The role of extracellular matrix in breast cancer progression has recently been realized. Here, the effect of various ECM properties including fiber structure, stiffness and biochemical composition are reviewed, with a special emphasis on the extracellular vesicles.
- breast cancer
- tumor microenvironment
- extracellular matrix
- extracellular vesicles
- stiffness
- biochemical composition
- cytokines
- fiber structure
- matrix metalloproteinases
- exosomes
1. Introduction
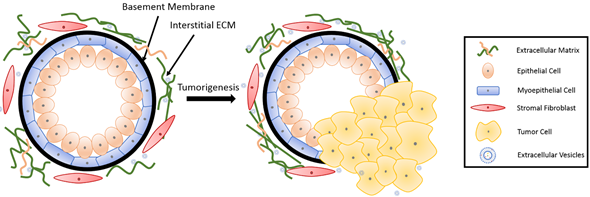
The basic mammary structure consists of luminal epithelial cells lining a central lumen, surrounded by a layer of myoepithelial cells, the stroma, and the basement membrane that separates the epithelium from the stroma (Figure 1). The basement membrane and the stroma make up an important part of the ECM in the mammary gland. The basement membrane is a thin layer of matrix mainly composed of type IV collagen, laminin and entactin[1] [13]. Stromal ECM, which mainly contains type I collagen, fibronectin, laminins, and glycoproteins, serves as a structural scaffold that maintains breast tissue integrity and sustainability[2] [14]. However, the role of ECM is way more significant than simply providing structural support. It plays multiple roles in regulating cell behavior in the breast tissue, such as survival, proliferation, differentiation[3] [15], invasion[4][5] [16,17], as well as immune responses[6] [18]. Furthermore, the ECM mediates stromal–epithelial communication and serves as a guide that regulates breast development[7] [10].
In recent decades, a growing body of research has revealed an important role of the ECM in breast cancer progression and metastasis[8][9] [8,9]. During tumorigenesis, the structure and composition of the ECM is also significantly altered, further contributing to cancer progression[7] [10]. In this section, we will discuss the influence of both the biophysical properties and biochemical composition of ECM on breast cancer progression and metastasis, as well as drug resistance. Moreover, we will discuss alterations taking place in the ECM during breast cancer development.
Figure 1. Breast tissue undergoing tumorigenesis. The basement membrane is a thin layer of pericellular matrix separating the epithelium and the stroma. Following tumorigenesis, a microenvironment is created, supporting tumor progression. Tumor cells surpass the basement membrane, which becomes more permissive in the tumor microenvironment (TME), invade the stroma, and eventually metastasize to distant sites through vasculature.
2. Role of Mechanical and Biophysical Properties of ECM in Breast Cancer
The ECM provides mechanical support to cells and guides them through mechanical stimuli. The cells adjust their behavior and remodel their microenvironment as a result of these forces[10] [19]. These mechanical cues, including ECM density and stiffness, alter mechanotransduction signaling[11][12] [20,21] and thus the protein[13] [22] and miRNA[14] [23] expression in cells. These alterations influence cell behavior including cell morphogenesis[15] [24], stem cell differentiation[16][17][18] [25–27], and cancer-associated fibroblast (CAF) activation[19][20] [11,28], thereby potentiating and stimulating aggressive behaviors in malignant epithelial cells. For example, in a meta-analysis study, the risk of breast cancer demonstrated a compelling increase of up to 4–5-fold in women with 75% mammographic density compared to those with less than 10% density[21] [29].
Stiffness is a well-known regulator of breast cancer cell behavior. ECM stiffness leads to profound changes in cancer cell growth, metastatic potential, as well as chemotherapeutic responses[22] [30]. Collagen crosslinking also increases the ECM stiffness, and thus promotes tumor metastasis[23] [31]. The collagen fibril bending stiffness of 3D collagen matrices was demonstrated to direct the spreading and clustering of breast cancer cells[24] [32]. A previous study by Yue et al.[12] [21] revealed that the influence of breast cancer cells on stromal cells was stiffness-dependent; breast cancer cells reduced the degree of adipogenesis only on stiff substrates. A more recent study showed that scaffold stiffness exerts its impact on breast tumor cell invasion through EGFR-linked Mena upregulation and matrix remodeling[25] [33], altering matrix organization. Additionally, tissue stiffness regulates integrin-linked kinase (ILK) expression to control stem-like breast cancer cells under hypoxic conditions[26] [7]. Tissue mechanical properties also modulate miR-18a expression to reduce PTEN and HOXA9 levels, and subsequently regulate cancer invasive progression[14] [23]. In the same vein, stromal miR-200s-regulated ECM stiffness contributes to breast cancer metastasis through CAF activation[20] [28].
The organization of the ECM is another factor influencing breast cancer cell behavior. Interestingly, collagen alignment was reported to promote migration in invasive breast cancer cells more than in non-invasive cells[27][28] [34,35]. The stromal tissue is rich in type I collagen, and the collagen network in the stroma serves as a physical barrier against cancer cell invasion[29] [36]. On the other hand, normal epithelial cells grown on type I collagen bind to type I collagen and go through epithelial-to-mesenchymal transition (EMT)[30] [37]. Type I collagen leads to increased secretion of matrix metalloproteinases (MMPs) which facilitate ECM degradation, and induces invasive behavior[31] [38]. Upon ECM fragmentation by MMPs, ECM bound growth factors are released[32] [39] and a path is opened for the cancer cells to migrate through.
3. Role of Biochemical Composition of ECM in Breast Cancer
Aside from the mechanical and biophysical properties, the biochemical composition of the ECM also has a significant impact on breast tumor progression, metastasis, and response to treatments. Growing evidence indicates that many ECM proteins serve a major functional role in breast cancer progression, metastatic niche construction, and metastatic growth promotion. A study from Staren group[33] [40] proved that ECM proteins, such as vitronectin and fibronectin, can enhance the metastatic potential of breast cancer cells by regulating cell adhesion and migration with integrin subunits. Many ECM proteins, including collagen, osteonectin, and hyaluronic acid, are involved in breast cancer development. Type I collagen poses a versatile role in breast cancer development. Fibronectin expression level in breast cancer cells is significantly associated with a higher probability of metastasis[34] [41]. Upregulation of fibronectin also promotes formation of the pre-metastatic niche[8] [8]. Proteoglycans various pathological processes, including cancer progression and metastasis[35] [42]. Proteoglycan expression is altered in the breast TME during tumor development, and such an alteration affects cancer cell growth, adhesion, signaling, migration and angiogenesis[36] [43]. A higher expression of proteoglycan in breast cancer cells is often correlated with increased tumor risk[37] [44], grade[38] [45] and size[37] [44], directing the cells toward metastasis[39] [46].
Cytokines and growth factors are becoming a significant part of breast-cancer-related studies. Many cytokines are considered as prognostic markers in breast cancer. These cytokines also impact breast cancer progression. Table 1 summarizes the principal cytokines involved in the prevention or progression of breast cancer. Transforming growth factor b (TGF-b), one of the most significant and widely studied cytokines in cancer research, is pro-tumorigenic and involved in breast cancer cell proliferation[40] [47]. Tumor necrosis factor α (TNF-α) enhances the dendritic cell (DC) antitumor effect, inhibits growth and promotes apoptosis of breast cancer cells[41] [48]. Fibroblast growth factor acidic (acidic FGF) is involved in the estrogen-independent and antiestrogen-resistant growth of MCF7 breast cancer cells[33] [40]. Many interleukins (IL) are involved in the cellular immunity and communication of stromal cells with breast cancer cells[42] [49]. IL-1α is known to promote metastasis[43] [50], as it contributes to the induction of pro-metastatic genes in breast cancer[44] [51]. IL-6 induces T cell and B cell differentiation, stimulates cytotoxic T cells and assists in killer cell activation to promote antitumor activity[45] [52], demonstrating its therapeutic potential.
Table 1. Common cytokines involved in breast cancer.
|
Cytokine Type |
Role in Breast Cancer |
Ref. |
|
IL-1 family |
Promote angiogenesis, tumor proliferation and local tumor invasion |
|
|
IL-4 |
Inhibit breast cancer cell growth |
|
|
IL-6 |
Promote tumor cell proliferation, induce T- and B-cell activation |
[50][57] |
|
IL-8 |
Promote tumor growth and metastasis |
|
|
IL-10 |
Inhibit tumor growth, induce drug resistance |
|
|
IL-12 |
Inhibit breast cancer cell proliferation and invasion |
|
|
IL-18 |
Inhibit metastasis |
[57][64] |
|
IL-33 |
Promote breast cancer cell proliferation |
[58][65] |
|
Type I Interferon (a,b) |
Inhibit tumor proliferation and invasion |
[59][66] |
|
Interferon g p |
Promote breast cancer proliferation and invasion |
[60][67] |
|
TGF-b |
Promote breast cancer cell proliferation |
[40][47] |
|
gp130 |
Promote breast cancer cell proliferation and invasion |
[61][68] |
|
TNF-a |
Promote breast cancer metastasis |
[62][69] |
|
Vascular endothelial growth factor (VEGF) |
Promote breast cancer metastasis |
[63][70] |
|
MMP-2 |
Stimulate breast cancer metastasis |
[64][71] |
|
Acidic FGF |
Inhibit breast cancer proliferation |
[33][40] |
|
Platelet-derived growth factor (PDGF)-BB |
Promote breast cancer cell invasion |
[65][72] |
|
Leukemia inhibitory factor (LIF) |
Promote breast cancer cell proliferation and invasion |
[66][73] |
|
Cystatin C |
Inhibit breast cancer cell proliferation |
[67][74] |
|
Resistin |
Facilitate breast cancer progression and promote breast cancer metastasis |
Under normal conditions, breast tissue maintains homeostasis. As the ECM starts to change and becomes suitable for cancer development, disruption of homeostasis follows. While tumor cells create their own microenvironment by remodeling the ECM, the TME also impacts cancer cell behavior, leading to a more aggressive phenotype. In a pathological microenvironment, collagen fibers tend to become relatively straight, forming a more organized alignment[28] [35]. ECM protein components could be degraded or modified by cancer-associated enzymes. MMPs are an important category of enzymes involved in ECM degradation and remodeling, and play a role in tumor cell invasiveness. MMP1, 2, 7–11, 13, 14, and 16 are constitutively expressed in tumor cell lines but not in normal breast epithelial cells[25] [33]. MMP expression alters the rigidity, porosity, and many other characteristics of the ECM, facilitating cell migration and invasion. Breast cancer cells can activate the surrounding stromal cells to create CAFs or cancer-associated adipocytes (CAAs), which remodel the ECM and promote tumor invasiveness[70] [77]. Breast cancer cells also modify the dynamics of stromal fibronectin and collagen interactions, with the help of MMPs[25] [33]. The sequestered pro-angiogenic factors are released as the ECM remodels, further facilitating downstream breast cancer invasion.
Nucleic acid cargo is another influential component of ECM. Lots of effort has been put into identifying the nucleic acid profiles of the breast TME. MicroRNAs (miRNAs) are a family of small-size, non-coding RNA molecules that function as post-transcriptional gene regulators, playing roles in cancer proliferation and invasion[71] [78]. In breast tissue, miRNAs regulate the expression of cytokines and growth factors[72] [79] that can affect ECM composition and pave the way for pathogenesis. miRNAs are often dysregulated in breast cancer[73] [80]. Researchers found out that a single-nucleotide polymorphism (SNP) with miR-196a2 is associated with a decreased risk of cancer[74] [81], while an SNP in miR-146a has been reported to be linked to earlier onset of breast cancer[75] [82]. In a study with more than 1000 patients, the upregulation of miR-103/107 was shown to be associated with metastasis and poor outcome of breast cancer patients[76] [83]. The downregulation of miR-210 was reported to be inversely correlated with cancer aggressiveness and metastatic capability[77] [84]. In a study by Song group[78] [85] with 32 patients, miR-21 was shown to target MMP3 expression to regulate breast cancer invasion. Stromal miR-200s might also regulate CAF activation and ECM remodeling to promote breast cancer cell invasion[12] [21].
4. The ECM as a Physical Barrier for Breast Cancer Treatment
The biophysical and biochemical properties of the breast significantly impact the treatment outcome of the patient. ECM components affect the penetration of immune cells, antibodies and drugs into tumor sites[79] [86]. The dense and stiff collagen network may also serve as a physical barrier against drug penetration[80] [87]. Hence, collagenase treatment can significantly enhance drug penetration for collagen-rich tumors[81] [88]. Glycoseaminoglycans, such as hyaluronic acid and chondroitin sulfate, may also limit drug penetration to the tumor site.
In the meanwhile, interactions between cancer cells and the ECM can drastically affect the sensitivity of cells to apoptosis and their response to chemotherapeutic drugs. ECM proteins mediate drug resistance in breast cancer in multiple therapies. Stromal-derived MMPs are involved in tamoxifen resistance. Loss of function experiments showed that MMPs facilitated the release of heparin-bound EGF, which further regulated cell behavior, resulting in the paracrine induction of 4-OH-tamoxifen resistance through EGFR and PI3K/AKT pathways[82][83] [89,90]. In HER2-positive breast cancer, ECM/integrin signaling promoted drug resistance to combination therapy aiming at HER2 and PI3K inhibition[84] [91]. Doxorubicin was shown to be more effective against MDA-MB-231 cells when ECM-cell signaling was disrupted by inhibiting β1-integrin[85] [92].
miRNAs are also involved in the modulation of chemotherapy responses. The dysregulation of miRNAs also affects the success of therapeutic interventions. miR-19, miR-21 and miR-203 expression in the breast results in resistance to chemotherapy[73] [80]. Moreover, the expression of miR-34 and miR-155 suppresses radiotherapy sensitivity[73] [80]. miRNA-34a has been reported to be associated with docetaxel resistance in human breast cancer cells[86] [93].