Chitosan is established as a non-toxic, biodegradable, and biocompatible compound. It offers fascinating properties; antimicrobial, antiviral, antifungal, antioxidant, anti-inflammatory, bio-adhesion, adsorption enhancer, etc. Chitosan coupled with nanotechnology could offer a sustainable alternative to the use of conventional agrochemicals towards a safer agriculture industry.
- sustainable agriculture
- chitosan-based agronanochemicals
- crop protection
1. Introduction
2. Chitosan-Based Agronanochemicals
According to the United Stated (USA) Food and Drug Association (FDA), chitosan is established as a non-toxic, biodegradable, and biocompatible compound [13][7]. It offers fascinating properties; antimicrobial, antiviral, antifungal, antioxidant, anti-inflammatory, bio-adhesion, adsorption enhancer, etc. [14][8]. Chitosan is soluble at acidic pHs due to the protonation of its amino group. It is derived from chitin via chemical deacetylation under alkaline conditions, where chitin is the second most abundant natural biopolymer and can be found in the shell of crustaceans, insect cuticles and fungal cell walls [15][9]. Besides, the production of chitosan is one of the ways to utilize the bio-waste that comes from the crustacean production industries, where its global production are approximately 6–8 million tonnes/year with 1.5 million being produced by Southeast Asian countries [16][10]. This is an effort towards achieving a “zero-waste” food industry, hence benefiting to both the economy and the environment [17][11]. Nevertheless, it is worth noting that the production of 1 kg of chitosan consumes over than 1 tonne of water. Therefore, the utilization of chitosan-based agronanochemicals as a sustainable alternative in crops management has raised a debate among researchers. However, wthe researchers believe that the advantages of chitosan production to convert waste to wealth, together with the positive outcomes of chitosan nanoformulations in crops management; especially the synergistic effect, toxic-protection abilities, minimization of agrochemical leaching and runoff to the soil and water body, high potency, high efficiency etc., outweigh the need for a huge amount of water during the production of chitosan. The controlled release formulation and high bioavailability could overcome environmental and health issues such as run-off and accumulation of agrochemicals, as well as helping in reducing the labor cost in the agricultural industry. The low toxicity properties make them harmless to the farmers and the person who will be applying it. Again, all in all, the benefits of chitosan-based agronanochemicals outweigh the huge water consumption required for the production of chitosan and therefore it is a way forward, especially for crop management. In agriculture, chitosan nanoparticles by themselves can act as growth enhancers and potent antimicrobial agents against pathogenic fungi and bacteria [18][12]. Alternatively, they can also act as a nanocarriers for existing agrochemicals, which hence are referred to as chitosan-based agronanochemicals [19,20,21][13][14][15]. The nanocarrier system enables the agriculturally active ingredient to be encapsulated via ionic or covalent inter/intramolecular bonds or entrapped in a polymeric matrix of chitosan to develop an effective nanodelivery system formulation [21][15]. Chitosan-based agronanochemicals can be prepared using several methods, including ionic gelation, emulsion cross-linking, spray drying, precipitation, reverse micellar and sieving methods [22][16]. Out of these methods, the sieving method is the simplest and direct method. However, the method has been reported to produce nanoparticles of irregular shape and size. On the other hand, the ionic gelation method is the subject of intense research in the formulation of chitosan nanoparticulate systems due to its simplicity and relatively cheap cost. The method does not require many chemicals, hence reducing the possible toxic side effects. It also employs the use of polyanions with a negative charge (e.g., tripolyphosphate) to bond with the positive charge of the protonated amino group of chitosan under acidic conditions. The emulsion cross-linking method produces stable nanoparticulate systems, however, the process is quite tedious and requires crosslinking agents such as glutaraldehyde, formaldehyde, alginate, etc. which might cause complications due to their incompatibility with the active ingredients. The resulting particle size mainly depends on the emulsion droplet size which in turn depends on the crosslinking degree, molecular weight of the chitosan, surfactant type, and the speed of stirring. Nanoformulations aim to enhance the benefits of chitosan and agrochemicals while simultaneously reducing the adverse outcomes. Due to its amphiphilic properties, the encapsulation of chitosan could overcome the poor solubility of many agrochemicals in water, providing an alternative use of inert chemicals in conventional agrochemicals, hence, subsequently reducing theirs toxicity level [19][13]. The bioadhesive properties in chitosan provide excellent protection to the encapsulated agrochemicals, thus, increasing the stability and bioavailability in the plant [23][17].2.1. Controlled Release Formulations
Chitosan-based agronanochemicals exhibit highly controlled release behavior that subsequently increases its bioavailability with high circulation and retention time in the plant tissue (higher half-lives, t1/2). Thus, the controlled release of active ingredients in agrochemicals aims to address the problems associated with the excessive usage of agrochemicals by reducing the quantiies and frequency application in the field. The agrochemical release from the chitosan matrix can be triggered by two types of stimuli: (1) biotic stress, such as the presence of plant pathogens (fungi and bacteria), nematodes, insects, pest and weeds; and (2) abiotic stress factors, such as pH, temperature, salinity, flooding, drought and other environmental factors [7,24][18][19]. The release mechanism upon stimulus-response is through pore diffusion, surface desorption, capsule swelling and degradation, as illustrated in Figure 1 [7,25][18][20]. The diffusion-controlled mechanism relies on a diffusion rate gradient while the surface desorption refers to the active ingredient adsorbed on the surface of the nanoformulation. Upon hydration, the release of agrochemicals depends on the swelling of the chitosan capsule. Moreover, enzymatic reactions or other environmental factors might result in the rupture or degradation of the capsule matrix. Hence, the controlled release based on the stimuli response in nanoformulations enables the release of the agrochemicals effectively and efficiently at the target site of interest. A pH-dependent release of Cu was observed upon its encapsulation into chitosan nanoparticles, in which the decrease from pH 3 to pH 1 leads to the increased release of Cu from 21.5% to 44.1%, respectively [26][21]. This is due to the protonation of the chitosan’s amino group. At higher pH of 6 and 7, a drastic decrease of Cu release was observed (6.1% and 4.9%, respectively), due to the deprotonation of the chitosan’s amino group. Moreover, the sustained release of Cu for up to 96 h was obtained at pH 4.5. A stimulus-response release mechanism was observed for chitosan-Zn nanoparticles, in which the Zn release was mainly due to the stomatal uptake, followed by diffusion and swelling of polymers upon water penetration [27][22]. The slightly acidic environment of the intracellular medium is also reported to be able to help release Zn from chitosan nanoparticles. Chitosan-hexaconazole nanoparticles and chitosan-dazomet nanoparticles demonstrated diffusion-controlled release of the fungicides at pH 5.5 with half release times (t1/2) of 42 and 11 h, respectively [28,29][23][24]. Moreover, the co-release of hexaconazole and dazomet from the chitosan-hexaconazole-dazomet nanoparticles prolonged the release time with t1/2 of 53 and 15 h, respectively [30][25]. The diffusion-controlled release of methomyl at pH 6.0 with t1/2 of 36–70 h has been obtained by its encapsulation into carboxymethyl chitosan and azidobenzaldehyde [31][26]. The release of the insecticide acetamiprid from nanocapsules of chitosan-alginate was reported to be pH-dependent, in which half of the acetamiprid was released after 36 h at pH 7.0 and 4.0 compared to only 24 h needed to release the same amount at pH 10 [32][27].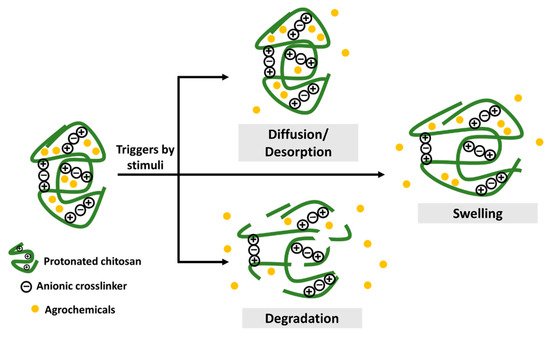
2.2. Plant Growth Promoter
The use of nanoformulations of chitosan itself as a plant growth promoter has been extensively researched. The protonated chitosan, rich in positive charges, shows increased affinity towards plant cell membranes, resulting in enhanced reactivity in the plant system. Also, 9–10% nitrogen, which is the main component of chitosan, serves as a macronutrient in a plant [22][16]. Alternatively, chitosan can be incorporated with plant macronutrients (nitrogen [N], phosphorus [P], potassium [K], magnesium [Mg], calcium [Ca] and sulfur [S]) and micronutrient (copper [Cu], manganese [Mn], nickel [Ni], zinc [Zn], boron [B], iron [Fe] and chlorine [Cl]). The summary of some of the recent works on the use of nanochitosan and macro/micronutrient nanocarriers as a plant growth promoter are listed in Table 1. As shown in Table 1, chitosan nanoformulations have been widely used as an alternative method in seed treatment to promote seed germination and increase biomass accumulation. Moreover, chitosan nanoformulations have also been used as growth promoters by enhancing the nutrient uptake, chlorophyll content and photosynthesis rate.|
Nanoformulations, Molecular Weight (MW), Deacetylation Degree and Final pH of the Product |
Plant and Application Type |
Average Size * and Zeta Potential |
Findings |
Ref. |
|---|
|
Agrochemicals Type and Its Active Ingredient |
Nanocarrier Formulations, Loading Content % (LC), Loading Efficiency % (LE), Encapsulation Efficiency % (EE), and its Average Size * |
Plant Pathogen |
In Vitro/In Vivo |
Findings |
Ref. |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Nano-chitosan, 600 kDa, 85%, pH 6.0 |
Alternaria solani, Fusarium oxysporum, and Robusta coffee (Coffea canephora), foliar spray |
420, 750 and 970 nm c |
Pyricularia grisea, | |||||||||||
|
Fungicide, | Increase chlorophyll content (30–50%), enhance nutrient uptake (10–27% N, 17–30% P, 30–45% K) and photosynthesis rate (30%). |
Dazomet Nano-CS, 10-30 nm b, –37 mV (fungicides) |
CS nanoparticles, [1] 276 nm b, 28% (LC), 78% (EE); [2] 32 nm b, 48% (LC), 98% (EE); [3] 31 nm b, 35% (LC), 85% (EE); [4] 7 nm b, 33% (LC), 83% (EE) In vitro |
Ganoderma boninense High inhibition on mycelial growth with the percentage of inhibition rate recorded at 92%, 87%, and 72% for P. grisea, F. oxysporum and A. solani, respectively. |
||||||||||
[ | ][33] |
In vitro |
Controlled release with saturation release of 97.9% and half release time (t1/2) of 11 h at pH 5.5. Increase fungicidal activity up to 30-fold compared to their counterparts. |
Nano-chitosan, 110 kDa, 85%–90%, pH 4.0 |
||||||||||
[ | ] | [ | 24] |
Aphis gossypii | ||||||||||
|
Fungicides, Hexaconazole and Dazomet | Chilli (Capsicum annuum), seed treatment |
CS-polyacrylic acid nanoparticles, 50 nm a (insecticides) |
CS nanoparticles, [1] 157 nm b, 17% (LC), 67% (EE); [2] 58 nm b, 17% (LC), 67% (EE); [3 163 nm a, +60.4 mV |
] 31 nm b, 17% (LC), 67% (EE); [4] 5 nm In vivo, reared on castor leaves Enhance in total root and leaf fresh mass up to 77% and 28%, respectively upon application of 1 mg/L of nano-chitosan. The increase of leaf catalase (33%) and peroxidase activities (23%) also been observed. |
b, 13% (LC), 64% (EE) |
Ganoderma boninense The mean number of eggs/females reduce significantly under the laboratory conditions and field conditions with 76% and 61%, respectively. |
In vitro |
|||||||
[ | ][42] |
Controlled release with half release time (t1/2) up to 66 and 19 h for hexaconazole and dazomet, respectively, at pH 5.5. Increase fungicidal activity up to 40-fold compared to their counterparts. |
Nano-chitosan, 100–399 kDa, |
Bean (Phaseolus vulgaris), seed treatment |
||||||||||
|
Fungicide, Hexaconazole | 46 nm a |
CS nanoparticles, 100 nm b, 73% (EE) Promote seed germination (123% after 72 h) and radical length (231% after 72 h) under salinity stress. |
Rhizoctonia solani |
|||||||||||
|
Callosobruchus chinensis |
In vivo, reared on castor leaves |
The mean number of eggs/females reduce significantly under the laboratory conditions and store conditions with 74% and 70%, respectively. |
In vitro |
Controlled release with prolongs the release time of hexaconazole up to 14 days at pH 8.3 while the conventional pesticides only last up to 5 days. Significant higher antifungal activity compared to the conventional counterpart. |
Nano-chitosan, pH 7.0–9.0 |
|||||||||
|
Fungicide, |
Maize (Zea mays), seed treatment |
Hexaconazole |
CS nanoparticles, [1 80–100 nm d |
|||||||||||
|
Callosobruchus maculatus: |
In vivo, reared on soybean ] | 272 nm b, 11% (LC), 56% (EE); [2] 169 nm b, 17% (LC), 67% (EE); [3] 32 nm b, 15% (LC), 65% (EE); [4] 18 nm b The mean number of eggs/females reduce significantly under the laboratory condition and store condition with 84% and 74%, respectively. Promote seed germination (37%), plant height (1.5-fold increase) and leaf area (2-fold increase). |
, 15% (LC), 65% (EE) [41][ |
Ganoderma boninense 42] |
In vitro |
|||||||||
Controlled release with saturation release of 99.9% and half release time (t | 1/2 | ) of 42 h at pH 5.5. Increase fungicidal activity up to 3-fold compared to their counterparts. |
Nano-chitosan, pH 4.8 |
|||||||||||
|
Colletotrichum Gloeosporioides and Alternaria spp. | ||||||||||||||
|
Fungicide, Pyraclostrobin |
Chickpea (Cicer arietinum), seed treatment |
Nano-CS, 406 nm a, –4.9 to –7.9 mV (fungicides) |
CS-lactide nanoparticles, [1] 128 nm a, 18% (LC), 45% (EE); [2] 90 nm a, 11% (LC), 85% (EE); [3] 10–30 nm b, −37 mV |
Enhance germination (100%), seedling vigor index (57%) and vegetative biomass of seedlings (3-fold). |
77 nm a, 2% (LC), 91% (EE); In vitro [43 |
Colletotrichum gossypii ][33] |
||||||||
Higher inhibition on mycelial (up to 82%) and sporulation of fungus, compared to the counterpart. Enhance seeds germination. |
In vitro |
Better stability of AI under light stress with 81% compared to the counterpart with 41%. Controlled release (75%) of AI up to 10 h at pH 8.3. High fungicidal activity with up to 85% inhibition rate at day 7 of incubation. |
Chitosan-polymethacrylic acid-NPK nanoparticles |
Wheat (Triticumaestivum), foliar spray |
26 and 31 nm b |
|||||||||
|
Curvularia lunata |
||||||||||||||
|
Fungicide, |
CS-Cu nanoparticles, 361 nm a, +22.1 mV (fungicides) |
Pyraclostrobin |
Quarternized CS-silica nanoparticles, 110 nm b, 27%–42% (LC) In vitro and In vivo (Maize, Zea mays) |
Enhance harvest index (24%), crop yield (59%), and mobilization index (42%). |
Phomopsis asparagi |
|||||||||
Induce more defense response: 1.5–2 fold of peroxidase, a significant amount of superoxide dismutase, 2–3 fold of phenylalanine ammonia-lyase, and a significant amount of polyphenol oxidase. | [ |
In vitro |
Controlled release (72%) with prolongs release time up to 13 h. Inhibition percentage of fungi up to 95% |
20 nm b |
||||||||||
] |
Fusarium oxysporum Enhance polysaccharides (10%) and total saccharides (11%). |
|||||||||||||
|
Fungicides, Tricyclazole and Hexaconazole | CS-CuO, 350 nm b, –26.8 mV; CS-ZnO, 441 nm b, –24.5 mV; and CS-Ag, 348 nm b, –49.1 mV (fungicides) | |||||||||||||
b |
In vitro and In vivo (chickpea, Cicer arietinum) |
In vitro results showed that the antifungal activity follows: CS-ZnO > CS-CuO > CS-Ag, while in vivo results showed that the wilt disease reduction follows: CS-CuO (47%) > CS-ZnO (40%) > CS-Ag (33%). |
Pyricularia oryzae [ |
In vitro |
Significantly increased the inhibition zone by 2-fold compared to the counterpart |
French bean (Phaseolus vulgaris), foliar spray |
20 nm b |
|||||||
|
Fungicide, Avermectin | Enhance plant growth, nutrient uptake, and biomass accumulation. The nanoformulations was found on the leaf phloem via HRTEM image |
[51 | ||||||||||||
|
Fusarium graminearum |
Nano-CS, 181 nm a, +45.6 mV (fungicides) | ] | [36] |
|||||||||||
In vitro and in vivo (wheat) |
CS-lanthanum-nanoparticles, 333 nm a, 46% (LE), 65% (EE) 85% inhibition of mycelial growth in plate treated with 5000 mg/mL of CS nanoparticles (in vitro) and 53% reduction in disease severity on wheat (in vivo). Deformation and dehydration of fungus mycelial growth also can be seen. |
[54 |
Magnaporthe grisea |
In vitro and In vivo ][45] |
Rapid release on the first 36 h followed by sustained release until day-10. No inhibitory of fungus was observed in the in vitro study. However, significant disease reduction was observed in the in vivo study (Rice, Oryza sativa). |
Pea (Pisum sativum), seed treatment |
||||||||
|
Nano-CS, [1] 181 nm a, +45.6 mV; [2] 310 nma, +33.2 mV; [3] 340 nm a, +21.7 mV (fungicides) | ||||||||||||||
|
Fungicide, Tebuconazole | 20 nm b |
In vitro and in vivo (wheat) |
CS-porphyrinic-pectin nanoparticles, 100 nm c, 30% (LE) Induce mitotic cell division (1.5 fold) and enhance of total soluble protein (i.e., legumin β, vicilin 1, 2 and 3, and convicilin) |
|||||||||||
Inhibition rate (%) at 1000 mg/mL follows: (1) Nano-CS (71.1%) > (3) Nano-CS (17.7%) > (2) Nano-CS (14.1%) |
Xanthomonas campestris, Pseudomonas syringae, and Alternaria alternate |
In vitro |
Metal-organic frameworks (MOFs) capsule comprise of chitosan, porous porhpyrinic, and pectin demonstrated a stimuli-responsive sustained release of AI with prolonged-release time up to 174 h at pH 7. The nanocapsule exhibited high antimicrobials activities and no phytotoxic effect on Chinese cabbage. |
Chitosan-Cu nanoparticles, low MW, 80% |
Maize ( | |||||||||
[ | ] | [ | 61] |
CS-Cu nanoparticles, 220 nm a, +40.0 mV (fungicides)Surya local), seed treatment |
150 nm b, +22.6 mV |
Increase α-amylase and protease activity as well as promote seedling growth. |
In vitro | |||||||
|
Herbicides, Imazapic, and Imazapyr |
CS-alginate nanoparticles, 378 nm a, 62% (EE) of imazapic, 71% (EE) of imazapyr;CS-tripolyphosphate nanoparticles, 479 nm a, 59% (EE) of imazapic, 70% (EE) of imazapyr Minimum inhibitory concentration after one week incubation follows: Cu (250 µg/mL) > CS-Cu nanoparticles (17.5 mg/mL) > chitosan (10 mg/mL). |
Bidens pilosa |
||||||||||||
In vivo | After 300 min under gentle agitation, 30% (imazapic) and 20% (imazapyr) were released in CS-alginate nanoparticles, while 59% (imazapic) and 9% (imazapyr) were released in CS-tripolyphosphate nanoparticles. Meanwhile, free imazapic and imazapyr were released up to 55% and 97%, respectively, hence, showing the slow-release formulation of the nanoparticulate system. The encapsulation of herbicides also reduced the toxicity of herbicides against the nontarget organism while maintaining its herbicidal activity on the tested weeds. |
Chitosan-Cu nanoparticles, 50–190 kDa, 80% |
||||||||||||
[ | ] | [ | 62] |
Fusarium verticillioids Maize (Zea mays), foliar spray |
CS-Cu nanoparticles, 296 nm a, +19.6 mV (fungicides) | |||||||||
|
Herbicide, Paraquat |
CS-Ag nanoparticles, 100 nm c, 90% (EE) 361 nm a,+22.1 mV |
In vivo (Maize, Zea mays pH-responsive sustained release of Cu was obtained. Promote seedling growth (significant increase in plant height, stem diameter, and root length). |
) |
Eichhornia crassipes [26][ |
At 4 and 8 h after treatment, the disease has been reduced by 48% and 50%, respectively.21] |
|||||||||
In vivo |
Improved herbicidal activity on the tested weed with a 90% release of paraquat was observed for up to 24 h. Improved the microbial population, bacteria, and yeast compared to its free herbicide. |
Chitosan-Zn nanoparticles, 60 kDa, 85% |
Wheat (Triticum durum), foliar spray |
|||||||||||
|
Pyricularia grisea | 325 nm a, +42.3 mV |
Nano-CS, 83 nm a, –28.0 mV (fungicides) |
Stomatal localization of nanoparticles was observed. Increase grain zinc content by up to 42%. |
|||||||||||
|
Nematicide, Avermectin | In vitro and In vivo (rice, | Oryza sativa) | ||||||||||||
No inhibitory activity was observed in the in vitro. However, in vivo results revealed its ability in suppressing the disease with zero percent disease incidence at 10 days after infection, where 100% disease incidence was observed in control. | [57 |
CS-γ-polyglutamic acid nanoparticles, 61 and 56 nm b, 31% (LC), 35% (EE) |
Caenorhabditis elegans |
In vitro ][49] |
The controlled release rate governed by pH. The mortality rate of nematodes was significantly increased by 29%, compared to its counterpart. |
Chitosan-γ-polyglutamic acid-gibberellic acid nanoparticles, 290 kDa, 75%–85%, pH 4.5 |
French bean (Phaseolus vulgaris), seed treatment |
134 nm a, −29.0 mV |
61% of the encapsulation efficiency of hormone in the nanoformulation. Offer sustained-release with 58% after 48 h. Exhibited high biological activity with 50–70% enhance of seed germination, leaf area, and root development compared to counterpart. |
[39] |
||||
] |
Chitosan-gibberellic acid nanoparticles, 27 kDa, 75%–85%, pH 4.5 |
French bean (Phaseolus vulgaris), seed treatment | ||||||||||||
|
In vitro and In vivo (finger millet, Eleusine coracana) |
In the in vitro evaluation, 65% of radial growth inhibition was obtained. Meanwhile, delayed disease symptom (25 days) and low disease infection (23%) was observed in the in vivo evaluation, while for control, the symptoms started appear in 15 days and 100% disease infection was recorded. Enhance in peroxidase activity level (reached maximum on day 50) also been observed. |
|||||||||||||
|
CS-Cu nanoparticles, 88 nm a, –29.0 mV (fungicides) | 450 nm a, +27.0 mV |
90% of the encapsulation efficiency of hormone in the nanoformulation. Offer stability up to 60 days with pH and temperature-controlled release mechanism. Upon treatment, the seedlings showed an increase of leaf area, chlorophyll and carotenoids amount. |
In vitro and In vivo (finger millet, Eleusine coracana) |
|||||||||||
Induce resistance against the pathogen attack: a 2-fold increase in chitinase and chitosanase and produce more protease inhibitors, peroxidase, β-1,3 glucanase, and polyphenol oxidase compared to the untreated plant. |
Chitosan-thiamine nanoparticles, 27 kDa, 85% |
Chickpea (Cicer arietinum), seed treatment |
596 nm a, +37.7 mV |
99% of the encapsulation efficiency of hormone in the nanoformulation. Enhance seeds germination and induce more defense enzymes (peroxidase, glucanase, chitinase, polyphenol oxidase, protease, and chitosanase activity) and increase 10-fold auxins level compared to the untreated seeds. |
2.3. Biocides Against Plant Pathogens and Pests
Chitosan with or without the incorporation of macronutrients can act as an alternative sustainable potent biocidal agent against pathogenic fungi, viruses and bacteria. A summary of some of the recent works on the use of nanochitosan and its incorporation in plant management is provided in Table 2. As shown in the summary, chitosan with or without the incorporation of other active agents exhibited good potential as a sustainable alternative to the use of conventional fungicides against Fusarium head blight and wilt disease in wheat and chickpea, post-flowering stalk rot in maize, blast leaf in rice, blast disease in finger millet and leaf spot in maize, among others.|
Plant Pathogen |
Nanoformulations, Average Size *, Zeta Potential and its Application |
In Vitro/In Vivo |
Findings |
Ref. |
|---|---|---|---|---|
Pyricularia oryzae | ||||
Nano-CS, 28 nm | ||||
b | ||||
, +49.0 to +53.0 mV and CS-protocatechuic acid, 33 nm | ||||
b | ||||
, +11.0 mV (fungicides) | ||||
In vitro |
The diameter of inhibition zone follows: CS-protocatechuic acid nanoparticles > protocatechuic acid > chitosan nanoparticles. Up to a 3-fold increase of the inhibition zone compared to the counterpart. |
|||
|
Verticillium dahliae |
Nano-oleoyl-CS, 297 nm c (fungicides) |
In vitro |
The nanoparticles internalized the fungal cell, hence leads to the deformation of spore and hyphae, thickened cell walls, cease of organelles and cytoplasmic vacuolation. |
*,a hydrodynamic mean size, b high-resolution transmission electron microscopy (HRTEM) mean diameter size and c field emission electron microscopy (FESEM) diameter size.
References
- Oerke, E.-C.; Dehne, H.-W. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285.
- Roy, R.N.; Finck, A.; Blair, G.; Tandon, H. Plant nutrition for food security. A guide for integrated nutrient management. FAO Fertil. Plant Nutr. Bull. 2006, 16, 368.
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446.
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1.
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12.
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255.
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469.
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330.
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403.
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2014.
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbio. 2010, 144, 51–63.
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66.
- Hernández-Téllez, C.N.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O. Chitosan-based bionanocomposites: Development and perspectives in food and agricultural applications. In Chitosan in the Preservation of Agricultural Commodities; Elsevier: Cambridge, MA, USA, 2016; pp. 315–338.
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51.
- Agarwal, M.; Nagar, D.; Srivastava, N.; Agarwal, M. Chitosan nanoparticles based drug delivery: An update. Int, J. Adv. Multidiscip. Res. 2015, 2, 1–13.
- Dudhani, A.R.; Kosaraju, S.L. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251.
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; Santo Pereira, A.d.E.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100.
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28.
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90.
- Choudhary, R.C.; Kumaraswamy, R.; Kumari, S.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754.
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401.
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, Z.; Hailini, N.; Jeffery Daim, L.D. Preparation of chitosan–hexaconazole nanoparticles as fungicide nanodelivery system for combating Ganoderma disease in oil palm. Molecules 2019, 24, 2498.
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Jeffery Daim, L.D. A Potent antifungal agent for basal stem rot disease treatment in oil palms based on chitosan-dazomet nanoparticles. Int. J. Mol. Sci. 2019, 20, 2247.
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Abu Seman, I.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Enhanced fungicidal efficacy on Ganoderma boninense by simultaneous co-delivery of hexaconazole and dazomet from their chitosan nanoparticles. RSC Adv. 2019, 9, 27083–27095.
- Sun, C.; Shu, K.; Wang, W.; Ye, Z.; Liu, T.; Gao, Y.; Zheng, H.; He, G.; Yin, Y. Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. Int. J. Pharm. 2014, 463, 108–114.
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R.; Dilbaghi, N. Development and evaluation of alginate–chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637.
- Neri-Badang, M.C.; Chakraborty, S. Carbohydrate polymers as controlled release devices for pesticides. J. Carbohydr. Chem. 2019, 38, 67–85.
- Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294.
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402.
- Zayed, M.; Elkafafi, S.; Zedan, A.M.; Dawoud, S.F. Effect of nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. J. Plant Production 2017, 8, 577–585.
- Khati, P.; Chaudhary, P.; Gangola, S.; Bhatt, P.; Sharma, A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech 2017, 7, 81.
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325.
- Abdel-Aziz, H.M.; Hasaneen, M.N.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Spani. J. Agric. Res. 2016, 14, 0902.
- Abdel-Aziz, H.; Hasaneen, M.N.; Omar, A. Effect of foliar application of nano chitosan NPK fertilizer on the chemical composition of wheat grains. Egypt. J. Bot. 2018, 58, 87–95.
- Hasaneen, M.; Abdel-aziz, H.M.M.; Omer, A.M. Effect of foliar application of engineered nanomaterials: Carbon nanotubes NPK and chitosan nanoparticles NPK fertilizer on the growth of French bean plant. Biochem. Biotechnol. Res. 2016, 4, 68–76.
- Khalifa, N.S.; Hasaneen, M.N. The effect of chitosan–PMAA–NPK nanofertilizer on Pisum sativum plants. 3 Biotech 2018, 8, 193.
- Saharan, V.; Kumaraswamy, R.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agr. Food Chem. 2016, 64, 6148–6155.
- Pereira, A.; Sandoval-Herrera, I.; Zavala-Betancourt, S.; Oliveira, H.; Ledezma-Pérez, A.; Romero, J.; Fraceto, L. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydr. Polym. 2017, 157, 1862–1873.
- Santo Pereira, A.E.; Silva, P.M.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf. B Biointerfaces 2017, 150, 141–152.
- Muthukrishnan, S.; Murugan, I.; Selvaraj, M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr. Polym. 2019, 212, 169–177.
- Sahab, A.; Waly, A.; Sabbour, M.; Nawar, L.S. Synthesis, antifungal and insecticidal potential of Chitosan (CS)-g-poly (acrylic acid)(PAA) nanoparticles against some seed borne fungi and insects of soybean. Int. J. Chem. Tech. Res 2015, 8, 589–598.
- Barrera-Necha, L.L.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M.; Jiménez, J.E.M.; Mejía, A.F.M. Synthesis and characterization of chitosan nanoparticles loaded botanical extracts with antifungal activity on Colletotrichum gloeosporioides and Alternaria species. Adv. Microbiol. 2018, 8, 286.
- Kaur, P.; Duhan, J.S.; Thakur, R. Comparative pot studies of chitosan and chitosan-metal nanocomposites as nano-agrochemicals against Fusarium wilt of chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2018, 14, 466–471.
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272.
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int. J. Biol. Macromol. 2017, 102, 526–538.
- Brunel, F.; El Gueddari, N.E.; Moerschbacher, B.M. Complexation of copper (II) with chitosan nanogels: Toward control of microbial growth. Carbohydr. Polym. 2013, 92, 1348–1356.
- Choudhary, M.K.; Joshi, A.; Sharma, S.; Saharan, V. Effect of laboratory synthesized Cu-Chitosan nanocomposites on control of PFSR disease of Maize caused by Fusarium verticillioids. Int. J. Curr. Microbiol. Appl. Sci 2017, 6, 1656–1664.
- Manikandan, A.; Sathiyabama, M. Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int. J. Biol. Macromol. 2016, 84, 58–61.
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016, 154, 241–246.
- Sathiyabama, M.; Manikandan, A. Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. J. Agri. Food Chem. 2018, 66, 1784–1790.
- Nguyen, T.H.; Thi, T.V.; Nguyen, T.-T.; Le, T.D.; Vo, D.M.H.; Nguyen, D.H.; Nguyen, C.K.; Nguyen, D.C.; Nguyen, T.T.; Bach, L.G. Investigation of chitosan nanoparticles loaded with protocatechuic acid (PCA) for the resistance of Pyricularia oryzae fungus against rice blast. Polymers 2019, 11, 177.
- Xing, K.; Liu, Y.; Shen, X.; Zhu, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Effect of O-chitosan nanoparticles on the development and membrane permeability of Verticillium dahliae. Carbohydr. Polym. 2017, 165, 334–343.
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23.
- Ye, Z.; Guo, J.; Wu, D.; Tan, M.; Xiong, X.; Yin, Y.; He, G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015, 132, 520–528.
- Feng, B.-H.; Peng, L.-F. Synthesis and characterization of carboxymethyl chitosan carrying ricinoleic functions as an emulsifier for azadirachtin. Carbohydr. Polym. 2012, 88, 576–582.
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.-H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624.
- Xu, L.; Cao, L.-D.; Li, F.-M.; Wang, X.-J.; Huang, Q.-L. Utilization of chitosan-lactide copolymer nanoparticles as controlled release pesticide carrier for pyraclostrobin against Colletotrichum gossypii Southw. J. Disper. Sci. Technol. 2014, 35, 544–550.
- Pham, D.C.; Nguyen, T.H.; Ngoc, U.T.P.; Le, N.T.T.; Tran, T.V.; Nguyen, D.H. Preparation, characterization and antifungal properties of chitosan-silver nanoparticles synergize fungicide against Pyricularia oryzae. J. Nanosci. Nanotechnol. 2018, 18, 5299–5305.
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Hu, P.; Jia, J.; Xu, H. A novel water-based chitosan-La pesticide nanocarrier enhancing defense responses in rice (Oryza sativa L) growth. Carbohydr. Polym. 2018, 199, 437–444.
- Tang, J.; Ding, G.; Niu, J.; Zhang, W.; Tang, G.; Liang, Y.; Fan, C.; Dong, H.; Yang, J.; Li, J. Preparation and characterization of tebuconazole metal-organic framework-based microcapsules with dual-microbicidal activity. Chem. Eng. 2019, 359, 225–232.
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.; Fraceto, L.F.; De Lima, R. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 2016, 6, 19768.
- Namasivayam, K.R.S.; Aruna, A.; Gokila. Evaluation of silver nanoparticles-chitosan encapsulated synthetic herbicide paraquate (AgNp-CS-PQ) preparation for the controlled release and improved herbicidal activity against Eichhornia crassipes. Res. J. Biotechnol. 2014, 9, 19–27.
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Jia, J.; Xu, H. Chitosan-based nanoparticles of avermectin to control pine wood nematodes. Int. J. Biol. Macromol. 2018, 112, 258–263.
