The plastic monomer and plasticizer bisphenol A (BPA) is one of the most widely used chemicals. BPA is present in polycarbonate plastics and epoxy resins, commonly used in food storage and industrial or medical products. However, the use of this synthetic compound is a growing concern, as BPA is an endocrine-disrupting compound and can bind mainly to estrogen receptors, interfering with different functions at the cardiovascular level.
1. Introduction
According to the World Health Organization, cardiovascular diseases (CVDs) are the leading cause of death worldwide. The majority of CVDs are chronic and asymptomatic over a long time, and usually, the first symptoms only appear as the disease progresses. However, CVDs can also induce immediate sudden death, which is the main cause of premature mortality worldwide. It is estimated that by the year 2030, 23.6 million people will die from CVDs each year. However, there is a slight downward trend in mortality and CVD incidence in north-eastern and Southern Europe
[1].
Currently, the influence of environmental contaminants on humans has been proposed as a cause of CVDs
[2]. Every year, millions of tons of plastic are produced worldwide which results in continuous daily human exposure to these environmentally toxic chemicals
[3]. Endocrine-disrupting compounds (EDCs) are defined by the North American Environmental Protection Agency as a natural or synthetic compounds which can interfere with the actions of the endocrine system. Specifically, EDCs can mimic or antagonize the action of endogenous hormones and alter their synthesis, transport, binding, and elimination. Then, these emerging compounds can disrupt normal hormonal homeostasis, reproduction, and/or behavior
[4][5][4,5]. Moreover, EDCs are potential modulators of cardiovascular physiology, from which emerges the need for the study of their cardiotoxicity
[6].
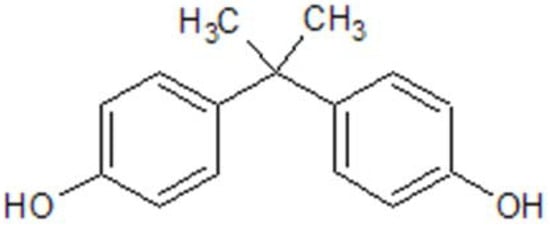
Among the various EDCs, bisphenol A (BPA) (
Figure 1) stands out as one of the most widely produced EDCs worldwide
[7]. BPA, also designated as 4,4’-ispropylidenediphenol by IUPAC, is a synthetic organic compound formed of two phenol groups, used in polycarbonate plastics and epoxy resins
[8]. In 1891, BPA was first synthesized by the chemist Alexender P.Dianin, and about 40 years later, some of its estrogenic effects began to be discovered
[9]. Its properties give plastics greater thermal resistance and elasticity, and for this reason, BPA is still in use after 130 years of its discovery.
Figure 1. BPA chemical structure, drawn in ChemDraw®®.
Regarding its appearance, BPA is a solid, white, crystalline substance whose melting point is 156 °C, with a boiling point of 220 °C (at a pressure of 5 hPa). Furthermore, BPA has a water–octanol coefficient of log Pow = 3.32, indicating that it has good solubility in fats, and contrariwise, low solubility in water (~200 mg/dL
3 at 25 °C). The presence of hydroxyl groups determines the good reactivity of BPA. Like other phenols, bisphenol can be converted into ethers, esters, and salts
[10]. The structure of BPA is similar to that of 17β-estradiol, and for that reason, this EDC binds to estrogenic receptors such as ERα, ERβ, ERγ, G-protein-coupled estrogen receptor (GPR30), and peroxisome proliferator-activated receptor gamma (PPAR-γ)
[11]. Although the mechanisms of action are not yet fully understood, BPA has been shown to induce insulin resistance, adipogenesis, pancreatic β-cell dysfunction, inflammation, and oxidative stress
[12].
2. Exposure to Bisphenol A (BPA)
Globally, the use of BPA has progressively increased, reaching more than 10 million tonnes per year
[7][10][7,10]. BPA is present in 95% of products requiring epoxy resins and polycarbonates, such as food containers, bottles, toys, dental products, CDs, DVDs, and water pipes
[13][14]. The use of BPA in consumables and medical products makes its exposure continuous, having been detected, for example, in urine in over 90% of the United States (US) population
[14][15]. In addition, BPA has also been identified in other biological samples, such as maternal blood (0.3 to 18.9 ng/mL)
[15][16][17][18][16,17,18,19], maternal urine (31.9 μg/L)
[18][19], amniotic liquid (median = 0.26 ng/mL)
[16][17], placental tissue (median = 12.7 ng/g)
[15][16], umbilical cord blood (0.2 to 9.2 ng/mL)
[15][17][19][16,18,20], breast milk (0.61 to 0.7 μg/L)
[18][20][19,21], and human colostrum (3.41 ng/mL)
[21][22]. However, in biomonitoring studies, urinary samples of BPA are often used. The reason is that BPA is a non-persistent chemical, so its chemical concentration is higher in these samples, compared to human plasma or serum
[6][22][6,23]. Nevertheless, the degree of exposure to BPA is quite variable depending on socioeconomic factors, lifestyle, medical status, and exposure pathways
[6]. With regard to this, oral exposure is considered the most prevalent, with BPA levels associated with dietary choices
[6][23][6,24]. On the other hand, cutaneous absorption and/or inhalation may also be associated with a higher level of exposure to unconjugated or biologically active BPA, which may persist for longer periods (~5.4 h) compared to ingested, subject to first-pass metabolism
[22][23].
BPA, similarly to other EDCs, interacts with receptors activated by estrogens, androgens, thyroid hormones, and peroxisome proliferator, and acts as an agonist or antagonist via a receptor-dependent signaling pathway; this is attributed to its chemical structure. However, its chemical structure may be an advantage, as demonstrated for binding to ER, in which BPA does not achieve proper accommodation in the confines of the hormone-binding site (it only induces a displacement of α-helices forming the ligand-binding domain (LBD))
[11]. Moreover, Tan et al. demonstrated that EDCs share three levels of key fragments: primary and secondary fragments (responsible for the receptors binding, which discriminate active and inactive compounds), and tertiary fragments that determine their activity type (agonist, antagonist, or agonist–antagonist (A-Anta)). This determination is achieved via the interaction of EDCs with the functional lobes, directly affecting the AF-2 surface, which is responsible for coregulator recruitment. In the case of BPA, this EDC contained primary fragments of oxygen-containing aromatics and secondary ones (bisphenol group)
[24][25]. The coexistence of primary and secondary fragments is responsible for activating BPA (active compound). Activation of the estrogen receptor (ER) and androgen receptor (AR) is achieved via interactions of the secondary fragment of stabilized BPA conformations in the LBD by forming hydrogen bonds with R394 amino acid and via van der Waals interactions with N705 amino acid, respectively. The comprehension of secondary fragments forming interacting networks with LBD amino acids is the basis for the activity of BPA. Ligand fragments of BPA interact with LBD and cause changes in the conformation of the AF-2 surface, recruiting two cofactors and, thus, determining its tertiary fragment (A-Anta activity).
Similar to natural hormones, some of the experimental studies with BPA suggest a non-monotonic response, highlighting that risk assessment is required with exposures from ‘lower’ to ‘higher’ doses, given the characteristic U-shaped response also observed by other EDCs
[25][26]. Not surprisingly, this property can complicate BPA toxicity risk assessment, as this EDC can interact with hormone receptors in specific cell types and/or have multiple biological endpoints with linear dose–response that collectively produce a non-monotonic dose–response relationship
[26][27].
Over the past few years, there has been growing concern regarding the adverse effects of BPA exposure on human health. These adverse effects have led countries such as Denmark and Belgium to restrict the use of BPA in food packaging for children between the ages of 0 and 3. Sweden has also limited the use of BPA in varnishes and food packaging coatings for children in the same age group. In addition, Austria has restricted the use of BPA in pacifiers and bottles since October 2010
[27][28].