Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zorba J Hernández-Estrada and Version 2 by Vivi Li.
During the fermentation of cocoa beans, the yeasts produce volatile organic compounds (VOCs). Through reactions associated with amino acid metabolism, yeasts generate important aroma precursors as acetate esters and fatty acid ethyl esters; these are essential in developing fruity flavors and aromas in the final product (usually chocolate). In addition, some yeasts may have pectinolytic and antifungal activity, which is desirable in the post-harvest process of cocoa. The main yeast species in cocoa fermentation are Saccharomyces cerevisiae, Pichia kudriavzevii, and Hanseniaspora opuntiae. These produce higher alcohols and acetyl-CoA to make acetate–esters, compounds that produce floral and fruity notes.
- yeast
- cocoa bean
- fermentation
- flavor precursors
- starter cultures
1. Introduction
Yeasts have been involved in the fermentation of products for thousands of years in the production of wine, bread, sake, chocolate, and other fermented foods. Some of them have been produced for more than 10,000 years [1][2]. It has been shown that increasing yeast diversity in food fermentations increases the sensory complexity and diversity of aroma compounds found in the final products. Aromatic compounds play many key roles for yeasts, as survival strategies, defense mechanisms, and cellular communication. Humans have used their production to enhance the flavor and sensory attributes of fermented foods [2]. One of the most relevant fermentations in which yeasts are involved is the fermentation of the cocoa bean [3].
Cocoa is the fruit of the Theobroma cacao L., which is a perennial tree native to the South American tropical region. The dry fermented cocoa bean is the raw material for chocolate production and is composed of two cotyledons and an embryo, enveloped in a sweet and white mucilaginous pulp [4][5]. Based on Statista data [6], the global cocoa production scenario for the period 2021–2022 is 4,955,000 metric tons. Leading cocoa-producing countries for the same period are the Ivory Coast, Ghana, Indonesia, Nigeria, Cameroon, and Brazil [7]. There are three main cocoa varieties: Forastero (bulk or ordinary accounts for 95% of world cocoa production), Criollo, and Trinitario [4][5].
When the cocoa beans are removed from the pod, the pulp is degraded by a spontaneous fermentation conducted by yeast, lactic acid bacteria (LAB), and acetic acid bacteria (AAB). Several authors have found that good quality in fermented dry cocoa beans was correlated with good on-farm agricultural and post-harvest practices, bean selection, placenta removal prior to fermentation, and blending of the cocoa bean pulp mass [8][9][10]. In addition, well-performed fermentation is a prerequisite for producing high-quality chocolate [11]. Several studies have shown that yeasts produce various aromatic precursor compounds, such as alcohols and esters, which positively contribute to the aromatic profile of the chocolate [11][12][13][14][15][16]. The ethanol produced by yeast strains during cocoa fermentation is used as a carbon source for acetic acid bacteria and triggers diverse biochemical reactions inside cocoa bean that drives the aroma and flavor precursors in cocoa cotyledons [17]. Cocoa aroma is the result of various reactions that occur during the processing of beans and is related to the cocoa genotype, as well as environmental conditions, microbial diversity during fermentation, and subsequent processing steps, mainly drying and roasting [18]. Furthermore, cocoa flavor comprises of non-volatile compounds (polyphenols, carbohydrates, alkaloids, and proteins) and volatile compounds (esters, phenols, alcohols, aldehydes, ketones, furanones, and pyrazines) [19].
2. Cocoa Bean Fermentation and Biochemical Transformations on Cocoa Bean during Fermentation
Fermentation is essential for developing flavor and reaching the final acidity of cacao beans [5][20][21][22]. Four different methods are used to ferment cocoa beans: platform, box, heap, and basket fermentation. The selection of the fermenting method is related to the region of cocoa production [22]. The cocoa bean fermentation process involves the degradation of the mucilaginous pulp surrounding the beans by complex microbial interactions, mainly by yeasts, lactic acid bacteria (LAB), and acetic acid bacteria (BAA). Other microorganisms such as spore-forming bacteria (Bacillus and Paenibacillus), enterobacteria, and filamentous fungi are also present; however, their role remains unclear [4][5][20][22][23][24]. Cocoa pulp is a rich medium for microbial growth. It consists of water (80–90%), sugars, mainly glucose, sucrose, and fructose (0–15%), citric acid (1–3%), and pectin (1–1.5%). Proteins (0.5–0.7%), amino acids, vitamins (mainly vitamin C), and minerals (K+, Na+, Ca+2, Mg+2, Fe+2, and Zn+2) are also present [4][18][20][22][23]. There are two important phases in the fermentation of cocoa beans, anaerobic, and aerobic. The anaerobic phase lasts about 48–72 h after cocoa pod breaking and involves yeast and LAB strains [25]. The aerobic phase occurs after approximately 48 h of fermentation with the growth of AAB strains [4][20].2.1. Anaerobic Phase of Cocoa Bean Fermentation
2.1.1. Yeast
The first stage of cocoa bean fermentation involves the growth of yeasts mostly belonging to the genera Hanseniaspora, Saccharomyces, Candida, Kluyveromyces, Kazachstania, Meyerozyma, Rhodotorula, Wickerhamomyces, and Pichia [16][26][27]. Yeasts are the microorganisms that predominate this process during the first 24 h of fermentation, and subsequently, their population decreases [4][21]. Yeasts are favored by the initial acidity of the cocoa pulp (pH 3.6), the concentration of citric acid, the low oxygen levels, and environmental temperature ranging from 25–35 °C [4][20]. Yeast metabolizes glucose, fructose, and sucrose present in the cocoa pulp, yielding ethanol and carbon dioxide [28]. Yeast central metabolism begins with the basic conversion of sugars to pyruvate, producing ATP and reduced NADH cofactors. Under aerobic conditions, pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase and directed to the citric acid cycle. The anaerobic conversion of pyruvate to ethanol is a two-step process. First, pyruvate is converted to acetaldehyde by pyruvate decarboxylase (PDC), releasing carbon dioxide. Next, acetaldehyde is converted to ethanol by alcohol dehydrogenase (ADH). This oxidoreductase type can catalyze the reversible interconversion of alcohols and the corresponding aldehydes or ketones (Figure 12A) [17]. Some yeast species can produce organic acids, including acetic, phosphoric, oxalic, malic, and succinic [27][29]. Yeasts also contribute to the development of the characteristic flavor of chocolate due to the generation of volatile compounds [30]. Furthermore, it has been reported that some yeast strains such as Pichia kudriavzevii can hydrolyze the pectin present in the mucilaginous pulp surrounding the cocoa bean since they can produce pectinolytic enzymes [25].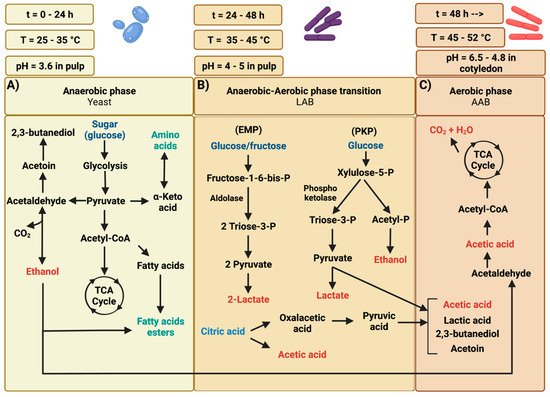
Figure 12. Main phases of cocoa fermentation. (A) Yeasts produce ethanol from sugar (glucose), fermenting it to pyruvate through glycolysis to obtain ATP, reduce equivalents production, and produce ethanol and carbon dioxide. (B) LAB strains utilize glucose through the Embden–Meyerhoff–Parnas EMP pathway (Homofermentative LAB) or phosphoketolase PKP pathway (Heterofermentative LAB). (C) Lastly, AAB strains oxidize ethanol produced by yeasts to acetic acid.
2.1.2. Lactic Acid Bacteria (LAB)
LAB is a group of Gram-positive bacteria whose main product of fermentable carbohydrate metabolism is lactic acid [31]. The LAB population increase when some of the pulp and lixiviate have drained mainly due to pectin degradation, and the yeast population decreases. Carbon dioxide production favors this increase in LAB populations by the yeasts and by the release of vitamins and other nutrients from the autolysis of yeast cells during cocoa fermentation [28]. The most abundant species after 24 h of fermentation are Limosilactobacillus fermentum, Lactiplantibacillus plantarum, Leuconostoc mesenteroides, and Lactococcus lactis [5][18][25][32][33]. During cocoa fermentation, LAB utilizes glucose via the Embden–Meyerhof pathway. The homofermentative LAB strains use glycolysis or Embden–Meyerhof–Parnas pathway (EMP) and yield more than 85% lactic acid. However, other species utilize glucose via the known pentose phosphoketolase pathway (PKP), hexose monophosphate shunt, or 6-phosphogluconate pathway producing only 50% lactic acid, and other metabolites such as ethanol, acetic acid, glycerol, mannitol, and CO2, as shown in Figure 12B [20][34]. LAB strains can consume fructose and metabolize citric acid. In the case of fructose, it is metabolized homofermentative (glycolysis) or heterofermentative (phosphoketolase pathway) to pyruvate, while citric acid is metabolized to acetic acid and oxaloacetic acid [5]. Oxoloacetic acid is converted into pyruvate, which will yield either lactic acid, acetic acid, or pyruvate metabolites as 2,3-butanedione (diacetyl; buttery notes), 2,3-butanediol, and 2-butanone (acetone; buttery notes) [5]. Some LAB strains can metabolize citric acid yielding diacetyl, acetoin, and butanediol [35].2.2. Aerobic Phase of Cocoa Bean Fermentation
On the third day of fermentation, when the pulp of the cocoa beans has been decreased, and both the temperature and the amount of air inside the fermentation mass have been increased, the environmental conditions are favorable for the proliferation of AAB. These bacteria metabolize the ethanol produced during yeast growth as their primary carbon source [18][36].Acetic Acid Bacteria (AAB)
AAB dominates this phase of cocoa bean fermentation; in recent years, these bacteria have been extensively studied due to their significant contribution to cocoa bean fermentation [37][38][39]. AAB conducts ethanol and lactic acid oxidation to acetic acid. Acetic acid is considered one of the main metabolites produced by an exothermic reaction oxidizing ethanol to acetic acid (Figure 12C). The rise in temperature to 40–52 °C, decrease in pH from 6.5 to 4.8 in the cotyledon, and penetration of acetic acid and ethanol to the cocoa bean is the cause of the death of the embryo, promoting their inactivation and increasing the permeability of the cell wall of the grain and the release of precursor molecules of cocoa color and flavor precursors [4][18][25][38][39][40]. The diversity of AAB is practically limited to two genera: Acetobacter and Gluconobacter [25]. Acetobacter pasteurianus is the most identified AAB during cocoa bean fermentation in Ivory Coast [41][42], Cameroon [16][43], Honduras [44], and Brazil [45][46].2.3. Biochemical Transformations on Cocoa Bean during Fermentation
The biochemical transformations that occur inside the cocoa bean are driven mainly by the production of ethanol, lactic acid, and acetic acid, and an increase in temperature during fermentation provoked by the oxidation of ethanol by AAB [5][20][21][47][48][49]. Acetic acid penetrates the bean and induces a drop in the pH of the cotyledons (approximately 6.5 to 4.8). This low pH of the cotyledons, combined with the presence of non-dissociated acetic acid and ethanol and the heat effect during fermentation, causes the embryo’s death (Figure 23) [20][21][47][50] damages the cotyledon’s internal structure to prevent the germination of cocoa beans. The physicochemical modifications result in desirable enzymatic and non-enzymatic conversions and the release of compounds from the cocoa bean.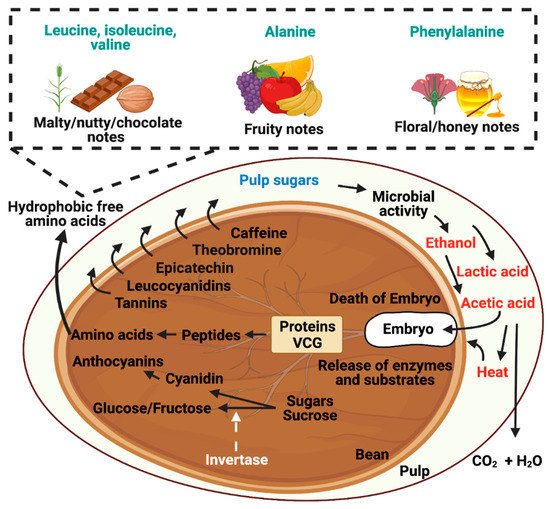
Figure 23. Formation of flavor precursors from hydrophobic-free amino acids. Microorganisms utilize the available substrates present in the cocoa pulp, such as carbohydrates, pectin, and organic acids, to produce the main metabolites of the process, such as ethanol, lactic acid, and acetic acid. Acetic acid penetrates the beans’ interior, causing the embryo’s death and the release of enzymes and endogenous substrates that, through proteolytic reactions, generate the flavor precursors (amino acids).
3. Contribution of Yeasts during Cocoa Fermentation
The fresh cocoa pulp is favorable for yeast growth since it consists of an anaerobic environment rich in sugars and a low pH that inhibits the development of other microorganisms [5][56]. Many studies have demonstrated a great diversity of yeast species during cocoa fermentation. The main yeast genera involved in the fermentation process of spontaneous cocoa are Pichia, Saccharomyces, Hanseniaspora, and Candida. Other genera found in lower abundance are Wickerhamomyces, Torulaspora, Kluyveromyces and Rhodotolura [9][11][13][15][16][26][27][28][29][33][41][45][46][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80]. Concerning the yeast species found in this process, several authors have highlighted that the most frequent are, in decreasing order, Saccharomyces cerevisiae, Pichia kudriavzevii, Hanseniaspora opuntiae, Hanseniaspora uvarum, Hanseniaspora guilliermondii, Pichia manshurica, Pichia kluyveri, and Candida tropicalis [25][56]. The main activities performed by yeasts during the cocoa fermentation process are the production of volatile organic compounds (VOCs), pectin hydrolysis, and carbohydrate fermentation [56]. Some species may have some antifungal effect [44][81][82] and can metabolize citric acid [29][75].3.1. Flavor Precursor Formation by Yeast during Fermentation
Yeasts are involved in the production of VOCs, which are essential in developing fruity flavors and aromas. These compounds are also determinants in developing fruity, caramel, or chocolate flavors and aromas [18]. Ho et al. [15] demonstrated that the absence of yeast during cocoa bean fermentation caused the absence of higher alcohols and esters in the fermented cocoa beans. This suggests that yeasts are the leading producers of these compounds. They concluded that yeasts were essential to the cocoa fermentation process. Koné et al. [13] identified 33 VOCs produced by yeasts. The species P. kudriavzevii, S. cerevisiae, C. tropicalis, and Wickerhamomyces anomalus were found to produce higher alcohols (isobutanol and isoamyl alcohol), acids (acetic acid and isovaleric acid) and esters (ethyl acetate, isobutyl acetate, and isovaleric acid). In the metabolism of yeasts, a fraction of the carbon is shuttled to the Krebs cycle, which forms important aroma precursors through reactions associated with amino acid metabolism [17]. Some yeast species such as Saccharomyces kudriavzevii produce higher alcohols, either catabolically or anabolically. The catabolic formation by the Ehrlich pathway involves consecutive transamination, decarboxylation, and dehydrogenation of amino acids. The anabolic production is by side products of amino acid biosynthesis starting from pyruvate. Some yeasts produce acetoin from acetaldehyde (green apple notes), which can be further reduced to 2,3-butanediol; similarly, diacetyl can be reduced to acetoin and 2,3-butanediol forming higher alcohol. Additionally, yeasts produce higher alcohols such as 3-methylbutanol and 2-phenylethanol and esters such as ethyl acetate, ethylphenyl acetate, and 2-phenylethyl acetate, contributing to the floral and fruity notes of the cocoa beans (Figure 34A) [5][17]. Esters are formed by a condensation reaction between acetyl/acyl-CoA and alcohol. The use of acetyl-CoA or acyl-CoA divides esters into acetate esters and fatty acid ethyl esters (Figure 34B). Acetate esters have significantly more influence over flavor and fragrance than the fatty acid counterparts due to their contribution of fruity and floral notes [17].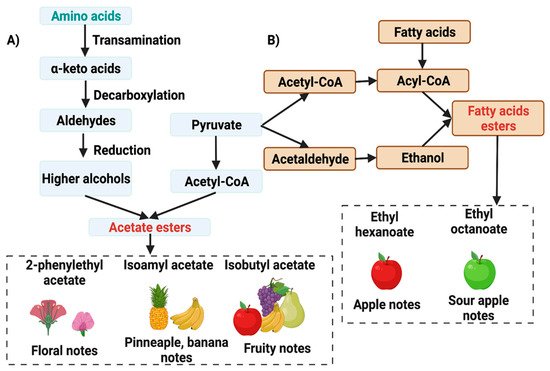
Figure 34. Formation of flavor precursors by yeast during fermentation via (A) Amino acids and (B) Fatty acids substrates. Yeast autochthonous to the cocoa fermentation process can produce higher alcohols either catabolically, through the Ehrlich pathway involving transamination, decarboxylation, and dehydrogenation of amino acids; or anabolically, as by-products of amino acid biosynthesis from pyruvate during the cocoa fermentation process.
References
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 8, 61–80.
- Carrau, F.; Boido, E.; Dellacassa, E. Yeast Diversity and Flavor Compounds. In Fungal Metabolites, 1st ed.; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 569–597.
- Venturini Copetti, M. Yeasts and molds in fermented food production: An ancient bioprocess. Curr. Opin. Food Sci. 2019, 25, 57–61.
- Figueroa-Hernández, C.; Mota-Gutierrez, J.; Ferrocino, I.; Hernández-Estrada, Z.J.; González-Ríos, O.; Cocolin, L.; Suárez-Quiroz, M.L. The challenges and perspectives of the Selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol. 2019, 301, 41–50.
- de Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453.
- Shahbandeh, M. Global Cocoa Production, 2020/21-Statista. Available online: https://www.statista.com/statistics/262620/global-cocoa-production/ (accessed on 27 May 2022).
- Cocoa Producing Countries. 2020. Available online: https://worldpopulationreview.com/country-rankings (accessed on 27 May 2022).
- Papalexandratou, Z.; Camu, N.; Falony, G.; de Vuyst, L. Comparison of the bacterial species diversity of spontaneous cocoa bean fermentations carried out at selected farms in Ivory coast and Brazil. Food Microbiol. 2011, 28, 964–973.
- Papalexandratou, Z.; de Vuyst, L. Assessment of the yeast species composition of cocoa bean fermentations in different cocoa-producing regions using denaturing gradient gel electrophoresis. FEMS Yeast Res. 2011, 11, 564–574.
- Lefeber, T.; Papalexandratou, Z.; Gobert, W.; Camu, N.; de Vuyst, L. On-farm implementation of a starter culture for improved cocoa bean fermentation and its influence on the flavour of chocolates produced thereof. Food Microbiol. 2012, 30, 379–392.
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116.
- Pereira, G.V.M.; Alvarez, J.P.; Neto, D.P.d.C.; Soccol, V.T.; Tanobe, V.O.A.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT 2017, 84, 290–297.
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917.
- Batista, N.N.; Ramos, C.L.; Ribeiro, D.D.; Pinheiro, A.C.M.; Schwan, R.F. Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT 2015, 63, 221–227.
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87.
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and biodiversity of bacterial and yeast communities during fermentation of cocoa beans. Appl. Environ. Microbiol. 2018, 84, e01164-18.
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128.
- Sarbu, I.; Csutak, O. The microbiology of cocoa fermentation. In Caffeinated and Cocoa Based Beverages: Volume 8. The Science of Beverages, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Duxford, UK, 2019; Volume 8, pp. 423–446.
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products-an overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91.
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221.
- de Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17.
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Favero, G.R.; de Carvalho, J.C.; Goés-Neto, A.; Rogez, H.; Soccol, C.R. Global cocoa fermentation microbiome: Revealing new taxa and microbial functions by next generation sequencing technologies. World J. Microbiol. Biotechnol. 2021, 37, 118.
- Camu, N.; de Winter, T.; Addo, S.K.; Takrama, J.S.; Bernaert, H.; de Vuyst, L. Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 2008, 88, 2288–2297.
- Lima, L.J.R.; Almeida, M.H.; Nout, M.J.R.; Zwietering, M.H. Theobroma cacao L., “The food of the gods”: Quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761.
- Chagas Junior, G.C.A.; Ferreira, N.R.; Lopes, A.S. The microbiota diversity identified during the cocoa fermentation and the benefits of the starter cultures use: An overview. Int. J. Food Sci. Technol. 2021, 56, 544–552.
- Delgado-Ospina, J.; Triboletti, S.; Alessandria, V.; Serio, A.; Sergi, M.; Paparella, A.; Rantsiou, K.; Chaves-López, C. Functional biodiversity of yeasts isolated from colombian fermented and dry cocoa beans. Microorganisms 2020, 8, 1086.
- Nielsen, D.S.; Hønholt, S.; Tano-Debrah, K.; Jespersen, L. Yeast populations associated with Ghanaian cocoa fermentations analysed using denaturing gradient gel electrophoresis (DGGE). Yeast 2005, 22, 271–284.
- Agyirifo, D.S.; Wamalwa, M.; Otwe, E.P.; Galyuon, I.; Runo, S.; Takrama, J.; Ngeranwa, J. Metagenomics analysis of cocoa bean fermentation microbiome identifying species diversity and putative functional capabilities. Heliyon 2019, 5, e02170.
- Daniel, H.M.; Vrancken, G.; Takrama, J.F.; Camu, N.; de Vos, P.; de Vuyst, L. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res. 2009, 9, 774–783.
- Leal, G.A.; Gomes, L.H.; Efraim, P.; de Almeida Tavares, F.C.; Figueira, A. Fermentation of cacao (Theobroma cacao L.) seeds with a hybrid Kluyveromyces marxianus strain improved product quality attributes. FEMS Yeast Res. 2008, 8, 788–798.
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117.
- Mota-Gutierrez, J.; Ferrocino, I.; Giordano, M.; Suarez-Quiroz, M.L.; Gonzalez-Ríos, O.; Cocolin, L. Influence of taxonomic and functional content of microbial communities on the quality of fermented cocoa pulp-bean mass. Appl. Environ. Microbiol. 2021, 87, e00425-21.
- Díaz-Muñoz, C.; van de Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; de Vuyst, L. Curing of cocoa beans: Fine-scale monitoring of the starter cultures applied and metabolomics of the fermentation and drying steps. Front. Microbiol. 2021, 11, 3446.
- von Wright, A.; Axelsson, L. Lactic Acid Bacteria. In Lactic Acid Bacteria, 5th ed.; Vinderola, G., Ouwehand, A.C., Salminen, S., Von Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–16.
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285.
- Illeghems, K.; de Vuyst, L.; Weckx, S. Complete genome sequence and comparative analysis of Acetobacter pasteurianus 386B, a strain well-adapted to the cocoa bean fermentation ecosystem. BMC Genom. 2013, 14, 526.
- Qin, Z.; Yu, S.; Chen, J.; Zhou, J. Dehydrogenases of acetic acid bacteria. Biotechnol. Adv. 2022, 54, 107863.
- Soumahoro, S. Occurrence of high acetic acid-producing bacteria in Ivorian cocoa fermentation and analysis of their response to fermentative stress. Am. J. BioSci. 2015, 3, 70–79.
- Farrera, L.; de la Noue, A.C.; Strub, C.; Guibert, B.; Kouame, C.; Grabulos, J.; Montet, D.; Teyssier, C. Towards a starter culture for cocoa fermentation by the selection of acetic acid bacteria. Fermentation 2021, 7, 42.
- Adler, P.; Frey, L.J.; Berger, A.; Bolten, C.J.; Hansen, C.E.; Wittmann, C. The key to acetate: Metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl. Environ. Microbiol. 2014, 80, 4702–4716.
- Hamdouche, Y.; Guehi, T.; Durand, N.; Kedjebo, K.B.D.; Montet, D.; Meile, J.C. Dynamics of microbial ecology during cocoa fermentation and drying: Towards the identification of molecular markers. Food Control 2015, 48, 117–122.
- Soumahoro, S.; Ouattara, H.G.; Droux, M.; Nasser, W.; Niamke, S.L.; Reverchon, S. Acetic acid bacteria (AAB) involved in cocoa fermentation from Ivory Coast: Species diversity and performance in acetic acid production. J. Food Sci. Technol. 2020, 57, 1904–1916.
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016, 236, 98–106.
- Romanens, E.; Freimüller Leischtfeld, S.; Volland, A.; Stevens, M.; Krähenmann, U.; Isele, D.; Fischer, B.; Meile, L.; Miescher Schwenninger, S. Screening of lactic acid bacteria and yeast strains to select adapted anti-fungal co-cultures for cocoa bean fermentation. Int. J. Food Microbiol. 2019, 290, 262–272.
- Miguel, M.G.d.C.P.; de Castro Reis, L.V.; Efraim, P.; Santos, C.; Lima, N.; Schwan, R.F. Cocoa fermentation: Microbial identification by MALDI-TOF MS, and sensory evaluation of produced chocolate. LWT-Food Sci. Technol. 2017, 77, 362–369.
- Serra, J.L.; Moura, F.G.; Pereira, G.V.d.M.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-Based Metagenomic Sequencing. LWT-Food Sci. Technol. 2019, 106, 229–239.
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613.
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857.
- John, W.A.; Böttcher, N.L.; Behrends, B.; Corno, M.; D’souza, R.N.; Kuhnert, N.; Ullrich, M.S. Experimentally modelling cocoa bean fermentation reveals key factors and their influences. Food Chem. 2020, 302, 125335.
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—Review. Food Res. Int. 2016, 82, 44–52.
- Rawel, H.M.; Huschek, G.; Sagu, S.T.; Homann, T. Cocoa bean proteins-characterization, changes and modifications due to ripening and post-harvest processing. Nutrients 2019, 11, 428.
- Fowler, M.S. Cocoa Beans: From Tree to Factory. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; John Wiley & Sons: Chichester, UK, 2009; pp. 10–47.
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157.
- Voigt, J.; Janek, K.; Textoris-Taube, K.; Niewienda, A.; Wöstemeyer, J. Partial purification and characterisation of the peptide precursors of the cocoa-specific aroma components. Food Chem. 2016, 192, 706–713.
- Nigam, P.S.; Singh, A. Cocoa and Coffee Fermentations. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: New York, NY, USA, 2014; Volume 1, pp. 485–492.
- Díaz-Muñoz, C.; de Vuyst, L. Functional Yeast starter cultures for cocoa fermentation. J. Appl. Microbiol. 2021, 15312.
- de Almeida, S.d.F.O.; Silva, L.R.C.; Junior, G.C.A.C.; Oliveira, G.; da Silva, S.H.M.; Vasconcelos, S.; Lopes, A.S. Diversity of yeasts during fermentation of cocoa from two sites in the Brazilian Amazon. Acta Amaz. 2019, 49, 64–70.
- Arana-Sánchez, A.; Segura-García, L.E.; Kirchmayr, M.; Orozco-Ávila, I.; Lugo-Cervantes, E.; Gschaedler-Mathis, A. Identification of predominant yeasts associated with artisan Mexican cocoa fermentations using culture-dependent and culture-independent approaches. World J. Microbiol. Biotechnol. 2015, 31, 359–369.
- Ardhana, M.M.; Fleet, G.H. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99.
- Fernández Maura, Y.; Balzarini, T.; Clapé Borges, P.; Evrard, P.; de Vuyst, L.; Daniel, H.M. The environmental and intrinsic yeast diversity of Cuban cocoa bean heap fermentations. Int. J. Food Microbiol. 2016, 233, 34–43.
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of turning, pod storage and fermentation time on microbial ecology and volatile composition of cocoa beans. Food Res. Int. 2019, 119, 477–491.
- Ho, V.T.T.; Zhao, J.; Fleet, G. The effect of lactic acid bacteria on cocoa bean fermentation. Int. J. Food Microbiol. 2015, 205, 54–67.
- Illeghems, K.; de Vuyst, L.; Papalexandratou, Z.; Weckx, S. Phylogenetic analysis of a spontaneous cocoa bean fermentation metagenome reveals new insights into its bacterial and fungal community diversity. PLoS ONE 2012, 7, e38040.
- Jespersen, L.; Nielsen, D.S.; Hønholt, S.; Jakobsen, M. Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res. 2005, 5, 441–453.
- Lagunes Gálvez, S.; Loiseau, G.; Paredes, J.L.; Barel, M.; Guiraud, J.P. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130.
- Meersman, E.; Steensels, J.; Mathawan, M.; Wittocx, P.J.; Saels, V.; Struyf, N.; Bernaert, H.; Vrancken, G.; Verstrepen, K.J. Detailed analysis of the microbial population in Malaysian spontaneous cocoa pulp fermentations reveals a core and variable microbiota. PLoS ONE 2013, 8, e81559.
- Moreira, I.M.D.V.; Miguel, M.G.D.C.P.; Duarte, W.; Dias, D.R.; Schwan, R. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res. Int. 2013, 54, 9–17.
- Moreira, I.M.D.V.; de Figueiredo Vilela, L.; Miguel, M.G.D.C.P.; Santos, C.; Lima, N.; Schwan, R.F. Impact of a microbial cocktail used as a starter culture on cocoa fermentation and chocolate flavor. Molecules 2017, 22, 766.
- Papalexandratou, Z.; Kaasik, K.; Kauffmann, L.V.; Skorstengaard, A.; Bouillon, G.; Espensen, J.L.; Hansen, L.H.; Jakobsen, R.R.; Blennow, A.; Krych, L.; et al. Linking cocoa varietals and microbial diversity of Nicaraguan fine cocoa bean fermentations and their impact on final cocoa quality appreciation. Int. J. Food Microbiol. 2019, 304, 106–118.
- Nielsen, D.S.; Teniola, O.D.; Ban-Koffi, L.; Owusu, M.; Andersson, T.S.; Holzapfel, W.H. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2007, 114, 168–186.
- Ouattara, H.G.; Niamké, S.L. Mapping the functional and strain diversity of the main microbiota involved in cocoa fermentation from Cote d’Ivoire. Food Microbiol. 2021, 98, 103767.
- Pacheco-Montealegre, M.E.; Dávila-Mora, L.L.; Botero-Rute, L.M.; Reyes, A.; Caro-Quintero, A. Fine resolution analysis of microbial communities provides insights into the variability of cocoa bean fermentation. Front. Microbiol. 2020, 11, 650.
- Papalexandratou, Z.; Lefeber, T.; Bahrim, B.; Lee, O.S.; Daniel, H.M.; de Vuyst, L. Hanseniaspora opuntiae, Saccharomyces cerevisiae, Lactobacillus fermentum, and Acetobacter pasteurianus predominate during well-performed Malaysian cocoa bean box fermentations, underlining the importance of these microbial species for a successful cocoa bean fermentation process. Food Microbiol. 2013, 35, 73–85.
- Pereira, G.V.d.M.; Miguel, M.G.d.C.P.; Ramos, C.L.; Schwan, R.F. Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl. Environ. Microbiol. 2012, 78, 5395–5405.
- Samagaci, L.; Ouattara, H.; Niamké, S.; Lemaire, M. Pichia kudrazevii and Candida nitrativorans are the most well-adapted and relevant yeast species fermenting cocoa in Agneby-Tiassa, a local Ivorian cocoa producing region. Food Res. Int. 2016, 89, 773–780.
- Verce, M.; Schoonejans, J.; Hernandez Aguirre, C.; Molina-Bravo, R.; de Vuyst, L.; Weckx, S. A combined metagenomics and metatranscriptomics approach to unravel Costa Rican cocoa box fermentation processes reveals yet unreported microbial species and functionalities. Front. Microbiol. 2021, 12, 641185.
- Visintin, S.; Alessandria, V.; Valente, A.; Dolci, P.; Cocolin, L. Molecular identification and physiological characterization of yeasts, lactic acid bacteria and acetic acid bacteria isolated from heap and box cocoa bean fermentations in West Africa. Int. J. Food Microbiol. 2016, 216, 69–78.
- Lima, C.O.; Vaz, A.B.M.; de Castro, G.M.; Lobo, F.; Solar, R.; Rodrigues, C.; Martins Pinto, L.R.; Vandenberghe, L.; Pereira, G.; Miúra da Costa, A.; et al. Integrating microbial metagenomics and physicochemical parameters and a new perspective on starter culture for fine cocoa fermentation. Food Microbiol. 2021, 93, 103608.
- Fernández-Niño, M.; Rodríguez-Cubillos, M.J.; Herrera-Rocha, F.; Anzola, J.M.; Cepeda-Hernández, M.L.; Aguirre Mejía, J.L.; Chica, M.J.; Olarte, H.H.; Rodríguez-López, C.; Calderón, D.; et al. Dissecting industrial fermentations of fine flavour cocoa through metagenomic analysis. Sci. Rep. 2021, 11, 8638.
- Papalexandratou, Z.; Falony, G.; Romanens, E.; Jimenez, J.C.; Amores, F.; Daniel, H.M.; de Vuyst, L. Species diversity, community dynamics, and metabolite kinetics of the microbiota associated with traditional Ecuadorian spontaneous cocoa bean fermentations. Appl. Environ. Microbiol. 2011, 77, 7698–7714.
- Romanens, E.; Pedan, V.; Meile, L.; Schwenninger, S.M. Influence of two anti-fungal Lactobacillus fermentum-Saccharomyces cerevisiae co-cultures on cocoa bean fermentation and final bean quality. PLoS ONE 2020, 15, e0239365.
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525.
More
