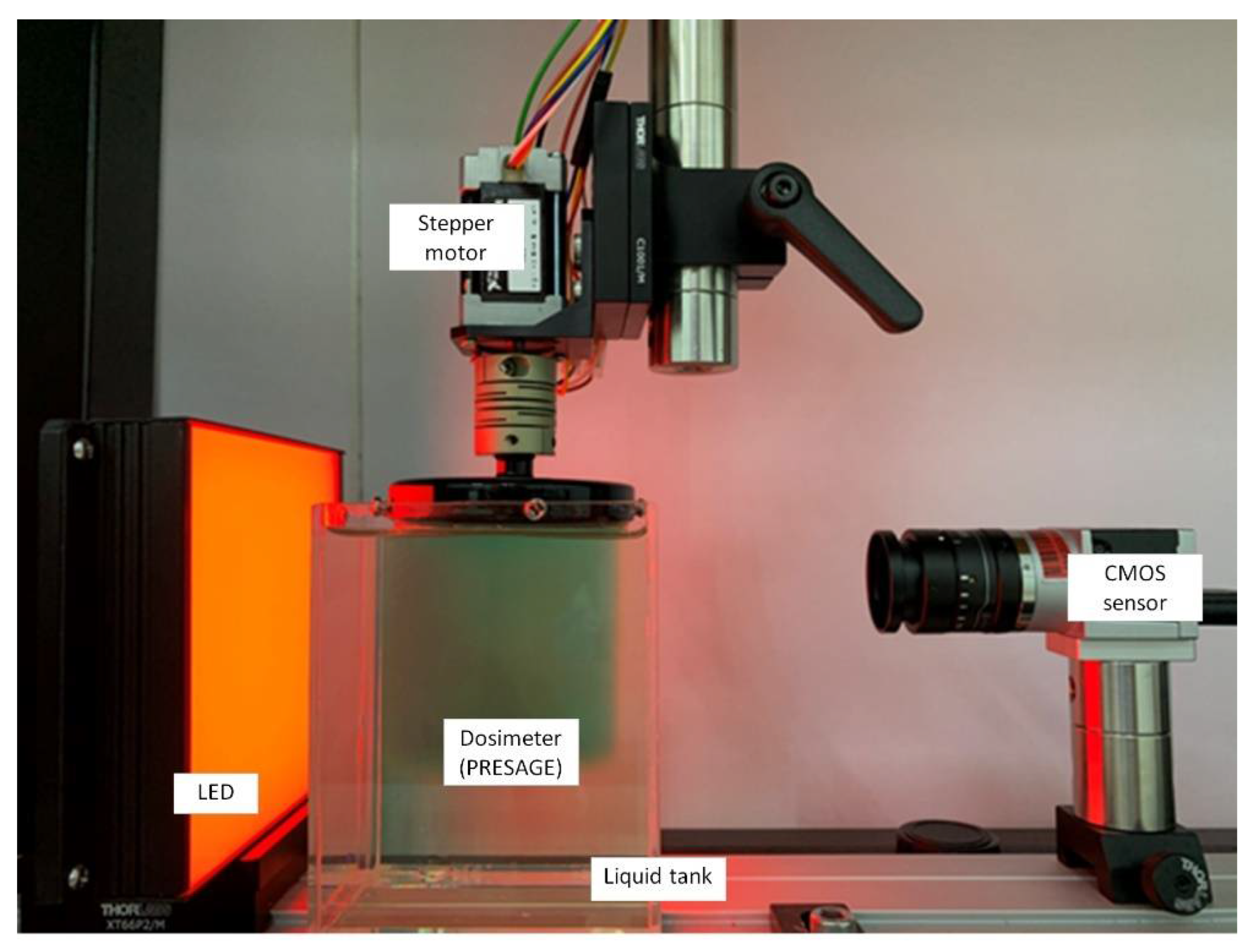
Figure 5 shows the CMOS-OCT imaging system, which consists of a CMOS sensor, stepper motor, LED, and dosimeter.
Figure 5. The in-house CMOS-OCT dosimetry system from the Laboratory of Medical Physics & Simulation, Universiti Teknologi MARA. The system primarily consists of CMOS sensor, the dosimeter, LED, and stepper motor.