Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Teodor Adrian Enache.
The immortalized PC-12 cell line demonstrated to be a classical neuronal cell model derived from rat pheochromocytoma with the ability to acquire the sympathetic neurons features in a differentiation process in the presence of nerve growth factor. PC-12 cell line was shown to be the preferred model in neurobiology study using biosensing devices. The analytical achievements and applicability of reported biosensing devices in PC-12 cultures for the detection of ions, neurotransmitters, and cellular events are summarized.
- PC12 cell line
- biosensing
- analytical determination
- neuronal stimulation
- neurotransmitters
- exocytosis
- ion channel
1. Metal Ions and Small Molecules Detection
1.1. Metal Ions
Metal ions are involved in cellular and subcellular functions with many functions still unrevealed and with a demand to elucidate the role of inorganic salts in living systems.
Some of the known functions of metal ions are: material transportation, energy conversion, information transmission, and metabolic regulation. Their low or high concentration can lead to health issues, so the detection and mapping of metal ions in individual cells, tissues, and whole organisms is crucial. Among the methods employed for metal ion detection, fluorescent sensors are the most applied ones since they also allow imaging of the metal ions in biological systems, such as Na+, K+, Ca2+, Mg2+, Fe2+/Fe3+, Zn2+, and Cu2+. A classic organic fluorescent probe contains one or more fluorescent cores, a metal chelating or binding moiety which is able to recognize and interact with different metal ions, and their use in the sensing mechanism for biological applications in metal detection is illustrated in Figure 1 [25][1].
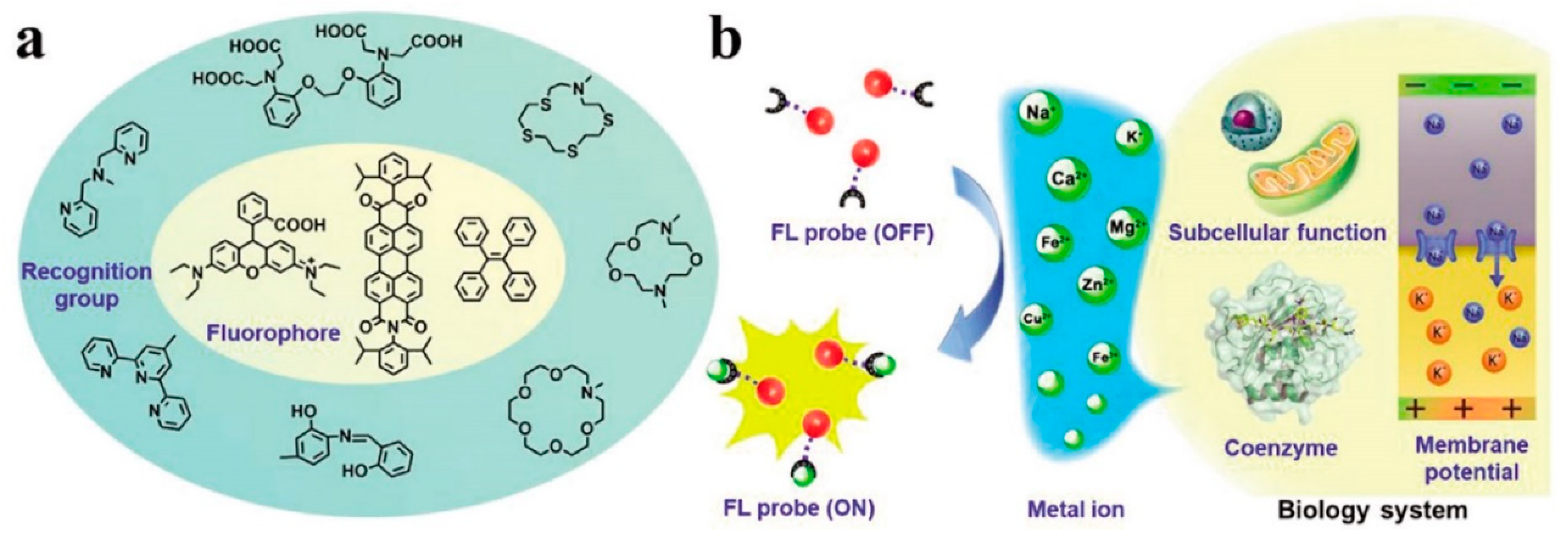
Several metal ions have been successfully quantified or imaged in PC-12 cells, bringing important insights into their physiological and pathological role, and are listed below.
Zinc ions are mostly known to play an important role in signaling in both intra- and intercellular communication, which require transients of free zinc ions, and not protein-bounded zinc. This represents a challenge since it is quite difficult to distinguish between the two forms of free and bounded zinc. For this purpose, two excitation ratiometric fluorescent biosensors based on carbonic anhydrase were reported [26,27][2][3]. No interferences form calcium or magnesium ions in millimolar concentration range were observed, which allowed to detect zinc ions in a very low concentration range of 5–10 pM in cytoplasm and nucleus [26][2]. The detection of free zinc level variation upon cellular oxygen glucose deprivation (OGD) in cytoplasm and mitochondria in the PC-12 rat pheochromacytoma cell culture line enabled to determine that there is an increase in the mitochondrial free zinc ions concentration immediately after OGD, which than gradually returned to physiological levels, while cytosolic zinc increased over a 24-h time period in viable cells. The findings are valuable to better understand bioenergetics dysfunction and cell death that occurs with both in vitro and in vivo models of reperfusion [27][3].
Copper is a required trace element with important biological roles, being a component of several human enzymes, making cupper deficiency or defects in copper transport leading to serious or fatal diseases. The use of a synthetic variant of human apocarbonic anhydrase II for sensing Cu2+ enabled and increased selectivity for Cu2+ over Zn2+. The biosensor based on the fluorescent-labeled Cu2+-specific variant of human apocarbonic anhydrase was able to measure very low concentrations of Cu2+ in the femtomolar range in PC-12 cell cultures with the possibility to also image the free Cu2+ levels by means of frequency-domain fluorescence lifetime microscopy [28][4].
Calcium ions were reported to be the key analyte for various events comprehensions in PC-12 cells, especially in synaptic transmission and spontaneous neurotransmitter release [29,30,31][5][6][7]. Single cell-based biosensors were also reported for calcium ion concentration detection by using fluorescence microscopy with the ion indicator fluo-3-acetoxymethyl ester to measure receptor activation in PC-12 lines [32][8] and for the identification of biologically active ligands present in a complex mixture. In the first case, the single-cell biosensor was based on the ligand–receptor binding and G-protein-mediated signal transduction with applicability in the identification of endogenous bradykinin, a peptide that promotes inflammation, in human hepatocellular carcinoma cells lysates and its bioactivity screening in degradation products from human blood plasma [32][8]. The second biosensor was able to detect specific components of a complex mixture fractionated by a microcolumn separation technique based on ligand–receptor binding and signal-transduction pathways to biochemically amplify the presence of an analyte after electrophoretic separation [33][9]. Another biosensor used the Ca2+ enhancement of the novel synthetized coumarine derivative fluorescence and a detection limit of 5.81 × 10−7 M, for calcium, was obtained [34][10].
1.2. Neurotransmitters
Neurotransmitters are small molecules that act as messengers in the synaptic transmission process, with imbalance in their activity causing serious mental disorders, such as Parkinson’s disease, schizophrenia, and Alzheimer’s disease. Therefore, monitoring the neurotransmitters concentrations is of great interest in the study and diagnosis of several mental illnesses. Biosensors for in vivo and ex vivo neurotransmitter detection rely on the use of nanomaterials, polymers, and biomolecule, with the electrochemical ones prevailing in the in vivo detection over the optical ones [35,36][11][12].
Exocytosis plays an essential role in the communication between cells in the nervous system. Understanding the regulation of neurotransmitter release during exocytosis and the amount of neurotransmitter content that is stored in vesicles is of importance, as it provides fundamental insights to understand how the brain works and how neurons elicit a certain behavior [30,37][6][13].
Catecholamines are a class of neurotransmitters comprising dopamine (DA), norepinephrine, and epinephrine. The correlation of catecholamine levels with many types of diseases, motivated many researchers to develop accurate quantification methodologies together with optimization procedures and sample preparation to detect them at extremely low concentrations in the presence of numerous co-existing biological interferences [38][14].
Among the catecholamines, DA plays a key role in the function of the human central nervous system. Since abnormal release of dopamine is associated with several neurological diseases, its detection and continuous monitoring in vivo has been of great interest, with many electrochemical and optical biosensors being developed for this purpose. The main focus was to achieve low detection limits, due to the fact that concentration of DA is extremely low in patients with neurological diseases [39][15]. In this context, PC-12 cell lines play an important role in the detection of DA in vivo, with several optical [40[16][17][18],41,42], electrochemical biosensing systems, fixed potential amperometry [43,44,45,46,47,48,49,50,51][19][20][21][22][23][24][25][26][27] and voltametric ones [52[28][29][30],53,54], and electrical devices [55,56][31][32] being investigated, with great potential for diagnostic purposes being demonstrated.
Catecholamine release from PC-12 cells has been observed at zeptomole levels using dc-amperometric detection at carbon fiber microelectrodes. The results obtained from 13 PC-12 cells corresponded to 190 zmol (114,300 molecules per release event) and the detection limit was as low as 31 zmol [57][33]. Moreover, cyclic voltametric measurements of relative concentration for zeptomole levels of transmitter in attoliter volumes provide evidence that loading vesicles by increased transmitter synthesis does not lead to elevated concentrations at individual release sites [58][34].
Structural and size analysis of the vesicular dense core and halo using transmission electron microscopy was combined with single-cell amperometry to study the vesicle size changes induced after zinc treatment, and the existence of a strong link between vesicle structure and exocytotic dynamics was established [59][35].
Several electrochemical biosensors were reported for DA detection in PC-12 cells, most of them using fixed potential amperometry [43,44,45,46,47,48,49,50,51][19][20][21][22][23][24][25][26][27] and voltammetry [52,53,54][28][29][30].
The amperometric biosensors operated at similar voltage values of around 0.3 V, normally chosen after evaluating the voltametric profile of DA at the corresponding developed sensing platform. The biosensor based on Au microelectrodes modified with conductive polymers and peptide nanofibers allowed the amperometrical detection of DA in the femtomole range, having the advantage in using the peptide nanofibers that increase the adherence properties of PC-12 cells [43][19]. A nitrogen-doped mesoporous carbon nanosheets-based biosensor operated at +0.25 V and showed high sensitivity and selectivity for DA sensing with a detection limit of 10 nM [44][20], with a similar detection limit value of 9 nM achieved by a paper-based electrochemical sensor [46][22] and a slightly lower value of 5 nM by the one based on graphene oxide and AuNP with EDTA immobilized-poly(1,5-diaminonaphthalne) [45][21]. The use of a nanocomposite comprising Pt nanoparticles (PtNPs) decorated multi-wall carbon nanotubes (MWCNTs), allowed the lowest detection limit among the amperometric biosensors, with a value of 2 nM [47][23]. The release of neurotransmitters from PC12 cells under stimulation was selectively monitored by amperometry at carbon fiber electrode in order to detect either solely dopamine or dopamine and FFN102 altogether. It was observed that FFN102 led to the partial replacement of dopamine in secretory vesicles and the partial replacement of dopamine could be used to monitor exocytic events through electrochemical detection of dopamine and fluorescence detection of FFN102 [60][36].
The development of electrochemical cytometry allows comparison between vesicle content and vesicular release and it was found that only part of the vesicle content is released in typical exocytotic cases measured by amperometry. The approach involves the adsorption and subsequent rupture of vesicles on an electrode surface following electrode redox processes. The measured current allows to count the number of molecules in the vesicles using Faraday’s law and to correlate this to the amount of molecules released when single exocytosis events take place in communicating cells [61][37].
Other amperometric arrays use a microfluidic sensor based on carbon nanotubes (CNTs)-modified indium tin oxide (ITO) microelectrodes for DA detection in single living rat pheochromocytoma (PC 12) cells [48][24], while the microdialysis-amperometric system reported in [49][25] uses high-performance liquid chromatography with electrochemical detection (HPLC–EC) [49][25], and the scale-integrated amperometric sensor in [50][26] was able to image simultaneously the DA release from PC-12 cells. Besides the amperometric detection assays exemplified above, which operated at relatively low overvoltage of around +0.3 V, another assay based on micro graphitic-diamond multi electrode arrays was applied for the detection of DA released form PC-12 cells under K+ stimulation at a higher overvoltage of +0.65 V [51][27].
One voltametric biosensor uses a 5-nm-thick poly-celestine blue (CB)-modified glassy carbon electrode (GCE), with a detection limit of 1.2 nM with successful application for DA detection released from PC-12 lines under nicotine stimulation [52][28], while the other is based on molecularly imprinted layers of 4-mercaptophenylboronic acid (4-MPBA) and the polymer acid chrome blue K, which is polymerized around the DA molecule to achieve high selectivity toward DA. The later sensor had a detection limit of 140 nM [53][29]. The lowest detection limit of voltametric biosensors was achieved by a graphene quantum dots/multiwalled carbon nanotubes (GQDs-MWCNTs)-based biosensor with excellent selectivity for DA and a detection limit of 0.87 nM using DPV [54][30].
Almost all electrochemical biosensors were successfully applied for DA detection released from PC-12 cells under K+ [44,45,47,50,51,53][20][21][23][26][27][29] or nicotine [52][28] stimulation. An important applicability was achieved by the amperometric biosensor in [47][23] which was able to evaluate the effects of antipsychotic drug (aripiprazole) on the dopamine release from cells treated with high K+ and by the paper-based one in [46][22] which monitored the DA released from damaged PC12 cells induced by amyloid-beta peptide (Aβ25–35) and cell intervene models protected by curcumin and marrow mesenchymal stem cells. The sensing platform developed in [49][25] also showed an important applicability in studying the effects of different drugs on DA secretion with the device in [50][26] exemplifying the effects of the dopaminergic drugs l-3,4-dihydroxyphenylalanine (l-DOPA) on the DA levels released form the PC-12 cells.
Lower values of detection limits toward DA detection were achieved using field effect transistors (FET) [55[31][32],56], one of them based on DNA-aptamers immobilized on a multiple-parallel-connected silicon nanowire (SiNW) with a detection limit lower than 10 fM, with high specificity for DA in the presence of ascorbic acid, catechol, phenethylamine, tyrosine, epinephrine, and norepinephrine. The FET enabled the DA monitoring released from PC-12 cells under hypoxic stimulation which is coupled to an increase in intracellular Ca2+ that is required to trigger DA secretion [55][31]. Another FET based on SiNW was developed using the chemical linker 3-aminopropyltrimethoxysilane (APTMS) and boronic acid, with high binding affinity achieved and a dissociation constant of dopamine–boronic acid complexes of 33 ± 8 fM [56][32].
Besides DA, epinephrine and norepinephrine were also the key analytes to be detected by several newly developed biosensing systems, with in vivo detection in PC-12 cells under various external stimuli.
Norepinephine was detected electrochemically in PC-12 cells on electrode platforms based on C-, N-doped NiO, which make use of both the homogenous doping and the nanostructure NiO surface to enhance the electroactivity of the sensor toward a sensitive noradrenaline detection released form PC-12 cells under K+ extracellular stimulation, with high selectivity and long-term stability [62][38]. Another electrochemical sensor for norepinephrine detection released from PC-12 cells under K+ stimulation was based on nitrogen-doped carbon hollow trunk-like structure which demonstrated high selectivity for monitoring of NA with a detection limit of 5 nM. Furthermore, this sensor is a portable sensor, an advantage for POC applications [63][39].
The neurotransmitter epinephrine is also a well-known medication in resuscitation, especially after a heart attack and to treat bronchial asthma attacks. Hence, its detection is crucial for the discovery and evaluation of new epinephrine-based drugs to control the metabolic processes linked to diseases, with applicability in the POC devices. Epinephrine was colorimetrically and electrochemically detected in PC-12 cells at CuO nanorods with laccase-mimicking properties. The dual biosensor had no interferences from dopamine, ascorbic acid, and uric acid, with detection limits of 0.31 μM and 20 nM for the colorimetric and voltametric method, respectively, underlying the advantage in using the electrochemical technique for epinephrine detection at very low concentrations [64][40].
Besides the above mentioned catecholamines, glutamate plays an important role in the excitatory neurotransmission in the mammalian brain, and its fast removal from the synaptic cleft is critical for preventing toxicity and spillover to neighboring synapses. Its detection in PC-12 cells was achieved using a fluorescent indicator protein for glutamate from Escherichia coli, which in the presence of ligands, has a concentration-dependent decrease in the fluorescence signal. Consequently, the depolarization of neurons leads to a fluorescence signal decrease corresponding to 300 nM glutamate at the cell surface, with no change in the signal when cells were exposed to 20 mM glutamate, the minimal glutamate uptake in the cytosol [65][41].
In addition to neurotransmitters, hydrogen peroxide release plays an important physiological role in cell-to-cell signal transduction, and amperometric detection at gold, platinum, and/or graphene transductors is generally preferred. Using a three-dimensional nanoporous gold electrode decorated with ultra-thin platinum nanoparticles, a wide linear range, from 0.05 μM till 7.37 mM, for the amperometric determination of H2O2, at an applied potential of −0.4 V, was obtained [49][25]. A limit of detection of 1.5 × 10−8 mol/L and a high sensitivity of 1.125 μAμM−1 cm−2 were achieved. Nevertheless, the biosensor has been applied to the dynamic determination of H2O2 released from PC-12 cells and a value of 52.5 amol H2O2, generated by each cell, was obtained [49][25].
Similar linear range was obtained at a reduced graphene oxide-platinum nanocomposite-modified glassy carbon electrode [66][42]. Although the detection limit was higher, 0.2 μM, the biosensor allowed the detection of H2O2 release from living cells.
Fluorimetric sensors were also reported, one based on BSA-stabilized Au nanoclusters [40][16] and one based on single-walled carbon nanotubes (SWCNT) [41][17]. In the first case, the detection principle is based on the decrease of the fluorescence intensity emitted by the sensor upon the attachment of DA to BSA which leads to a photo-induced electron transfer process. The sensor exhibited a detection limit of 10 nM, with no interferences form possible interfering substances and successful application for the DA monitoring in PC-12 cells [40][16]. In the second methodology, arrays of fluorescent nanosensors based on SWCNT are placed under and around neuroprogenitor cells to enable the imaging of the released DA from the PC-12 cells following K+ stimulation. The acquired fluorescence map revealed areas where DA is released on the cell’s surface and how its morphology affects the location of release sites. Moreover, the results elucidate how membrane morphology influences the directionality of chemical signaling by DA [41][17].
Another optical biosensor based on three-dimensional tungsten disulfide (WS2) was reported using Raman spectroscopy detection. The sensor uses 2D WS2 directly grown on a 3D WO3NH by sulfurization, with advantages over the 2D support array of WO3NH on the adsorption of biomolecules and cells proliferation which enhances sensor sensitivity to DA [42][18].
Combining the lithography and electrochemistry method, a metal-free graphene based hybrid microelectrode array for sensitive and in-situ amperometric sensing of H2O2 was fabricated and a detection limit of 0.18 μM, in PC-12 cell culture, was achieved [67][43].
2. Cellular Events
2.1. Attachment, Proliferation, and Differentiation
The main characteristics of PC-12 as a neuronal cell line is the ability to develop neurite outgrowths during the differentiation process; a process that demands the cells to be attached on a surface. Usually, the PC-12 cells grow as small, irregularly shaped cells, adherent or in suspension, floating in the growth media, and tend to form aggregate and adhere poorly to non-coated surfaces. Therefore, the control of neuronal cell patterning needs modified surfaces commonly coated with fibronectin, laminin, poly-L-lysine, collagen, etc.
For neurite growth guiding, collagen-coated electrospun gelatin/polycaprolactone nanofiber mats on microstructured polystyrene surface were used obtaining an increased adhesion, differentiation, and guided neurite outgrowth compared to controls [68][44].
Another approach for increasing cell adhesion is to take advantage of the physicochemical properties of the of electroconductive hydrogels. This showed integration of electropolymerized polypyrrole networks within poly(hydroxyethylmethacrylate)-based hydrogels and controlled the elastic modulus and the electrical impedance properties of electroconductive hydrogels [69][45]. Growth of attachment-dependent PC-12 cells at the hydrogel showed to be dependent with electropolymerization charge density [69][45].
Quartz crystal microbalance (QCM) has been applied to develop cell-based biosensors as secondary sensors to deliver functional information of cells such as the cell attachment, proliferation, and cell–substrate interaction under different conditions. QCM was used to monitor the mass change and rigidity of populations of excitable cells during exocytosis and subsequent retrieval of dense-core vesicles and it was observed that stimulating the cells to exocytosis with elevated potassium concentration resulted in an increase in the frequency response corresponding to loss of mass from the cells owing to release of vesicles [70][46].
Optical biosensors based on surface plasmon resonance (SPR) have the potential for investigations of cell responses and real-time monitoring of individual cell responses to various exogenous substances under ambient conditions. This technology addresses cell monolayers cultivated on the gold sensor chip and allows the evaluation of compound potency, specificity, selectivity, toxicity, and effectiveness at the level of individual cell. Thus, based on reflection intensity changes of SPR, the intracellular translocation of protein kinase C (PKC) of PC 12 during differentiation process was observed [71[47][48],72], and the detection of neuronal differentiation in live cells, at the level of individual cells, was achieved [71][47]. Moreover, a high sensitivity and enhancement of SPR response to muscarine stimulation was found for the cells treated with the nerve growth factor [71][47].
Live cell-fluorescent biosensors allow the collection of temporal information about cellular events, from changes of the membrane potential of the cell till cell cycle progression and arrest. Measuring the changes of cell membrane potential, the level of superoxide anion generated during the in vitro differentiation of PC-12 cells was determined in a noninvasive way [73][49]. Nevertheless, using a fluorescent biosensor, it was demonstrated that NGF-induced neurite extension occurs independently of NGF-induced cell cycle G1 phase arrest. This finding allowed the investigation of the PC-12 proliferation at the resolution of individual cells and neuronal differentiation as a dynamic process of parallel cell cycle arrest and neurite outgrowth [74][50].
The axonal elongation requires axonal membrane growth by exocytosis of plasmalemmal precursor vesicles at the nerve growth cone, a process regulated by nucleotide guanosine triphosphate (GTP) hydrolase enzymes family [65][41]. Similar to fluorescence, Forster resonance energy transfer (FRET)-based biosensors allow the acquisition of valuable information about cellular events such as exocytosis, neurite outgrowth, proliferation or differentiation, as well as the biochemical mechanisms involved. Using a FRET biosensor it was showed that the activity of TC10, a GTP enzyme, at the plasma membrane decreased at extending growth cones in NGF-treated PC12 and it was demonstrated that cells signaling machinery containing TC10 is used for exocytosis [75][51].
Light-addressable potentiometric sensor (LAPS) allows the fabrication of interfaces between the physical and biological system, i.e., semiconductor—biological cell. To improve the biological cell adhesion, the LAPS insulator usually is coated with biocompatible molecules. Thus, efficient culturing of PC-12 on 4 nm poly-l-ornithine and laminin layer coated LAPS structure was achieved [76][52]. This layer enhanced sensitivity and allowed simulation of neural action potential applied to the LAPS [51][27].
Impedance biosensors are very useful for real-time monitoring of extracellular matrix-mediated PC12 cell attachment and proliferation [77][53], adhesion and differentiation [78,79][54][55]. The attachment and proliferation of the neuron-like cell line PC-12, on different extracellular matrices, confirmed by MTT assays and a scanning electron microscopy analysis, was monitored using cellular impedance sensing [77][53]. Similar, using interdigitate microelectrodes array, the attachment, differentiation, and formation of synapse-like contacts between the neuronal cell and the conductive surface of a microelectrode array were followed by recording changes of impedance [78,79][54][55] and it was demonstrated that the complex impedance is dependent on ion fluxes at the neuron-to-electrode contact surface.
Cell-coupled silicon nanowire field-effect transistor devices were exploited to elucidate the effect of cell proliferation on impedance spectra. Owing to the hindrance of ions or negatively charged membrane of the coupled cells, the changes of carrier density within transistors correspond to the signals in impedance spectra and can be used to probe cell condition during the growth [80][56].
2.2. Action Potential Cell Stimulation and Intracellular Signal Transduction
Real-time observation of intracellular process of signal transduction is very useful for biomedical and pharmaceutical applications as well as for basic research work of cell biology. For feasible and reagentless observation of intracellular alterations in real time, a SPR biosensor was applied in PC-12 cells culture for monitoring of intracellular signal transduction that was mainly translocation of protein kinase C. This was achieved via local refractive index change in PC-12 cells adhered on a gold sensor slide after stimulation with KCl and phorbol-12-myristate-13-acetate (a protein kinase C activator), at different concentrations, in order to induce intracellular PKC translocation [81][57]. In another study, similar achievements were obtained using a nonadiabatic tapered optical fiber biosensor [82][58].
The stimulation of PC-12 cells with epidermal growth factor (EGF) leads to transient extracellular signal-regulated kinase (ERK) activity and cell proliferation, whereas nerve growth factor (NGF) stimulation leads to sustained ERK activity and differentiation. Using a FRET-based biosensors it was showed that both NGF and EGF potently activate PKA at the plasma membrane, although they generate temporally distinct activity patterns [83][59].
Coupling an ultra-thin microelectrode array with total internal reflection fluorescence microscopy, simultaneous recording of action potentials and neurotransmitter release was obtained. The combination of the optical and electrical techniques enabled mapping of neuron connectivity in an entire neuronal circuit. Moreover, the real-time recording of action potential and neurotransmitter release reveals the relevance of electrical and chemical activities in the neuronal mode [8][60].
The real-time electrochemical investigation of PC-12 cell culture was accomplishing using a membrane-based electrode sensor. The electrochemical performance of the membrane electrodes was characterized by cyclic voltammetry and chronoamperometry, and the detection of synthetic dopamine and dopamine exocytosis was demonstrated down to a concentration of 3.1 pM [84][61].
Microelectrode arrays, composed of 60 independent electrodes, for stimulation and signal recording of in vitro cultured neurons were coupled with impedance measurements [85,86][62][63]. The extracellular signal recording in the presence of acetylcholine and other stimulants has been carried out and the results indicate that microelectrodes array systems can be used for extracellular stimulation, recording, simultaneous stimulation and recording, and isolation of PC12 cells network cultured in vitro [85,87][62][64].
Monitoring of electrophysiological properties of cultured neuron networks derived from PC-12 was achieved using a neurochip based on LAPS biosensor technology. After the differentiation of PC-12 cells and formation of neuronal networks on LAPS system, the extracellular potentials were recorded. The results showed that the neurochip of PC-12 cells coupled to LAPS is stable and suitable for long-term and non-invasive measurement of cell electrophysiological properties [88][65].
2.3. Monitorization of Cytotoxic Effect
Fast and selective monitoring of dopamine release is essential for screening pharmaceuticals that may be beneficial in the treatment of catecholamine-related psychiatric disorders [89][66]. The complexity of the exocytosis process, dopamine re-uptake, diffusion, and auto-oxidation are crucial parameters when employing biosensing methodologies, as well as considering the pharmaceutical’s distinct mechanism of action over dopaminergic pathways, which may involve inhibition of vesicular monoamine transport (VMAT), dopamine transport (DAT), catechol-O-methyltransferase pathway, dopamine precursor supply, and activation of dopamine receptors. In this context, the development of a rapid electrochemical approach has been reported toward screening of pharmaceuticals targeting dopamine-related psychiatric disorders. For this, microelectrode array biochips based on gold covered silicon wafers were used to investigate the effect of three drugs (L-dopa, reserpine and nomifensine) with different mechanisms of action upon dopamine release, the latter monitored using chronoamperometry upon K+-stimulation of drug-treated PC-12 cells [90][67]. A two-cell-based biosensor was also developed to monitor the influence of receptor antagonists (ATP, acetylcholine), K+-induced membrane polarization, and neurotoxins (black widow spider venom, α-latrotoxin) on neurosecretory output of PC-12 cells. For this, the transducing mechanism was achieved through optical monitoring changes in naturally colored fish chromatophores, where varying the catecholamine secretion level under stimuli led to pigment aggregation [91][68].
It is clear that when dealing with living cell assays, the biocompatibility of the sensor materials is of utmost importance to support a good environment for cell adhesion and proliferation, whilst avoiding toxicity which may be induced by the material itself, such as reported for dose-dependent exocytosis induced by silver nanoparticles [92][69]. Some strategies describe the use of paper-based scaffolds to improve cell implantation, thus maintaining a three-dimensional similarity to the cell physiological microenvironment. As an example, a 3D-based cell culture system based on paper-polylactic platform coupled with an electrochemical sensor surface was used to monitor cell damage upon interaction with amyloid-beta oligomers. The excellent biocompatibility of the materials as well as efficient cell implantation supported the investigation of the cytoprotective effects of donepezil and BMSCs-secreted active molecules upon the inflicted cell damage [93][70]. A different approach employed a 250 μm gold microelectrode fabricated within cell culture biochips, whilst surface attachment of PC-12 cells was conducted using self-assembled monolayers of cysteamine covalently derivatized with laminin. In this study, electrochemical impedance spectroscopy was used to characterize Ca2+ exocytosis fluctuations upon treatment of living cells with calcimycin, nifedipine, mannitol, and carbachol, as well as to correlate phenotypic alterations due to cell exposure to nerve growth factor (NGF), dexamethasone, and forskolin [94][71]. Similar, field-effect transistors based on semiconducting single-walled carbon nanotubes were successfully used for monitoring the effects of histamines on Ca2+ release from the intracellular stores [95][72].
Neurotoxins are often destructive compounds able to inflict damage upon nerve tissue, often leading to neuron excitotoxicity or apoptosis. The influence of rotenone, okadaic acid and peroxynitrite, neurotoxins that induce cell death on PC-12 cell lines, has been evaluated with electrochemical impedance spectroscopy at a cell-based array of gold electrodes complemented by artificial neural networks (ANNs), able to discriminate against false positives [96][73]. Another approach was used to electrochemically monitor bisphenol and its analogues, environmental pollutants that have been reported to inflict adverse neurodevelopment on animals and humans through interaction with O-GlcNAcase, an enzyme involved in the control of neuronal functions [97][74].
The cell response to nuclear growth factor, which may induce differentiation, has shown to be dependent on different parameters including ion-exchange inhibition, as reported using a silicon-based cytosensor for continuous and real-time monitoring of extracellular acidification rate changes on living cells [98][75]. Monitoring neuronal differentiation has also been achieved for 2D SPR-based sensors, where the increase in refractive index has been observed for differentiated cells stimulated with muscarine and carbacol [99][76]. Another approach investigated the intracellular signal transduction upon carbacol stimulation, whilst the reflection intensity change response has been suppressed by different natural compounds [100][77]. Finally, a high-throughput screening for small molecules able to bind on amyloid-β was conducted in order to identify natural inhibitors against Alzheimer’s disease. For this, biolayer interferometry together with an ultra-high-performance liquid chromatography coupled with diode-array detector and quadrupole/time-of-flight tandem mass spectrometry was used to assess the inhibition of amyloid-β fibrillation by polyporenic acid C, dehydrotumulosic acid, and tumulosid acid. Moreover, an improvement was observed in the viability of the amyloid-β-treated PC-12 cells [101][78].
2.4. Cellular Transport
Cell transport refers to the movement of neurotransmitters, ions, proteins, and other species through the cell membrane; the latter ensures cell structure and protection for the cytosolic content [102][79]. The importance on the membrane passive/active involvement to sustain cell homeostasis is considered a target for drug development and medical therapy, where, for example, K+, Ca2+ influx can be modulated, whilst catecholamine and cytokine release can be monitored to investigate the effects of such external stimuli upon the living cell environment.
As previously mentioned, K+-evoked dopamine release is important to understand overall dopaminergic function. For this, a carbon nanotube (CNT)-based field effect transistor (FET) has been employed to monitor catecholamine release upon activation of membrane K+ ion-channels through K+ stimulation, as well as through incorporation of pimozide, an antipsychotic drug. The CNT-FET developed device was coated by a Nafion® -radical hybrid film containing 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals, thus ensuring the selectivity for dopamine detection up to 10 nM even in the presence of neurotransmitter interferents [103][80].
The maxi-anion channel is found in almost every part of the body, and is responsible for the control of membrane potential and biomolecules movement. The channel is activated in response to a variety of factors, ranging from osmotic cell swelling to physiologically and pathophysiologically relevant stimuli. In this context, a PC-12 cell-based biosensor was employed to investigate the special distribution of maxi-anion channels activity of a single rat cardiomyocyte through a patch clamp technique with scanning ion conductance microscopy. For this, local ATP release was monitored at concentrations of 1 mM through the PC-12 P2X receptor channels at a holding potential of −50 mV, whilst changing the placing of the biosensor in different areas of the cardiomyocyte. The special distribution of maxi-anions was assessed with fine-tipped pipettes, and results showed that they were concentrated at T-Tubules along Z-lines in adult cardiomyocytes. This approach further illustrated how the smart-patch technique can provide information regarding localized unitary events for specific transport channels, as well as for physiological/pathophysiological cell signaling investigation [104][81].
2.5. Cancer Cell Detection
The biosensing field has been a sophisticated alternative for early cancer detection, mainly due to vast versatility in constructions for selectively monitoring specific biomarkers, such as proteins or other biomolecules, often correlated to cellular malignancies. An alternative approach regards the early detection of cellular malignancies through the direct recognition of Jurcat, HeLa, PC-12, MDA-MB-231, and MCF7 cancerous cell lines as models for leukemia, cervical, adrenal gland, and breast cancer (type-1 and type-2), respectively.
The increased amount of proteins in cancerous cells leads to an increase of 1.37 to 1.40 in their refractive index, a parameter that can be easily monitored through optical biosensors for early detection of cancer. One approach described the use of 2D materials-based fiber-optic surface plasmon resonance (SPR) to monitor the angular shift and power loss of cancerous cells, thus distinguished from healthy cell lines [105][82]. Incorporation of different allotropes such as SnSe alongside a heterostructure of Blue P/MOS2 has also been shown to support the increase in refractive index variations to include monitoring cancer cells directly in biological solutions, as reported for the detection of skin, cervical, blood, adrenal gland, and breast (type-1 and type-2) cancer with a maximum quality factor of 24.83 RIU−1 and detection accuracy of 0.54 deg−1 [106][83]. In order to decrease measurement errors when compared with single-read biosensors, another approach was reported to monitor similar cancerous cells (including PC-12) through a portable micro-structured plasmonic based on a three-hole wagon wheel design [107][84].
Photonic crystals are also interesting platforms for the development of refractive index-based sensors, especially those used to monitor cancer cells [108][85]. One methodology described the infiltration of cancerous cells within defects between graphene and nanocomposite layers present in a one-dimensional photonic crystal biosensor, whilst their recognition was achieved through monitoring resonance peaks located within the transmittance spectrum [109][86]. Another approach involved the development of an ultra-compact biochemical sensor based on 2D photonic crystal cavity to monitor PC-12 cancerous cell lines [110][87]. Incorporation of gold nanowires as plasmonic materials has been reported to obtain improved performance of such optical biosensors for the recognition of MCF-7 and PC-12 cells with great sensitivity and resolution [111][88]. Photonic crystal fibers (PCF) also have been used to monitor cancerous cells, whilst the maximum values of sensing parameters were achieved for recognition of breast cancer (MCF-7) [112][89]. A polyamide substrate was also employed for immobilization of hexagonal gold layers, whilst an elevated yield confinement ensured a high sensitivity for monitoring basal, breast, cervical, and adrenal cancerous cells, the analysis being carried out using the full vectoral finite element methodology [113][90].
In light of the necessity to maintain sensitivity but also to improve the overall cancer detection in more point-of-care testing environment, a novel paper-based electrochemiluminescence (ECL) approach was reported for the detection of leukemia, through the use of a labelled aptamer specific to HL-60 cells. In the presence of cancerous cells, the aptamer would conjugate with glycoproteins at the cell surface, followed by the release in the label, rendering a decline in the ECL signal. This methodology, which was able to detect HL-60 cancer cells down to 56 cells/mL supports how paper-based approaches can still be tailored in point-of-care testing environment to monitor PC-12 cell lines for early adrenal cancer detection [114][91].
References
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246.
- Bozym, R.A.; Thompson, R.B.; Stoddard, A.K.; Fierke, C.A. Measuring Picomolar Intracellular Exchangeable Zinc in PC-12 Cells Using a Ratiometric Fluorescence Biosensor. ACS Chem. Biol. 2006, 1, 103–111.
- McCranor, B.J.; Bozym, R.A.; Vitolo, M.I.; Fierke, C.A.; Bambrick, L.; Polster, B.M.; Fiskum, G.; Thompson, R.B. Quantitative imaging of mitochondrial and cytosolic free zinc levels in an in vitro model of ischemia/reperfusion. J. Bioenerg. Biomembr. 2012, 44, 253–263.
- Mccranor, B.J.; Szmacinski, H.; Zeng, H.H.; Stoddard, A.K.; Hurst, T.; Fierke, C.A.; Lakowicz Ab, J.R.; Thompson, R.B. Fluorescence lifetime imaging of physiological free Cu(II) levels in live cells with a Cu(II)-selective carbonic anhydrase-based biosensor. Metallomics 2014, 6, 1034.
- Courtney, N.A.; Briguglio, J.S.; Bradberry, M.M.; Greer, C.; Chapman, E.R. Excitatory and Inhibitory Neurons Utilize Different Ca2+ Sensors and Sources to Regulate Spontaneous Release. Neuron 2018, 98, 977–991.e5.
- Westerink, R.H.S.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285.
- Liu, C.; Jiao, X.; Cai, S.; He, S.; Zhao, L.; Zeng, X. Reversible fluorescent probe for visually monitoring the concentration-dependent dynamic correlations among HOCl, H2S, and Ca2+ in neurons. Sens. Actuators B Chem. 2021, 329, 129213.
- Fishman, H.A.; Schellert, R.H.; Zare, R.N. Chemistry Identification of receptor ligands and receptor subtypes using antagonists in a capillary electrophoresis single-cell biosensor separation system (microcolumn separation/fluorescence microscopy/calcium imaging/neurotransmitters). Proc. Natl. Acad. Sci. USA 1995, 92, 7877–7881.
- Shear, J.B.; Fishman, H.A.; Allbritton, N.L.; Garigan, D.; Zare, R.N.; Scheller, R.H. Single cells as biosensors for chemical separations. Science 1995, 267, 74–77.
- Yao, K.; Chang, Y.; Li, B.; Yang, H.; Xu, K. A novel coumarin-based fluorescent sensor for Ca 2+ and sequential detection of F − and its live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 385–394.
- Cho, Y.-W.; Park, J.-H.; Lee, K.-H.; Lee, T.; Luo, Z.; Kim, T.-H. Recent advances in nanomaterial-modified electrical platforms for the detection of dopamine in living cells. Nano Converg. 2020, 7, 40.
- Banerjee, S.; McCracken, S.; Faruk Hossain, M.; Slaughter, G. Electrochemical Detection of Neurotransmitters. Biosensors 2020, 10, 101.
- Wang, Y.; Ewing, A. Electrochemical Quantification of Neurotransmitters in Single Live Cell Vesicles Shows Exocytosis is Predominantly Partial. ChemBioChem 2020, 22, 807–813.
- Shi, N.; Bu, X.; Zhang, M.; Wang, B.; Xu, X.; Shi, X.; Hussain, D.; Xu, X.; Chen, D. Current Sample Preparation Methodologies for Determination of Catecholamines and Their Metabolites. Molecules 2022, 27, 2702.
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102.
- Tao, Y.; Lin, Y.; Ren, J.; Qu, X. A dual fluorometric and colorimetric sensor for dopamine based on BSA-stabilized Aunanoclusters. Biosens. Bioelectron. 2013, 42, 41–46.
- Kruss, S.; Salem, D.P.; Vuković, L.; Lima, B.; Ende, E.V.; Boyden, E.S.; Strano, M.S. High-resolution imaging of cellular dopamine efflux using a fluorescent nanosensor array. Proc. Natl. Acad. Sci. USA 2017, 114, 1789–1794.
- Lee, N.; Shin, M.H.; Lee, E.; Cho, S.H.; Hwang, H.; Cho, K.; Kim, J.K.; Hahn, S.K. Three-Dimensional Tungsten Disulfide Raman Biosensor for Dopamine Detection. ACS Appl. Bio Mater. 2020, 3, 7687–7695.
- Taskin, M.B.; Sasso, L.; Dimaki, M.; Svendsen, W.E.; Castillo-León, J. Combined cell culture-biosensing platform using vertically aligned patterned peptide nanofibers for cellular studies. ACS Appl. Mater. Interfaces 2013, 5, 3323–3328.
- Emran, M.Y.; Shenashen, M.A.; Morita, H.; El-Safty, S.A. 3D-Ridge Stocked Layers of Nitrogen-Doped Mesoporous Carbon Nanosheets for Ultrasensitive Monitoring of Dopamine Released from PC12 Cells under K+ Stimulation. Adv. Healthc. Mater. 2018, 7, e1701459.
- Mir, T.A.; Akhtar, M.H.; Gurudatt, N.G.; Kim, J.I.; Choi, C.S.; Shim, Y.B. An amperometric nanobiosensor for the selective detection of K+-induced dopamine released from living cells. Biosens. Bioelectron. 2015, 68, 421–428.
- Liu, M.M.; Guo, Z.Z.; Liu, H.; Li, S.H.; Chen, Y.; Zhong, Y.; Lei, Y.; Lin, X.H.; Liu, A.L. Paper-based 3D culture device integrated with electrochemical sensor for the on-line cell viability evaluation of amyloid-beta peptide induced damage in PC12 cells. Biosens. Bioelectron. 2019, 144, 111686.
- Li, J.; Huang, X.; Shi, W.; Jiang, M.; Tian, L.; Su, M.; Wu, J.; Liu, Q.; Yu, C.; Gu, H. Pt nanoparticle decorated carbon nanotubes nanocomposite based sensing platform for the monitoring of cell-secreted dopamine. Sens. Actuators B Chem. 2021, 330, 129311.
- Shi, B.X.; Wang, Y.; Zhang, K.; Lam, T.L.; Chan, H.L.W. Monitoring of dopamine release in single cell using ultrasensitive ITO microsensors modified with carbon nanotubes. Biosens. Bioelectron. 2011, 26, 2917–2921.
- Zhong, Y.; Liu, M.-M.; Chen, Y.; Yang, Y.-J.; Wu, L.-N.; Bai, F.-Q.; Lei, Y.; Gao, F.; Liu, A.-L. A high-performance amperometric sensor based on a monodisperse Pt–Au bimetallic nanoporous electrode for determination of hydrogen peroxide released from living cells. Microchim. Acta 2020, 187, 499.
- Abe, H.; Ino, K.; Li, C.-Z.; Kanno, Y.; Inoue, K.Y.; Suda, A.; Kunikata, R.; Matsudaira, M.; Takahashi, Y.; Shiku, H.; et al. Electrochemical Imaging of Dopamine Release from Three-Dimensional-Cultured PC12 Cells Using Large-Scale Integration-Based Amperometric Sensors. Anal. Chem. 2015, 87, 6364–6370.
- Tomagra, G.; Franchino, C.; Pasquarelli, A.; Carbone, E.; Olivero, P.; Carabelli, V.; Picollo, F. Simultaneous multisite detection of quantal release from PC12 cells using micro graphitic-diamond multi electrode arrays. Biophys. Chem. 2019, 253, 106241.
- Yang, C.; Liu, M.M.; Bai, F.Q.; Guo, Z.Z.; Liu, H.; Zhong, G.X.; Peng, H.P.; Chen, W.; Lin, X.H.; Lei, Y.; et al. An electrochemical biosensor for sensitive detection of nicotine-induced dopamine secreted by PC12 cells. J. Electroanal. Chem. 2019, 832, 217–224.
- Xu, C.; Gu, C.; Xiao, Q.; Chen, J.; Yin, Z.Z.; Liu, H.; Fan, K.; Li, L. A highly selective and sensitive biosensor for dopamine based on a surface molecularly imprinted layer to coordinate nano-interface functionalized acupuncture needle. Chem. Eng. J. 2022, 436, 135203.
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650.
- Li, B.R.; Hsieh, Y.J.; Chen, Y.X.; Chung, Y.T.; Pan, C.Y.; Chen, Y.T. An ultrasensitive nanowire-transistor biosensor for detecting dopamine release from living pc12 cells under hypoxic stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037.
- Li, B.R.; Chen, C.W.; Yang, W.L.; Lin, T.Y.; Pan, C.Y.; Chen, Y.T. Biomolecular recognition with a sensitivity-enhanced nanowire transistor biosensor. Biosens. Bioelectron. 2013, 45, 252–259.
- Chen, T.K.; Luo, G.; Ewing, A.G. Amperometric Monitoring of Stimulated Catecholamine Release from Rat Pheochromocytoma (PC12) Cells at the Zeptomole Level. Anal. Chem. 1994, 66, 3031–3035.
- Kozminski, K.D.; Gutman, D.A.; Davila, V.; Sulzer, D.; Ewing, A.G. Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat pheochromocytoma (PC12) cells. Anal. Chem. 1998, 70, 3123–3130.
- Ren, L.; Oleinick, A.; Svir, I.; Amatore, C.; Ewing, A.G. Amperometric Measurements and Dynamic Models Reveal a Mechanism for How Zinc Alters Neurotransmitter Release. Angew. Chem. Int. Ed. 2020, 59, 3083–3087.
- Hu, L.; Savy, A.; Grimaud, L.; Guille-Collignon, M.; Lemaître, F.; Amatore, C.; Delacotte, J. Electroactive fluorescent false neurotransmitter FFN102 partially replaces dopamine in PC12 cell vesicles. Biophys. Chem. 2019, 245, 1–5.
- Li, X.; Dunevall, J.; Ewing, A.G. Quantitative Chemical Measurements of Vesicular Transmitters with Electrochemical Cytometry. Acc. Chem. Res. 2016, 49, 2347–2354.
- Emran, M.Y.Y.; Mekawy, M.; Akhtar, N.; Shenashen, M.A.A.; EL-Sewify, I.M.M.; Faheem, A.; El-Safty, S.A.A. Broccoli-shaped biosensor hierarchy for electrochemical screening of noradrenaline in living cells. Biosens. Bioelectron. 2018, 100, 122–131.
- Emran, M.Y.; Shenashen, M.A.; Elmarakbi, A.; Selim, M.M.; El-Safty, S.A. Nitrogen-doped carbon hollow trunk-like structure as a portable electrochemical sensor for noradrenaline detection in neuronal cells. Anal. Chim. Acta 2022, 1192, 339380.
- Alizadeh, N.; Ghasemi, S.; Salimi, A.; Sham, T.K.; Hallaj, R. CuO nanorods as a laccase mimicking enzyme for highly sensitive colorimetric and electrochemical dual biosensor: Application in living cell epinephrine analysis. Colloids Surf. B Biointerfaces 2020, 195, 111228.
- Okumoto, S.; Looger, L.L.; Micheva, K.D.; Reimer, R.J.; Smith, S.J.; Frommer, W.B. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. USA 2005, 102, 8740–8745.
- Zhang, Y.; Bai, X.; Wang, X.; Shiu, K.K.; Zhu, Y.; Jiang, H. Highly Sensitive Graphene-Pt Nanocomposites Amperometric Biosensor and Its Application in Living Cell H2O2 Detection. Anal. Chem. 2014, 86, 9459–9465.
- Zhang, J.; Zhao, M.; Yang, J.; Wu, G.; Wu, H.; Chen, C.; Liu, A. Metal-free rGO/GO hybrid microelectrode array for sensitive and in-situ hydrogen peroxide sensing. Electrochim. Acta 2019, 326, 134967.
- Malkoc, V.; Gallego-Perez, D.; Nelson, T.; Lannutti, J.J.; Hansford, D.J. Controlled neuronal cell patterning and guided neurite growth on micropatterned nanofiber platforms. J. Micromech. Microeng. 2015, 25, 125001.
- Kotanen, C.N.; Wilson, A.N.; Dong, C.; Dinu, C.Z.; Justin, G.A.; Guiseppi-Elie, A. The effect of the physicochemical properties of bioactive electroconductive hydrogels on the growth and proliferation of attachment dependent cells. Biomaterials 2013, 34, 6318–6327.
- Cans, A.S.; Höök, F.; Shupliakov, O.; Ewing, A.G.; Eriksson, P.S.; Brodin, L.; Orwar, O. Measurement of the dynamics of exocytosis and vesicle retrieval at cell populations using a quartz crystal microbalance. Anal. Chem. 2001, 73, 5805–5811.
- Mir, T.A.; Shinohara, H. Two-dimensional surface plasmon resonance imaging system for cellular analysis. Methods Mol. Biol. 2017, 157, 131–146.
- Shinohara, H. 2.2.3 Invited: Novel Cell-based Biosensing with 2D-SPR imager. Tagungsband 2020, 1, 173–174.
- Moschopoulou, G.; Kintzios, S. Noninvasive Superoxide Monitoring of in Vitro Neuronal Differentiation Using a Cell-Based Biosensor. J. Sens. 2015, 2015, 768352.
- Hahn, A.T.; Jones, J.T.; Meyer, T. Quantitative analysis of cell cycle phase durations and PC12 differentiation using fluorescent biosensors. Cell Cycle 2009, 8, 1044–1052.
- Fujita, A.; Koinuma, S.; Yasuda, S.; Nagai, H.; Kamiguchi, H.; Wada, N.; Nakamura, T. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE 2013, 8, e79689.
- Ismail, A.B.M.; Yoshinobu, T.; Iwasaki, H.; Sugihara, H.; Yukimasa, T.; Hirata, I.; Iwata, H. Investigation on light-addressable potentiometric sensor as a possible cell-semiconductor hybrid. Biosens. Bioelectron. 2003, 18, 1509–1514.
- Zhang, Y.; Wang, L.; Zhu, J.; Hu, Y.; Xing, W.; Cheng, J. Real-time monitoring of extracellular matrix-mediated PC12 cell attachment and proliferation using an electronic biosensing device. Biotechnol. Lett. 2012, 34, 397–404.
- Chen, C.C.; Lin, S.Y.; Sheu, J.T. Using impedance biosensors to distinguish the PC12 cells adhesion and differentiation. In Proceedings of the NEMS 2011-6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Kaohsiung, Taiwan, 20–23 February 2011.
- Bieberich, E.; Guiseppi-Elie, A. Neuronal differentiation and synapse formation of PC12 and embryonic stem cells on interdigitated microelectrode arrays: Contact structures for neuron-to-electrode signal transmission (NEST). Biosens. Bioelectron. 2004, 19, 923–931.
- Vinzons, L.U.; Gupta, A.K.; Lai, T.Y.; Lin, S.P. Interpretation of biosensing technology in cell-coupled silicon nanowire transistors via impedance spectra. Mater. Lett. 2022, 308, 131087.
- Shinohara, H.; Sakai, Y.; Mir, T.A. Real-time monitoring of intracellular signal transduction in PC12 cells by two-dimensional surface plasmon resonance imager. Anal. Biochem. 2013, 441, 185–189.
- Zibaii, M.I.; Latifi, H.; Asadollahi, A.; Noraeipoor, Z.; Dargahi, L. Real-time monitoring of intracellular signal transduction in PC12 cells by non-adiabatic tapered optical fiber biosensor. In Proceedings of the 23rd International Conference on Optical Fibre Sensors, Santander, Spain, 2–6 June 2014; Volume 9157.
- Herbst, K.J.; Allen, M.D.; Zhang, J. Spatiotemporally Regulated Protein Kinase A Activity Is a Critical Regulator of Growth Factor-Stimulated Extracellular Signal-Regulated Kinase Signaling in PC12 Cells. Mol. Cell. Biol. 2011, 31, 4063–4075.
- Cui, M.R.; Zhao, W.; Li, X.L.; Xu, C.H.; Xu, J.J.; Chen, H.Y. Simultaneous monitoring of action potentials and neurotransmitter release from neuron-like PC12 cells. Anal. Chim. Acta 2020, 1105, 74–81.
- Alatraktchi, F.A.; Bakmand, T.; Dimaki, M.; Svendsen, W.E. Novel membrane-based electrochemical sensor for real-time bio-applications. Sensors 2014, 14, 22128–22139.
- Pan, H.X.; Lü, X.Y.; Wang, Z.G.; Ren, T.L.; Fang, T.; Zhang, J.; Zhou, C.J.; Wang, L.G. Silicon-based microelectrode arrays for stimulation and signal recording of in vitro cultured neurons. Sci. China Inf. Sci. 2011, 54, 2199–2208.
- Liang, C.K.; Chen, D.K.; Chen, J.J.J.; Chen, S.C. A multi-functional online measurement system for neuron-microelectrode interface study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology-Proceedings, San Francisco, CA, USA, 1–5 September 2004.
- Lü, X.Y.; Meng, C.; An, S.; Zhao, Y.F.; Wang, Z.G. Study on influence of external factors on the electrical excitability of PC12 quasi-neuronal networks through Voltage Threshold Measurement Method. PLoS ONE 2022, 17, e0265078.
- Liu, Q.J.; Ye, W.W.; Yu, H.; Hu, N.; Du, L.P.; Wang, P. Neurochip based on light-addressable potentiometric sensor with wavelet transform de-noising. J. Zhejiang Univ. Sci. B 2010, 11, 323–331.
- Nagamura, Y.; Terawaki, K.; Uezono, Y.; Tsukada, T. Enhancement of catecholamine release from PC12 cells by the traditional Japanese medicine, rikkunshito. BMC Complement. Altern. Med. 2014, 14, 256.
- Cui, H.F.; Ye, J.S.; Chen, Y.; Chong, S.C.; Sheu, F.S. Microelectrode array biochip: Tool for in vitro drug screening based on the detection of a drug effect on dopamine release from PC12 cells. Anal. Chem. 2006, 78, 6347–6355.
- Ryan Preston, R.; McFadden, P.N. A two-cell biosensor that couples neuronal cells to optically monitored fish chromatophores. Biosens. Bioelectron. 2001, 16, 447–455.
- Shah, P.; Yue, Q.; Zhu, X.; Xu, F.; Wang, H.S.; Li, C.Z. PC12 cell integrated biosensing neuron devices for evaluating neuronal exocytosis function upon silver nanoparticles exposure. Sci. China Chem. 2015, 58, 1600–1604.
- Liu, M.M.; Zhong, Y.; Chen, Y.; Wu, L.N.; Chen, W.; Lin, X.H.; Lei, Y.; Liu, A.L. Electrochemical monitoring the effect of drug intervention on PC12 cell damage model cultured on paper-PLA 3D printed device. Anal. Chim. Acta 2022, 1194, 339409.
- Slaughter, G.E.; Hobson, R. An impedimetric biosensor based on PC 12 cells for the monitoring of exogenous agents. Biosens. Bioelectron. 2009, 24, 1153–1158.
- Pham Ba, V.A.; Pham Van Bach, N.; Nguyen Luong, T.; Nguyen, K.V. Semiconducting Carbon Nanotube-Based Nanodevices for Monitoring the Effects of Chlorphenamine on the Activities of Intracellular Ca2+Stores. J. Anal. Methods Chem. 2022, 2022, 9019262.
- Slaughter, G.E.; Hobson, R.S. Artificial neural network for temporal impedance recognition of neurotoxins. In Proceedings of the IEEE International Conference on Neural Networks-Conference Proceedings, Vancouver, BC, Canada, 16–21 July 2006.
- Gu, Y.X.; Liang, X.X.; Yin, N.Y.; Yang, Y.; Wan, B.; Guo, L.H.; Faiola, F. New insights into mechanism of bisphenol analogue neurotoxicity: Implications of inhibition of O-GlcNAcase activity in PC12 cells. Arch. Toxicol. 2019, 93, 2661–2671.
- Pitchford, S.; De Moor, K.; Glaeser, B.S. Nerve growth factor stimulates rapid metabolic responses in PC12 cells. Am. J. Physiol. Cell Physiol. 1995, 268, C936–C943.
- Mir, T.A.; Shinohara, H. P1.1.18 Real-time monitoring of cell response to drug stimulation by 2D-SPR Sensor: An approach to study neuronal differentiation. Proc. IMCS 2020, 2012, 863–865.
- Mir, T.A.; Shinohara, H. 2D-SPR biosensor detects the intracellular signal transduction in PC 12 cells at single cell level. In Proceedings of the International Conference on Sensing Technology, ICST, Kolkata, India, 18–21 December 2012.
- Guo, M.; Zhu, F.; Qiu, W.; Qiao, G.; Law, B.Y.K.; Yu, L.; Wu, J.; Tang, Y.; Yu, C.; Qin, D.; et al. High-throughput screening for amyloid-β binding natural small-molecules based on the combinational use of biolayer interferometry and UHPLC−DAD-Q/TOF-MS/MS. Acta Pharm. Sin. B 2021, 12, 1723–1739.
- Cho, Y.; Ba, V.A.P.; Jeong, J.Y.; Choi, Y.; Hong, S. Ion-selective carbon nanotube field-effect transistors for monitoring drug effects on nicotinic acetylcholine receptor activation in live cells. Sensors 2020, 20, 3680.
- Pham Ba, V.A.; Cho, D.G.; Hong, S. Nafion-Radical Hybrid Films on Carbon Nanotube Transistors for Monitoring Antipsychotic Drug Effects on Stimulated Dopamine Release. ACS Appl. Mater. Interfaces 2019, 11, 9716–9723.
- Dutta, A.K.; Korchev, Y.E.; Shevchuk, A.I.; Hayashi, S.; Okada, Y.; Sabirov, R.Z. Spatial distribution of maxi-anion channel on cardiomyocytes detected by smart-patch technique. Biophys. J. 2008, 94, 1646–1655.
- Kaur, B.; Kumar, S.; Kaushik, B.K. 2D Materials based Fiber Optic SPR Biosensor for Cancer Detection at 1550 nm. IEEE Sens. J. 2021, 21, 23957–23964.
- Hossain, B.; Paul, A.K.; Islam, M.A.; Rahman, M.M.; Sarkar, A.K.; Abdulrazak, L.F. A highly sensitive surface plasmon resonance biosensor using SnSe allotrope and heterostructure of BlueP/MoS2 for cancerous cell detection. Optik 2022, 252, 168506.
- Hoseinian, M.S.; Ahmadi, A.; Safaei Bezgabadi, A.; Bolorizadeh, M.A. Simulation of wagon wheel optical fiber biosensor for quick and easy detection of cancer cells. Opt. Quantum Electron. 2021, 53, 427.
- Jibon, R.H.; Podder, E.; Bulbul, A.A.M. Adrenal Glands Cancer Detection using PCF-based Biosensor. In Proceedings of the International Conference on Electronics, Communications and Information Technology, ICECIT, Khulna, Bangladesh, 14–16 September 2021.
- Segovia-Chaves, F.; Yague, J.C.T.; Vinck-Posada, H. Effects of chemical potential and cavity thickness on defective mode sensitivity for a cancer cell in a biosensor formed using a photonic crystal. Optik 2021, 240, 166823.
- Adoghe, A.U.; Noma-Osaghae, E.; Israel, Y.R. Photonic crystal and its application as a biosensor for the early detection of cancerous cells. Int. J. Online Biomed. Eng. 2020, 16, 86–94.
- Meshginqalam, B.; Barvestani, J. High performance surface plasmon resonance-based photonic crystal fiber biosensor for cancer cells detection. Eur. Phys. J. Plus 2022, 137, 417.
- Ramola, A.; Marwaha, A.; Singh, S. Design and investigation of a dedicated PCF SPR biosensor for CANCER exposure employing external sensing. Appl. Phys. A Mater. Sci. Process. 2021, 127, 643.
- Azab, M.Y.; Hameed, M.F.O.; Nasr, A.M.; Obayya, S.S.A. Highly Sensitive Metamaterial Biosensor for Cancer Early Detection. IEEE Sens. J. 2021, 21, 7748–7755.
- Feng, Q.M.; Liu, Z.; Chen, H.Y.; Xu, J.J. Paper-based electrochemiluminescence biosensor for cancer cell detection. Electrochem. Commun. 2014, 49, 88–92.
More
