Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lingyun Yang and Version 2 by Amina Yu.
G protein-coupled receptors (GPCRs) are a large membrane protein family found in higher organisms, including the human body. GPCRs mediate cellular responses to diverse extracellular stimuli and thus control key physiological functions, which makes them important targets for drug design. Signaling by GPCRs is related to the structure and dynamics of these proteins, which are modulated by extrinsic ligands as well as by intracellular binding partners such as G proteins and arrestins.
- G protein-coupled receptors
- 19F-NMR
- membrane mimetics
- stable-isotope labeling
- in-membrane chemical modification
- amino-acid-specific NMR labeling
- sequence-specific NMR labeling
1. 19F-Nuclear Magnetic Resonance (NMR) R with Observation of Extrinsic Probes Attached to G Protein-coupled Receptor (GPCR)PCRs
19F-NMR has long been used for studies of complex biological systems, since 19F has no natural background signals and displays high sensitivity toward changes in its microenvironment. As fluorine is not a natural component of proteins, it is essential to develop ever-improved methods to incorporate fluorine probes into GPCRs, either during expression or by post-translational chemical modification (Figure 13) [1][2][3][4][5][40,41,42,43,44].
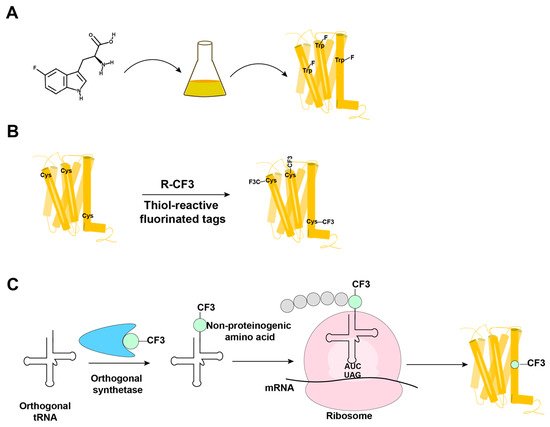
Figure 13. Overview of the methods in use for incorporation of 19F-NMR labels into GPCRs. (A) Biosynthetic incorporation by adding fluorinated amino acids, such as 5F-Trp, to the expression system; all Trp residues in the protein are then labeled with 19F. (B) Post-translational chemical modification by reacting the GPCR with thiol-reactive fluorinated tags; all reagent-accessible Cys residues are then labeled with the fluorinated tag. (C) Genetic labeling using an extrinsic orthogonal tRNA/aminoacyl-tRNA synthetase pair to incorporate non-proteinogenic 19F-labeled amino acids at positions defined by a TAG amber codon.
In biosynthetic incorporation, all residues of one amino acid type can be replaced by its fluorinated analogue, providing “amino-acid-specific 19F-labeling” (Figure 13A). Fluorinated amino acids are fed to the expression host by including a high concentration of the fluorinated amino acid in the growth medium (Figure 13A) [2][6][7][41,45,46]. This approach may be limited by the fact that high concentrations of 19F-containing amino acids can inhibit cell growth [8][47]. Induction of the amino acid auxotrophy in nonauxotrophic bacterial strains by shutting down selected amino-acid-specific biosynthesis pathways with specific inhibitors has also been used for 19F-labeling [9][10][48,49]. Tryptophan residues are highly present at the hydrophobic interfaces of protein–protein complexes, which makes fluorotryptophan attractive for NMR studies of membrane proteins [11][12][50,51]. The use of fluorinated indole as a fluorotryptophan precursor has been described as an inexpensive alternative for obtaining tryptophan-specific labeling [8][47].
Fluorine has been widely incorporated into proteins by post-translational chemical modification (Figure 13B). The most commonly employed method is cysteine-labeling, making use of the high nucleophilicity of the side chain sulfhydryl group [4][43]. Labels with CF3 groups are attractive because they yield strong signals that are not subject to large chemical shift anisotropy relaxation [5][44]. Examples are 3-bromo-1,1,1-trifluoroacetone (BTFA) [13][52] and 2-bromo-4-(trifluoromethyl)acetanilide (BTFMA), which react with the sulfhydryl group in a single step [14][53]. The conjugation of 2,2,2-trifluoroethanethiol (TET) to membrane proteins starts with sulfhydryl group activation by 4,4-dithiodipyridine (4-DPS), and a disulfide bond is formed in a second step [3][15][16][17][18][42,54,55,56,57]. Post-translational chemical modification can be applied to otherwise unlabeled proteins and regardless of the expression system used; high expression yields can thus be obtained, which is especially useful for GPCRs [3][15][16][17][18][19][24,42,54,55,56,57]. When using amino-acid-specific labeling, further sequence-specific assignments of 19F-resonances have been obtained by site-specific mutagenesis. For cysteine-rich GPCRs, individual assignments can therefore be very demanding. In 2015, Sušac et al. [17][56] reported the in-membrane chemical modification (IMCM) method, which makes use of the natural protection of most cysteines in the transmembrane helices by the membrane environment. Selective cysteine labeling on the receptor surface with minimal or no mutagenesis can thus be achieved.
The introduction of multiple 19F-labels within the same protein has been used to check on intramolecular distances related to the three-dimensional molecular structure [20][21][58,59].
In a genetic engineering approach, the site-specific incorporation of fluorinated amino acids is accomplished through using an orthogonal amber suppressor tRNA with a paired tRNA synthetase to insert the non-proteinogenic amino acid at positions defined by a TAG amber codon (Figure 13C) [1][40]. Fluorinated phenylalanine [22][23][24][60,61,62] and tyrosine [25][63] derivatives have been incorporated into proteins using this approach. Genetic labeling can be highly precise, but in improperly optimized expression systems, it may provide low yields of both the expression and incorporation of the fluorinated-amino acid [26][64]. Wang et al. [24][62] reported the genetic labeling of the cannabinoid receptor 1 (CB1) with the non-proteinogenic amino acid 3’-trifluoromenthyl-phenylalanine (mtfF) in the baculovirus expression system; this approach enabled studies of conformational transformations under the influence of ligands with variable efficacies [24][62].
2. NMR in Solution of GPCRs Using Stable-Isotope Labeling
Three different stable-isotope labeling strategies have primarily been used for studies of GPCRs: post-translational chemical labeling with 13C-labeled methyl groups, amino-acid-type selective labeling and uniform labeling. Post-translational chemical labeling of the reactive side chains of surface-accessible cysteine or lysine residues has been used in many NMR studies of GPCRs. 13C-isotope-labeled methyl probes can be chemically attached to the γ-SH moiety of cysteine side chains or the ε-NH2 groups of lysine side chains. 13C-formaldehyde has been used to label solvent-exposed lysines, yielding 13C-dimethyllysines as NMR probes [27][28][29][65,66,67]. 13C-methyl methanethiosulfonate (13C-MMTS) has been used to label solvent-exposed cysteines of GPCRs [30][68]. Unlike trifluoromethyl probes, which are usually attached to a single judiciously selected surface-accessible cysteine to avoid signal interference from other labeled residues [3][31][32][33][34][42,69,70,71,72], 13C-labeled methyl probes have often been used to label all surface-accessible (endogenous as well as non-endogenous) cysteines or lysines, and 2D 1H-13C correlation spectra were recorded to resolve multiple signals [35][73].
Limitations arise because the choice of stable-isotope probes for the chemical modification of amino acids side chains may influence the dynamics of GPCRs [33][71]. On principal grounds, post-translational chemical labeling normally only targets surface-accessible residues of GPCRs. In contrast, amino-acid-selective isotope labeling also targets the transmembrane region of the receptor. Examples of amino-acid-type selective isotope labeling include the use of [δ1-13CH3]-isoleucine [36][37][74,75], [ε-13CH3]-methionine [29][30][36][38][39][40][41][42][43][44][45][46][28,67,68,74,76,77,78,79,80,81,82,83], [15N]-valine [47][48][84,85] and [15N]-leucine [49][86]. An increased sensitivity was achieved by deuterating the α- and β- positions of methionine and leucine, using [2,3,3-2H, methyl-13C]-methionine [38][39][41][42][28,76,78,79] and [2,3,3-2H, 15N]-leucine [49][86] in the nutrient.
Uniform 15N-labeling of GPCRs has been achieved in E. coli for the rat neurotensin receptor 1 [50][87] and in Pichia pastoris for A2AAR [51][52][53][88,89,90] and the histamine H1 receptor [54][91]; the minimal medium contained [15N]-ammonium sulfate or [15N]-ammonium chloride as the only nitrogen sources. Since deuteration is mandatory for transverse relaxation-optimized spectroscopy (TROSY) studies of large macromolecular systems [55][92], it is essential that the expression systems used can produce partially or fully deuterated recombinant proteins. Uniform 2H, 15N-labeling was also achieved by expression of the β1-adrenergic receptor in Sf9 insect cells; the addition of 2H, 15N-labeled yeast extract to the insect cell medium allowed deuteration levels of >60% [56][93]. Eddy et al. [51][52][53][88,89,90] expressed uniformly 2H, 15N-labeled A2AAR with D2O-adapted Pichia pastoris in D2O growth media. All six tryptophan indole 15N-1H signals and eight of the eighteen glycine backbone 15N-1H NMR signals were resolved in the 2D [15N, 1H]-TROSY spectrum of A2AAR, and sequence-specific NMR assignments were obtained by single-residue amino acid replacements [51][88]. Drug-dependent local conformational changes in A2AAR could thus be observed, as illustrated for the toggle switch Trp2466.48 [51][88]. In addition to the natural tryptophans, extrinsic tryptophan residues were introduced into judiciously selected sites of the receptor by genetic engineering; these were then used as supplementary NMR probes for monitoring conformational changes of A2AAR [52][53][89,90].
Overall, in contrast to “probe methods”, uniform labeling can provide global information on a receptor.
3. GPCR–Ligand Interactions Studied by NMR Observation of the Ligand
NMR observation of bound and free ligands can provide unique insights into the biophysical properties and biological functions of GPCRs. Specifically, in addition to providing data on the influence of bound ligands on GPCR, NMR spectroscopy is uniquely powerful in detecting weak binding [57][58][59][95,96,97]. Measurements of the chemical shifts, line widths, and relaxation times of free and bound ligands are all informative on ligand binding events. Depending on the time scale of the GPCR–ligand interactions, different approaches are used [60][98].
For studies of weak binding with a rapid ligand exchange, a large arsenal of experiments based on observation of the modulation of the NMR signal of the free ligand through exchange with the bound ligand is available [61][62][63][64][101,102,103,104]. Among these, the transfer NOE (trNOE) stands out by the fact that information on the structure of the bound ligand can be obtained. For example, in studies of the peptide ligand dynorphin interacting with the kappa opioid receptor (KOR) [65][105], 1H and 15N chemical shift variations for the free ligand indicated that the free peptide is in fast exchange with the bound peptide. The receptor–peptide interaction was within the range that allowed the determination of a conformation of the KOR-bound dynorphin via the trNOE method.
