Celiac disease (CD) is an immune mediate disease characterised by gluten dependent T-cell mediated activation, autoimmunity and derangement of the intestinal mucosa in a specific genetic background. Although the activation of the T-cells has been studied in dept, the central question remains still unanswered, namely, why a pro-inflammatory T cell response to gluten is generated instead of a regulatory response, which normally promotes oral tolerance to dietary protein antigens.
Recent Tliterature demonstrated that there is an inflamed environment in CD intestine, enriched in cytokines, such as IL-15, or type I interferons, in which T cells tend to acquire a pro- inflammatory phenotype. The factors that create a pro-inflammatory environment in the CD intestine, leading to an expansion of gliadin-specific T cells in genetically susceptible individuals and further shifting them towards a pro-inflammatory phenotype, remain to be identified. The recent literature is starting to address this question. From these studies it appears clearly, that subtle alterations on the CD cells are present also before the introduction of gluten. Gluten exacerbates these constitutive alterations, by increasing the same markers already altered before the gluten introduction, both in vitro and in vivo.
All these new observations add this disease “tout court” to the big family of increasing chronic inflammatory diseases where nutrients can have pro-inflammatory or anti-inflammatory effects, directly or indirectly mediated by the intestinal microbiota, where the intestine functions as a cross road for the control of the inflammation both local and at distance.
- celiac disease
- gluten
- inflammation
- microbiota
1. Celiac Disease as an Inflammatory Chronic Disease
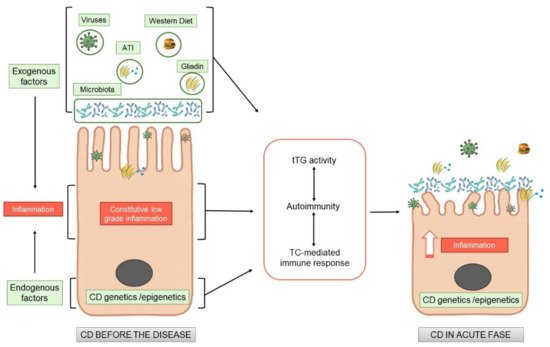
2. Endogenous Alterations in CD Independent of Gluten
| Endogenous Factors | |
|---|---|
| Models Investigated | Pathways Described |
| Children at risk of CD (before gluten introduction) |
|
| GFD–CD biopsies |
|
| Intestinal organoids derived from CD patients |
|
| Nonintestinal cells from GFD–CD patients |
|
2.1. Patients at Risk of CD: What Happens before the Disease?
2.2. GFD–CD Patient Biopsies, Lessons from In Vivo and In Vitro Studies
Celiac patients after diagnosis have to initiate a strict diet without gluten. These patients are defined as gluten-free diet–celiac disease patients (GFD–CD). The dietary restrictions have to be followed for life. GFD–CD patients do not eat gluten; thus, the main cause of the disease is not present, but some alterations mainly at the intestinal level can still be found.GFD–CD Patients before and after Gluten Challenge In Vivo
New observations have been obtained and published challenging gluten in vivo in GFD–CD patients by analyzing before and after gluten introduction on several different pathways such as proliferation, inflammation and differentiation at the intestinal level [9][10][11][25,26,27]. Dotsenko et al., demonstrated that the intestinal morphology in patients on a strict GFD was similar to that measured in control subjects [9][25]. However, gene transcription showed that the GFD–CD group differed significantly from the control group, showing a substantial number of differentially expressed genes. Gene Ontology and Reactome pathway analyses revealed that patients on a GFD presented altered expression of genes with functions such as brush border assembly, developmental processes, transport of small molecules, and FOXO (Forkhead box-O)-mediated transcription of cell cycle genes [9][25]. Moreover, the Wnt (Wingless and Int 1) pathway has been found altered in GFD–CD patients with respect to controls, suggesting that the alterations of a pathway nodal to intestinal differentiation and homeostasis is also present in the absence of gluten [9][25]. In the gastrointestinal tract, Wnt signaling activation drives homeostasis and damage-induced repair. Wnt signaling also has a key role during stem cell-driven intestinal homeostasis, regeneration, ageing and cancer [44][59]. After being gluten challenged, the Wnt pathway was further altered, as tested by RNA sec, in intestinal biopsies of patients on a GFD with respect to controls [9][25]. All together, these data show that even on a strict GFD, patients reveal patterns of ongoing disease and that gluten acts on the same pathways already altered. Another recent paper by Stamnaes J. et al. [10][11][26,27] shows by proteomics analysis that before being challenged in GFD–CD patients, epithelial inflammation is already in the intestines, with changes indicative of minor crypt hyperplasia and low-grade inflammation in the serum. After challenge with gluten, an increase in the same proteins, already altered at base line in GFD–CD responders, was found. Despite clinical and histological remission, celiac disease patients that develop a mucosal response after 14 days of gluten challenge have already at baseline altered protein compositions of their gut tissue with signs of ongoing inflammation. In conclusion, these observations indicate that in GFD–CD patients, several different pathways such as proliferation, inflammation and differentiation are altered at the intestinal level and that they can be further altered by gliadin challenge.GFD–CD Patient Biopsies
Several papers have been published studying the biopsies of GFD–CD patients. In these papers, several pathways have been found altered by different techniques. Most of the papers analyze the whole biopsies, as in the case of genetic or expression studies, while in some others, only the epithelium are evaluated. The following listed are the most significant literature contributions that we discuss, clustered by the pathways found altered.- A.
-
Inflammation
- B.
-
Innate immunity pathways
- C.
-
Enterocyte proliferation and differentiation
- D.
-
Structural alterations
2.3. Intestinal Organoids
An emerging role in CD pathogenesis has been attributed to the intestinal epithelium. In epithelial cells of CD patients, morphological and functional alterations have been described together with the activation of the inflammasome pathway [23][64][39,79]. Organoids derived from the small intestine represent a new tool to study the role of the intestinal epithelium in several different diseases [65][80]. Intestinal organoids are derived from crypt stem cells cultivated in 3D and embedded in a matrix; they resemble the small intestinal epithelium. Organoids from CD patients have shown the presence of increased staminality, permeability, inflammasome activity, and innate immunity genes with respect to organoids in healthy individuals [33][64][48,79]. Instead, extracellular matrix (ECM) genes were decreased in [34][49]. Moreover, increased markers of inflammation were found at the protein and mRNA levels in CD organoids. This inflammation was not a residual effect of the tissue of origin but is probably constitutive, as it was persistent even after many days in culture [1][17]. In CD biopsies and in intestinal organoids, increased sensitivity to inflammatory stimuli from bacteria [64][79], viral ligand loxoribine [1][17] and gliadin peptide P31-43 [1][17] have been described, indicating that intestinal organoids from CD patients are more sensitive to pro-inflammatory stimuli. Taken all together, these observations indicate that CD intestinal epithelial cells are constitutively different from those in healthy individuals.
