Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jian Zhang and Version 2 by Sirius Huang.
Toona sinensis belongs to the Meliaceae family and is commonly called Chinese toon or Chinese mahogany. Young leaves and buds of T. sinensis plants are excellent source of flavonoids, terpenoids, phenylpropanoids, and more. In addition, the bioactive components of T. sinensis possess numerous health benefits, such as antiviral, antioxidant, anti-cancer, anti-inflammatory, and hypoglycemic effects.
- T. sinensis
- pharmacology
- functional gene
- omics
- genetics
1. Characteristic Chemical Compounds of T. sinensis
1.1. Volatile Compounds
The headspace solid phase microextraction (HS-SPME) and gas chromatography- mass spectrometry (GC-MS) technologies can be used to extract and analyze the volatile components of plants. Yang et al. (2016) used an ultrasonic-assisted method to extract volatile components from T. sinensis leaves and identified 73 volatile compounds using GC-MS. The main volatile compounds included arachidonic acid ethyl ester, benzothiazole, pentadecanoic acid methyl ester, n-heneicosane, β-caryophyllene, benzoic acid hexylester, 1, 2-benzenedicarboxylic acid butyl octyl ester, limonene, heptacosane, n-hexadecanoic acid, and others [1][55]. Ji et al. (2018) used HS-SPME and GC-MS techniques to identify 32 volatile compounds from T. sinensis leaves, and found that (3E)-3-Hexenyl acetate, (Z)-Hex-3-en-1-ol, caryophyllene, and (Z)-Butanoic acid-3-hexenyl ester were the major constituents [2][56]. Gao et al. (2016) used the same methods to identify volatile components in T. sinensis leaves, flowers, and seeds, and further analysis showed 36 volatile compounds in leaves, 37 volatile compounds in flowers, and 26 volatile compounds in seeds. Among them, beta-Elemene, germacrene B, L-calamenene, and alpha-Cubebene were the most common [3][57]. Comparing the volatile components of three T. sinensis varieties (Ximu red from Yantai city in Shandong province, Jiaozuo red from Jiaozuo city in Henan province, and Heiyou purple from Taihe county in Anhui province), Liu et al. (2013) discovered that the Ximu and Jiaozuo red cultivars contain thiophenes and terpenes, although their exact contents differ, and the Heiyou purple cultivar contains terpenes and esters [4][58].
1.2. Terpenoid Compounds
After the first terpenoid compound, toosendanin, was extracted in 1972, ongoing research has identified various terpenoids in T. sinensis leaves; triterpenoids are main type of terpenoid. Hu et al. (2020) used various chromatographic techniques (e.g., silica gel, Sephadex LH-20, MCI gel, and ODS gel) to isolate and characterize terpenoids from 80% ethanol extract of T. sinensis leaves; the results showed that eight terpenoids could be extracted and identified from the leaf extract of T. sinensis: cedrelone, cedrodorol B, toonayunnanin D, toonaciliatone D, toonaciliatone A, 8β-hydroxypimar-15-en-19-oic acid methyl ester, 11β-acetoxyobacunol, and 11β-hydroxygedunin [5][59]. Yang et al. (2013) extracted the triterpenoids betulinic acid and ursolic acid for the first time [6][60]. Two triterpenoids, 6-acetoxyobaconicate and 7α-actoxydihydron, were first isolated and identified from the leaves of T. sinensis [7][61]. Moreover, another study first extracted and identified the terpenoids 11β-hydroxy-7α-oba-conylacetate and 11β-oxocneorin G from T. sinensis leaves [8][62].
1.3. Flavonoid Compounds
Flavonoids are widely distributed in plants, and the flavonoid content in T. sinensis leaves is 2~3 times than that of Ginkgo biloba L.. Zhao et al. (2016) used high-performance liquid chromatography (HPLC) to extract four flavonoids (rutin, quercetin, kaempferol, and gallic acid) from old leaves of T. sinensis [9][63]. Chen et al. (2019) separated and purified extracts from old leaves using column chromatography and HPLC, then used nuclear magnetic resonance (NMR) and infrared spectroscopy (IR) to identify substances such as rutin, epicatechin, quercetin, isoquercetin, and guava glucoside [10][64]. Ge et al. (2017) used ultra-high performance liquid chromatography to determine the flavonoid compounds in T. sinensis shoots, ultimately detecting glycosides, rutinoside, myricitrin, hyperoside, isoquercitrin, guaijaverin, astragalin, quercitrin, and afzelin [11][65]. Moreover, Miao et al. (2016) found five flavonoids identified from the T. sinensis leaf extract using the NKA9 macroporous adsorption resin method [12][66]. The total flavonoid content in stems, leaves, and flowers of T. sinensis plants were comparatively analyzed, and the results showed that the total flavonoid content in the leaves was highest, followed by the flowers and stems [13][67].
1.4. Phenylpropanoid Compounds
Phenylpropanoids commonly exist in natural plants, and mainly include lignins and coumarins. Nine phenylpropanoid compounds have been isolated and identified from different tissues of T. sinensis, namely, cedralins A and B [14][68], lyoniresinol, toonin C (Figure 1), matairesinol [15][6], 7-dimethoxy-5-methylcoumarin [16][69], scopoletin [17][70], and ficusesquilignans A and B [18][71]. Phenylpropanoid compounds often have pharmacological activities, such as antiviral, anti-inflammatory, antitumor, and antibacterial activity.
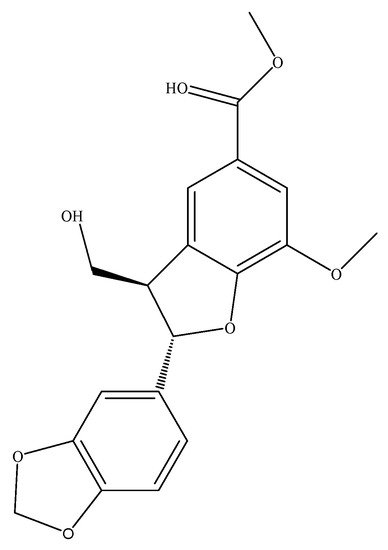
Figure 1. Structure of toonin C.
2. Pharmacological Characteristics of T. sinensis
The medicinal use of T. sinensis was first recorded in the Tang Materia Medica, which is a famous Traditional Chinese Medicine (TCM) monograph written in Tang dynasty China; this plant has thus been used as an herbal medicine for thousands of years [19][20][72,73]. The “Compendium of Materia Medica” and “Dictionary of Traditional Chinese Medicine” introduced the medicinal uses of the roots, bark, petioles, leaves, fruits, and seeds of T. sinensis [21][22][74,75]. In Chinese folk medicine, T. sinensis is described as an herbal medicine with good anti-inflammatory, detoxifying, and hemostatic effects, and it was commonly used to treat enteritis, dysentery, urinary tract infections, leukorrheal diseases, and skin itch [23][76]. Modern studies of T. sinensis have mainly focused on the extraction and identification of bioactive components from the leaves of T. sinensis [24][25][77,78], while few studies have reported the bioactive ingredients in the bark and seeds [26][79]. Extensive studies have shown that bioactive components from T. sinensis possess numerous health benefits, such as antiviral, antibacterial, antioxidant, anti-cancer, anti-inflammatory, and hypoglycemic effects [27][28][29][30][31][80,81,82,83,84].
2.1. Antioxidant Effect
Previous research reports have indicated that the extracts of T. sinensis are natural antioxidant agents [32][33][85,86]. Several reports have shown that phenolic compounds in the extract of T. sinensis have the ability to scavenge free radicals [34][35][36][87,88,89]. In addition, Hsieh et al. (2004) reported that extract of T. sinensis has antioxidant effects on hydrogen peroxide-induced oxidative stress [37][90].
2.2. Antiviral and Antibacterial Effect
T. sinensis possess notable antiviral and antibacterial effects. Chen et al. found that extract of T. sinensis leaves had antiviral activity against SARS-CoV in vitro, with an IC50 value of 30 μg· mL−1 [38][91]. You et al. (2013) reported that extract of T. sinensis leaves could be used an alternative treatment and prophylaxis against the H1N1 virus [39][92]. In addition, the extract of T. sinensis leaves has been found to possess promising antibacterial potential against E. coli, Salmonella, and Staphylococcus [40][41][93,94]. At present, the antiviral and antibacterial effects of T. sinensis are an increasing concerned of the pharmaceutical industry.
2.3. Anti-Inflammatory Effect
Many natural anti-inflammatory products isolated form the extracts of T. sinensis have been reported, and play important roles in preventing and treating inflammatory disease [42][95]. In 2012, Yang and Chen (2012) published a research report showing that total polyphenols extracted from the seeds of T. sinensis had a significant effect on the treatment of rat arthritis [43][96]. Chen et al. (2017) reported 7-deacetylgedunin (7-DGD) extracted from the fruit of T. sinensis, conducted in vivo and in vitro tests on mice, and the results showed that 7-DGD alleviated mice mortality induced by LPS [44][97]. This substance improves inflammation by activating the Keap1/Nrf2/HO-1 signaling pathway. In addition, many natural substances isolated from T. sinensis have been reported to possess notable anti-inflammatory effects [45][46][98,99].
2.4. Anti-Cancer Effect
As a natural anti-cancer drug, extract of T. sinensis is attracting increasing attention. Zhang et al. (2014) extracted four compounds from T. sinensis leaves (quercetin-3-O-α-L-rhamnopyranoside, kaempferol-3-O-α-L-rhamnopyranoside, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose and ethyl gallate), and reported that Kaempferol-3-O-α-L-rhamnopyranoside can inhibit the proliferation of HepG2 human liver cancer cells and MCF-2 human breast cancer cells as well as induce apoptosis [47][100]. The leaves of T. sinensis are rich in gallic acid, an important anti-cancer substance that can promote DU145 prostate cell apoptosis through the production of reactive oxygen species and mitochondrial pathways [48][101] as well as induce the apoptosis of oral squamous cancer cells by up-regulating the pro-apoptotic genes (TNF-α, TP53BP2 and GADD45A) and down-regulating the anti-apoptotic genes (Survivin and cIAP1) [49][102]. In addition, betulonic acid and 3-oxours-12-en-28-oic acid are both found in T. sinensis, which block the proliferation of MGC-803 and PC3 cancer cells and induce their apoptosis through the mitochondrial p53, bax, caspase 9, and caspase 3 pathways [50][103].
2.5. Hypoglycemic Effect
For nearly twenty years studies on the hypoglycemic effects of T. sinensis extracts have been reported, which could be beneficial for diabetes patients. In 2003, Yang et al. (2013) reported that ethanol extracts of T. sinensis leaf could enhance cellular glucose uptake in basal and insulin-stimulated 3T3-L1 adipocytes [51][104]. Hsieh et al. (2005) reported the inhibitory effect of T. sinensis extracts on LDL glycation induced by glucose and glyoxal [52][105]. Two studies have indicated that flavonoids of T. sinensis might be the active constituents corresponding to the hypoglycemic effects of this plant [53][54][106,107]. Furthermore, studies on the extract of T. sinensis have revealed that the mechanisms of TSL stimulating glucose uptake and ameliorating insulin resistance might be related to AMPK activation in skeletal muscles and to up-regulation of PPARγ and normalized adiponectin in adipose tissues [55][108].
3. Research on Genes and Omics
3.1. Function Genes
T. sinensis is rich in lignin and anthocyanin, two substances that are important indicators of bud quality. Cinnamic alcohol-CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) are the key enzymes in lignin biosynthesis, and chalcone isomerase (CHI) and anthocyanidin reductase (ANR) are required for plant anthocyanins biosynthesis. Based on the RNA-seq data of T. sinensis, TsCCR [56][109], TsCCR1 [57][110], TsCAD1 [58][111], TsCHI [59][112], TsANR [60][113] were identified and cloned. The TsCCR gene contained an open reading frame of 975 bp and encoded putative polypeptides of 324 amino acid residues [56][109], and the TsCCR1 gene contained an open reading frame of 924 bp and encoded putative polypeptides of 307 amino acid residues [57][110]. The TsCAD1 gene contained an open reading frame of 1,068 bp and encoded putative polypeptides of 355 amino acid residues [58][111]. The TsCHI gene contained an open reading frame of 717 bp and encoded putative polypeptides of 238 amino acids [59][112]. The TsANR gene contained an open reading frame of 1011 bp and encoded putative polypeptides of 336 amino acids [60][113].
Few studies have reported the expression patterns of the four genes (TsCCR, TsCAD1, TsCHI, and TsANR) in different tissues of the T. sinensis plant; the results showed that the expression level of TsCCR and TsCHI genes in stems were significantly higher than those in roots and leaves, and the transcript level of TsCAD1 gene in roots was significantly higher than in the stems and leaves. The results of real-time PCR showed the highest relative expression of TsANR gene in the leaves of T. sinensis seedlings.
Moreover, the expression pattern of these four genes under different stress treatments were investigated, and the results showed that the expression pattern of the TsCCR gene was first temporarily up-regulated and then down-regulated after cold treatment, which is the opposite of the expression pattern of this gene during heat treatment. During a salt stress treatment (200 mmol·L−1 NaCl), TsCCR expression decreased significantly in the first 4 h, then increased rapidly, decreased, increased, and then decreased again during 200 g·L−1 PEG6000 drought stress treatment [56][109]. During 24 h 38 °C heat treatment, TsCAD1 expression first decreased and then increased significantly, while it first increased and then decreased during 24 h 4 °C cold treatment. TsCAD1 expression first decreased, then increased, and then decreased again during 24 h drought stress treatment (200 g·L−1 PEG 6000), although the relative expression level was lower than control. The expression trend during a 200 mmol·L−1 NaCl salt stress treatment for 24 h first decreased and then increased, although the relative expression level was lower than the control [58][111]. Under high temperature (38 °C), drought stress (200 g·L−1 PEG 6000 solution) and salt stress (200 mmol·L−1 NaCl solution), TsCHI expression was higher than that of the control, and showed an upward trend as the processing time was extended [59][112]. These results provide a theoretical basis for genetically engineering T. sinensis to cultivate resistances against extreme environmental conditions. With 24 h of high temperature treatment (38 °C), TsANR gene expression increased significantly at 1 h, then returned to the level of control [60][113].
3.2. Omics
T. sinensis omics research has mainly focused on genomic and transcriptomic studies. Ran et al. (2020) sequenced the transcriptomes of the buds of the T. sinensis varieties ‘Heiyouchun’ and ‘Qingyouchun’ during four developmental periods, then analyzed the expression pattern of anthocyanin biosynthesis genes. Among the key genes expressed in anthocyanin synthesis by KEGG analysis, five genes, namely, phenylalanine ammonia lyase (PAL), coumarin-Coa ligase (4CL), Chalketone synthase (CHS), flavonoid 3-hydroxylase (F3′H), and anthocyanin synthase (ANS), were up-regulated in ‘Heiyouchun’, while C3’H and flavonol synthase (FLS) were down-regulated in ‘Heiyouchun’ [61][114]. Zhao et al. (2017) analyzed the RNA-seq data of ‘Heiyouchun’ sprouts and found 467 unigenes involved in terpenoid biosynthesis related to flavor formation, including 226, 71, 86, and 84 unigenes for terpenoid backbone, monoterpenoid, sesquiterpenoid (triterpenoid), and diterpenoid biosynthesis, respectively [62][115]. Sui et al. (2019) analyzed the RNA-seq data of young leaves and mature leaves of T. sinensis, and found that the KEGG pathways for phenylpropanoid, naringenin, lignin, cutin, suberin, and wax biosynthesis were significantly enriched in mature leaves [63][116].
Xiang et al. (2021) assembled the complete T. sinensis chloroplast genome using second-generation high-throughput sequencing technology. The chloroplast genome contained 138 genes in total, including 89 protein-coding genes, seven rRNA genes, forty tRNA genes, and two pseudogenes [64][117]. Liu et al. (2019) sequenced the chloroplast genome of T. sinensis using an Ilumina sequencing platform, and found that the chloroplast genome is a characteristic four-party structure with a length of 157,228 bp which contains two 26,994 bp inverted repeats (IRs), an 85,971 bp large single-copy, and a 17,269 bp small single-copy. A total of 126 genes, including 82 protein-coding genes, 36 tRNA genes, and eight rRNA genes, were identified [65][118]. Ji et al. (2021) reported a high-quality T. sinensis genome assembly with scaffolds anchored to 28 chromosomes, an assembled length of 596 Mb, and a total of 34,345 genes predicted in the genome after homology-based and de novo annotation analyses [66][119].
