Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sadanand Pandey and Version 2 by Catherine Yang.
Breast cancer (BC) is a highly metastatic multifactorial disease with various histological and molecular subtypes. Due to recent advancements, the mortality rate in BC has improved over the past five decades. Detection and treatment of many cancers are now possible due to the application of nanomedicine in clinical practice. Nanomedicine products such as Doxil® and Abraxane® have already been extensively used for BC adjuvant therapy with favorable clinical outcomes.
- breast cancer
- cancer imaging
- theranostics
- Nanotechnology
1. Biomarkers for BC Detection
Various biomarkers have been exploited during the initial diagnosis of BC [1][87]. The method of diagnosis becomes much more valuable depending on how earlier and accurately the biomarker can be detected. Various tumor antigens have been investigated that may elicit a response specific to that tumor and, therefore, can be employed in the initial recognition of cancer. Different approaches were studied to identify tumor antigens [2][88]. Scientists have suggested a proteomics-based approach for detecting autoantibodies to proteins isolated from tumor lysate that may help identify antigenicity related to post-translational modification of tumor cell proteins. Several autoantibodies have been reported to be biomarkers for BC. RS/DJ-1 has been identified in 13.3% of newly recognized BC patients [3][89]. In addition, 15% of patients with a poor prognosis for BC had elevated levels of p53 autoantibodies; however, the biomarker was seen in malignancies other than BC [4][90]. Thus, p53 does not offer BC-specific autoimmunity [5][91]. Specific heat shock proteins (HSP) such as HSP 60 and HSP90 have also been recognized to elicit the BC-specific immune response [6][92]. Another serum protein, CA 15-3, was identified to be a circulating biomarker for BC [7][93]. Researchers have used this protein to detect BC recurrences and monitor treatment for metastatic cancer. The CA 15-3 concentration also had a prognostic impact at the initial stage [8][94].
HER/neu, a human epidermal growth factor receptor 2, has been recognized as a critical determinant in malignant alterations in BC that can indicate its destitute prognosis [9][95]. Specific ductal proteins have been isolated from nipple exudate and were identified as important BC biomarkers. Among various ductal proteins, lipophilin-B, beta-globin, hemopexin, and vitamin-D-binding protein have been found overexpressed in nipple exudate from samples of tumor-bearing breasts [10][96]. Several studies have also described the use of abnormally expressed miRNA-21 for early recognition of BC. NP or enzyme-labeled miRNAs have been found to have high sensitivity via miRNA hybridization [1][11][87,97].
2. Nanotechnology for Early Detection of BC
There has been extensive research on using nanotechnology for early cancer detection [12][13][98,99].
2.1. Graphene NPs
Due to its unique surface characteristics and simple surface functionalization, many applications of graphene and its derivatives have been introduced into the biomedical field [14][100]. Graphene-based nanomaterials possess inherent anticancer properties and can enhance cell adhesion and capture BC cells. Graphene may form toxic byproducts in tumor cells through oxidative stress and autophagy. Graphene nanomaterials have been shown to inhibit macrophage activity, causing oxidative damage [15][101]. Rostamabadi and coworkers have demonstrated an electrochemical method for detecting BC biomarker HER-2 utilizing a transformed carbon electrode. Graphene oxide and carbon nanotubes (single-walled) were densely packed with AuNPs and placed on the glassy carbon electrode. The electrode was functionalized by an HER-2-specific aptamer that selectively recognizes HER-2 at the electrode interface, leading to enhanced charge transfer resistance using ferro- or ferri-cyanide as an electrochemical probe. This method was found to be highly specific and reproducible in discriminating the serum of patients affected with BC from serum samples of healthy individuals [16][102]. Safavipour and colleagues have proposed MUC-1 apta-sensors based on TiO nanotubes coupled with graphene oxide for the electrochemical recognition of MFC-7 BC cells. The biosensor effectively detected MUC-1 biomarkers in clinical samples and, thus, can help detect BC cells [17][103].
Xu and coworkers have investigated nano-enzyme decorated graphene quantum dots for electrochemical recognition of H2O2 in clinical BC analysis. The nanocarrier system exhibited high sensitivity and good biocompatibility for instantaneous tracing of H2O2 released from varying BC cells [18][104]. Graphene-based nanomaterials show high drug-loading capacity, easy functionalization, high target selectivity, and chemo sensitization. Graphene-based nanomaterials can be used in BC diagnosis and treatment due to their distinctive structures and attractive physicochemical properties.
2.2. Mesoporous Silica NPs
In addition to chemotherapy, photodynamic therapy (PDT) and photothermal therapy (PTT) can be used as a non-invasive therapeutic strategy as a complement to overcome the deficiency of monotherapy. As a result of light exposure, photoactive therapeutics (photosensitizers) can produce reactive oxygen species (ROS) or hyperthermia, thus killing cancer cells. Mesoporous silica NPs are suitable for multi-drug loading since they have a large surface area, pore size, and volume, making them a suitable target for photo-chemotherapy. The structural merits of MSNs have generated considerable attention as potential partners for PDT in recent years. In solid tumors, many photosensitizers aggregate easily, reducing their efficacy, and have poor intracellular uptake, making them unsuitable for use. The incorporation of MSNs prevents PSs from aggregating and improves their targeting ability and biocompatibility, resulting in fewer side effects and stronger anti-cancer effects.
Wang and coworkers have proposed mucin-1 protein (MUC-1) targeted magnetic silica-based mesoporous NPs to capture MFC-7 cells. These silica NPs were coupled with a folate-receptor-directed fluorescent probe by conjugating folic acid and fluorescein isothiocyanate over the surface of bovine serum albumin for selective and specific labeling of folate receptors in HER-2over-expressed MCF-7 cells. The quantitative assay developed showed superior specificity and sensitivity towards MCF-7 cells [19][105]. Chen and colleagues have developed anti-HER-2 single-chain variable segment functionalized ultra-small silica nanostructures to increase tumor-specific targeting efficiency and improve renal clearance. This nanostructure was proposed to be highly efficient in imaging BC and an ideal candidate for delivering therapeutic agents to the desired region [20][106].
Qiao and coworkers have explored pH-responsive plumbagin-loaded theranostic NPs composed of mesoporous silica-covered gadolinium-III, conjugated with zoledronic acid for early recognition of BC-associated bone metastasis with increased sensitivity and selectivity [21][107].
2.3. AuNPs
Salahandish and coworkers have demonstrated gold and silver NPs grafted graphene and nanostructured polyaniline for highly selective biosensing and a wide linear response range. The nanostructures were found to be ultrasensitive for label-free sensing of BC cells. These nano-biosensors had a faster response rate and a detection limit as low as 2-cells/mL for the SKBR-3 cell line. The sensor also exhibited a high detection efficiency of 90% [22][108].
In another experiment by Saeed et al., developed an electrochemical DNA biosensor based on AuNPs-modified graphene oxide for simultaneous detection detect CD24, a new prognostic marker in BC, and a transmembrane protein tyrosine kinase (ERBB2). The developed DNA nanosensor utilized a sandwich-type detection method, and amperometric detection was used to assess the sensor’s response. For this purpose, the gold nanoparticles were grafted onto the glassy carbon after being attached to graphene oxide. Surface immobilization was used to attach thiolated nucleic acid capture probes. Because of the amplification of the signal that was obtained, sensitive detection of both BC markers (ERBB2c and CD24c) was possible. The detection limit was found to be 0.16 nM and 0.23 nM for ERBB2 and CD24, respectively [23][109].
Dong and coworkers have investigated HER-2 functionalized magnetic gold-shelled poly (lactic-co-glycolic acid) nanocarriers for ultrasound or magnetic resonance imaging and BC photothermal therapy. The nanocarrier system exhibited a receptor-specific binding HER-2 overexpressed in BC cells (SKBR-3) with a significantly high binding affinity. In vitro studies revealed that NPs possessed both enhanced ultrasound and magnetic resonance imaging properties [24][110]. Photothermal cytotoxicity experiments revealed multifunctional nanocarriers to have excellent photo-absorption and thus can be beneficial in the photothermal therapy of BC [24][110]. Rao and coworkers have suggested gold nanocluster-loaded functionalized liposomes for early recognition of HER-2 overexpressed BC cells [25][111]. In another study, an aptamer-based bipolar electrode system modified by AuNPs was used to amplify the signals, and the sensor can detect overexpressed nucleolin in BC (MFC-7) cells. The apta-sensors were found to have low cost, high sensitivity, and selectivity towards BC cells [26][112]. AuNPs functionalized with copper frameworks were also investigated for BC sensing with quaternary chalcogenide-platinum-doped graphitic carbon nitride (g-C3N4). Cu2ZnSnS4 NPs can be used for the detection of HER-2 in BC [27][113].
Tian and associates have investigated AuNPs for electrochemical sensing (label-free) of microRNA-21 (miRNA-21) via a redox indicator. These sensors exhibited high sensitivity, selectivity, and reproducibility in blood serum samples [28][114]. Feng and coworkers have developed an electrochemical AuNP-based biosensor for sensitive and selective detection of BARC-1 to form a sandwich-type assembly (Figure 12) [29][115].
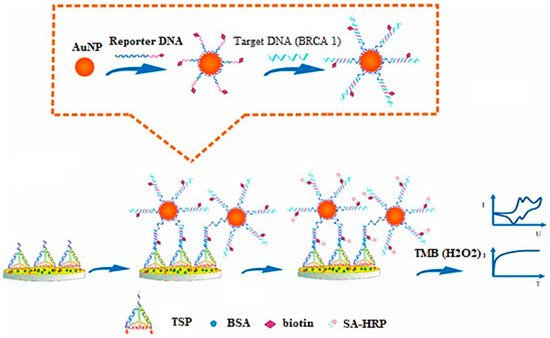
Figure 12. Principle for development of DNA-based electrochemical gold nano-sensors, reprinted from [29].
2.4. Silver NPs
Arteaga and coworkers have utilized silver NPs (citrate reduced) as a substrate for surface-enhanced Raman spectroscopy to quantify BC-associated elevation in sialic acid in saliva. This simplified test exhibited a 94% sensitivity and 98% specificity for the diagnosis of the BC patient, with a cut-off concentration of sialic acid assumed to be >7 mg/dL [30][116].
In another study, label-free geno-sensors were developed for the detection of BC-specific biomarker miRNA-21. The sensor was based on graphene functionalized silver NPs, resulting in significant electrochemical signal amplification. This biosensor was found to be very reproducible in assessing blood samples and can be used for direct recognition of miRNA-21 during the early stages of BC without the need for any sample preparation or RNA extraction [31][117].
2.5. Iron-Oxide NPs
Albernaz and coworkers have investigated nano-radio-labeled superparamagnetic iron oxide NPs. These NPs were further conjugated with diethylene triamine penta-acetic acid labeled with technetium 99 and gallium 68 for SPECT and PET. The results demonstrated a high accumulation of nanocarrier systems in tumorous cells compared to normal cells, giving a clear contrast image by both techniques (SPECT and PET) [32][118].
Hsu and coworkers have proposed a multimodal imaging probe, called “all in one NP (AION)”, which is developed by combining near-infra-red fluorophore, silver sulfide NPs, and iron oxide NPs into PEGylated micelles. The AION exhibited a minimal release of silver ions leading to a significant decrease in associate cytotoxicity. A strong contrast was generated compared to all imaging modalities and exhibited a strong contrast when injected intravenously for in vivo tumor imaging. Thus, AION was suggested as an excellent candidate for BC detection with various imaging opportunities [33][119].
In 2021, Li and colleagues developed a motif (RXDLXXL)-linked arginine-glycine-aspartate nano-peptide and conjugated it with superparamagnetic iron oxide NPs (cFK-9-USPIO) for molecular imaging of integrin protein (αvβ6), overexpressed in BC. In comparison to the controls, the in vivo MRI of four nude mice carrying T1 xenograft tumors revealed a significant decline in T2 signal intensity in the BC tissue. Prussian blue staining provided additional confirmation that v6 integrin-targeted NPs had specifically accumulated in 4T1 BC cells. Comparatively, significantly fewer particles were seen in the 4 T1 tumors of mice that had been injected with control USPIO [34][120].
In another study, ultra-small iron-oxide NPs coupled with BRBP-1 were investigated for NIRF and MRI of BC-associated brain metastasis. The peptide-modified nanocarrier system was found to enhance the imaging signal induced by targeting the ability of BRBP-1, thereby increasing the potential of nanocarriers for the diagnosis of brain metastasis associated with BC [35][121].
Semkina and coworkers have studied anti-vascular endothelial growth factor coupled iron-oxide NPs intended for targeted transport of DOX in the murine breast adenocarcinoma 4T1 cell line. After 24 h, MRI was used to study how NPs accumulated in cancer cells, demonstrating that the carrier system can simultaneously deliver drugs and diagnose cancer [36][122]. DOX is easily delivered to tumor cells due to the conjugation of anti-vascular endothelial growth factor (VEGF) antibodies with bovine serum albumin-coated PEGylated magnetic NPs. This excellent strategy can be pursued in future BC detection approaches.
Pacheco and coworkers have proposed molecularly imprinted polymeric sensors to sense and quantify HER-ECD. The quantification and detection limits were 1.6 ng/L and 5.2 ng/mL, respectively. The biomarker was found to be more selective in comparison to other protein biomarkers [37][123]. Zhang and coworkers have improved the F19 MRI sensitivity by developing peptide aptamer conjugated hyper-branched perfluoropolyether NPs for BC-specific detection. The NPs enhanced the mobility of fluorinated segments, thus increasing the sensitivity of MRI imaging. The conjugation of protein aptamer exhibited improved tumor targeting efficiency of NPs along with improved tumor penetration [38][124]. Wojtynek and coworkers have demonstrated self-assembled hyaluronic acid (HA) NPs encapsulating indocyanine green for BC imaging to enhance intraoperative contrast. The NPs enhanced the intraoperative contrast and helped identify small and occult lesions and, hence, can significantly improve surgical outcomes relevant to BC [39][125].
Jin and coworkers have developed a peptide (cyclic arginine-glycine-aspartic acid) conjugated polymeric NPs using poly(2-methoxy-5-(2-ethyl-hexyloxy)-1,4-phenylene vinylene) as a photosensitizer targeting TNBCs. The polymeric nanoconjugates exhibited bright flourescence, increased stability, and could generate reactive oxygen species on light irradiation. It was demonstrated that such nanoparticulate systems are useful as diagnostic tools for clinical use [40][126].
2.6. Miscellaneous Nanocarriers
Zhang and coworkers have explored tumor-derived exosomes as biomarkers for detecting MCF-7 cells by targeting overexpressed mucin-1. The proposed on–off apta-sensors were turned on in the presence of mucin-1 on tumor-derived, resulting in fluorescence signal emission. The exosome-based biosensor successfully quantified a small number of samples, making them highly sensitive sensors [41][127]. Xu and associates developed allochroic acid NPs to detect overexpressed ERs, progesterone receptors, and HER-2 in BC using pH indicators. Further functionalization with bovine serum albumin and antibody was carried out to increase the dispersity of NPs. Moreover, the assay was coupled with a smartphone to ensure point-of-care analysis [42][128].
Mohammadniaei and colleagues have developed a new electrochemical biosensor using a topological illustrator and metallic DNA. Bismuth selenide NPs were prepared and sandwiched between an Au electrode, and Au deposited a thin layer of bismuth selenide. This was followed by immobilizing eight silver ion-mediated dsDNA onto the substrate to detect H2O2 liberated from BC cells. The bismuth selenide NPs were regarded as electrochemical signal boosters, while the Au coating added to the stability of these signals. The proposed biosensors offered a low detection limit (10 × 10−9 M) along with the excellent capacity to distinguish two variations of BC cells (MFC-7 and MDA-MB-231) contingent on the variation in H2O2 generation [43][129].
