Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Behnam Asgari Lajayer.
Biological and physico-chemical remediation technologies can be efficient in terms of spill cleanup and microorganisms—mainly bacteria—are the main ones responsible for petroleum hydrocarbons (PHCs) degradation such as crude oil. Currently, bBiodegradation is considered as one of the most sustainable and efficient techniques for the removal of PHCs.
- oil spills
- biosurfactants
- biostimulation
1. Microorganisms Involved in the Removal of Oil Spills from Marine Surfaces
Yeasts, fungi, and bacteria have the potential to use the various HCs in crude oil and derived products. For instance, following the Deepwater Horizon disaster (20 April 2010), the bacterial community identified was prone to succession [42,43][1][2]. At first, a primary enrichment of Pseudomonads and Oceanospirillales prevailed, followed by a change in dominance to Cycloclasticus, Methylotrophs, Colwellia, and Pseudoalteromonas. Genes associated with the transportation of iron or phosphorus and nitrogen-based compounds and associated metabolisms such as nitrate reduction were transcribed [44][3]. Correspondingly, total petroleum hydrocarbon and ammonium concentrations on the surfaces of the sediment were correlated with denitrification-related genes that were highly transcribed [45][4]. The gene expression for sulfite reduction and sulfur cycling was raised when O2 levels stayed high, however, the relevant mechanism is still unclear [46][5]. Several months after the DWH (Deepwater Horizon) spill, the sediment of the wellhead area region was enriched with Chloroflexi, Firmicutes, Methylotrophic, and Actinobacteria [47][6]. An exploratory survey was conducted and showed that—after a decade—the microorganisms of the sediments had returned to pre-spill composition [10][7].
While aliphatic HCs can be properly biodegraded in nature, this is completely different for branched-chain and long-chain HCs [48,49,50][8][9][10]. Moreover, with increasing pH and increasing salinity, the rate of degradation decreases, and that is why aliphatic HCs are hardly degraded in crude oil under hypersaline circumstances [8][11]. Salinity not only influences the diversity and growth of microorganisms, but also has a direct effect on biodegradation, and enzymes engaged in HCs degradation undergo some changes and lose their functioning. This inhibition is reversible when the microorganisms go back to normal conditions (moderate salinity level). Knowledge about molecular mechanisms and pathways of HCs degradation at high salinity is rare and only recently there have been a few reports explaining genes, enzymes, and breakdown steps for some HCs. These investigations have clearly shown that degradation of oxygenated and non-HCs by halophilic and halotolerant bacteria occurs by pathways similar to those found in non-halophiles [51][12]. In seasons with high temperatures and evaporation, salinity tends to increase and HCs degradation rate is remarkably reduced in deep-sea water [52][13]. During recent years many investigations have been performed on PAHs bioremediation and resources (Table 1). Several bacterial enzymes with various catabolic productivity allied with PAHs degradation have been identified and various biochemical pathways for PAH degradation (Such as naphthalene, phenanthrene, anthracene, and acenaphthene) have been introduced. Furthermore, some PAH catabolic operons have been sequenced, and their regulatory mechanism for PAH degradation has been known. In the environment, microorganisms are able to degrade different types of PAHs including quinoline, benzothiophene, and benzofuran through biochemical pathways. Nonetheless, a more detailed study is required to determine what is going on in a PAH-contaminated environment. Besides, many aspects of PAHs bioremediation have remained unknown or insufficient, which demands prospective attention. There is little knowledge on the transmembrane trafficking of PAHs, genes, enzymes, and molecular mechanisms of PAHs degradation in anaerobic conditions or high salinity and acidic environments. During the last decade, many studies have focused on Fusarium species ability in metabolizing the hydrocarbon chrysene to help to identify fungal strains displaying crude oil degradation potential under saline conditions [53,54][14][15].
. For microbial cell reproduction and viability, the presence of energy is necessary, which is obtained in the bioremediation process through redox reactions like respiration. In this energy supplying system, the presence of nutrients and energy sources, including electron donor and acceptor (Such as carbon dioxide, oxygen, iron (III), and sulfate) is essential [67][28]. In HCs bioremediation, the availability of a microbial strain or consortia with suitable metabolic capabilities is the most challenging. Microbial communities adapt, change genetically and selectively enrich within hours of exposure to HCs. As a result, higher biodegradation rates are achieved than in communities with no introduction of HCs contamination [68][29]. In bioremediation of HCs polluted sites, isolation of appropriate numbers of special HCs degraders from an environment is a justification for effective bioremediation. On the other hand, usually, consortia of different strains are required in the bioremediation of oil spills because it is made of a mixture of compounds, and one strain can metabolize only a limited range of HC substrates. Bioremediation relies on nutrient presence and optimal site properties that support biological functions. For example, microbial growth is affected when exposed to a high concentration of the contaminants owing to the presence of high toxins. In contrast, low concentration can block the induction of bacterial enzymes [64][25]. Additionally, an optimum availability of water (between 14% and 27% moisture) and pH (in the range of 5.5–7.8) in the environment is vital for the proliferation and growth of microbial cells [69][30]. The biodegradation efficiency, to an extent, rises with increasing temperature and decreases with reducing temperature [69][30]. Nutrient availability (e.g., through organic substrate amendments) is also needed for cell growth, division, and electron donor as a biostimulant [64][25]. Finally, contaminant bioavailability is another factor impacting the bioremediation process. Bioavailability for microbial reactions is lower for contaminants that are more sorbed to solids attached in matrices of molecules in contaminated sites, more widely diffused in macropores of soil and sediments, or are present in non-aqueous phase liquid (NAPL) form [64][25]. In the next sections, we discuss the role of environmental factors affecting the bioremediation process.
3. Biologically Based Solutions for Ashore and Marine Pollution
In the following sub-section, we review three common bioremediation technologies that are practical for eliminating oil spills in marine environments by microorganisms. In addition, factors and strategies for increasing the efficiency of technologies will be discussed. Figure 1 depicts what agents are responsible for three techniques of biosurfactant, biostimulation, and bioaugmentation.
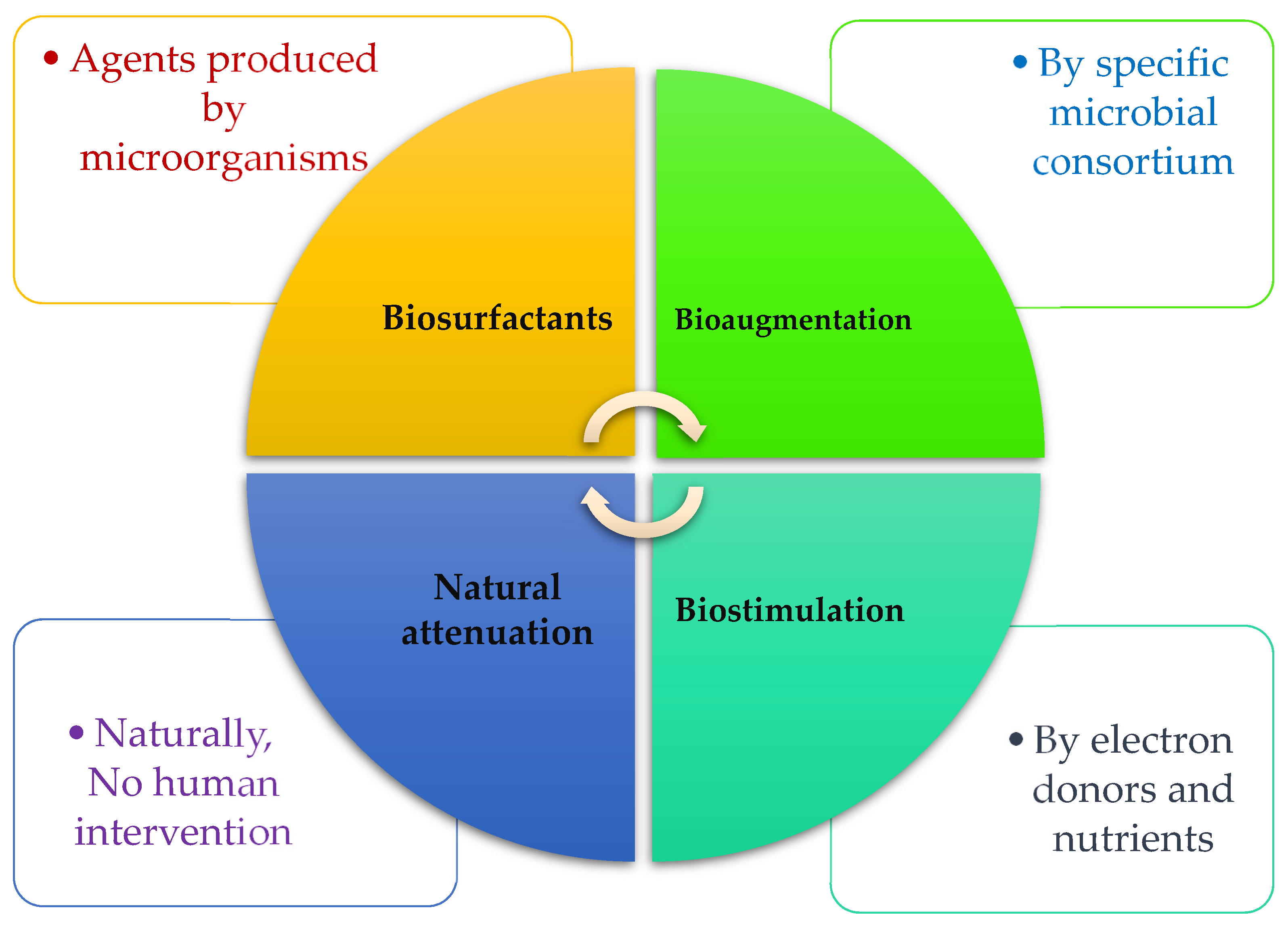
Figure 1.
The most prevailing bioremediation techniques are used for HCs degradation.
3.1. Bioaugmentation
Generally, adding oil-degrading bacteria to the existing microbial community is called bioaugmentation [70][31]. Bioremediation activities intend to boost the degradation efficiency that is performed by adding exogenic microorganisms. Bioaugmentation is considered a “polishing-up” or “accomplishing” process to deal with the very slow initial degradation of a fresh oil spill due to the very high concentrations of the originally spilled oil and its concomitant high abundance of toxic compounds. Usually, non-native microorganisms are looking for a suitable amount of biosurfactant when they are exposed to perilous oil spills to avoid detrimental impacts due to the toxicity of the spill. The HCs non-indigenous and indigenous degrading bacteria employ intracellular enzymes that permit bacteria to transform the HCs into another food source. Usually, on a HCs-containing culture medium or at the site of a spill in bioreactors, oil-degrading microbes are concentrated, microbial agents. These agents aim to prepare the influenced region with a significant oil-degrading microbial inoculum [71][32]. Therefore, bioaugmentation helps increasing the number of HCs degraders to a point that the spilled oil is used as the principal energy source. The most prevalent used options for bioaugmentation are the introduction of genetically engineered bacteria and the addition of biodegradation relevant genes packaged in a vector to be transferred into indigenous microorganisms by conjugation, as well as the addition of a pre-adapted consortium and a pre-adapted pure bacterial strain [72][33]. Screening followed by tailoring a competent microbial community for a particular site is a prerequisite for bioaugmentation. The first screening or selection step should pay attention to the metabolic capacities of the microorganism and vital characteristics that keep the cells active and alive under undesired environmental conditions. Prior knowledge about the microbial communities inhabiting the target site is an excellent strategy to select effective communities. Sometimes in co-contaminated sites, where both organic and metal contaminations are found, the best strategy is to take advantage of multi-component systems, i.e., a creating led microbial consortium is more beneficial than single-component systems [73][34]. Additionally, using a microbial consortium is more practical compared with a pure culture because it provides the metabolic diversity and reliability needed for field applications. Table 2 indicates relevant microbial consortia in the bioaugmentation. Some researchers using selected native strains reported full degradation of diesel oil and phenanthrene; an overall decrease of about 75% of the total HCs, and a decrease of 60% of isoprenoids in 40 days [74,75][35][36]. In 2009, Li et al. [76][37] confirmed that using indigenous microbial consortia (three bacteria including Bacillus sp., Zoogloea sp., and Flavobacterium with five fungal strains including Aspergillus niger, Phanerochaete, Cuuninghamella sp, Chrysosporium, Alternaria alternate, and Keissler, Penicillium chrysogenum) enhanced the degradation of PAHs in water by 41.5%. The biomass to be employed as inoculum for bioaugmentation is produced in bioreactors, and transferring of cultures to the site is often challenging. After being exposed to environmental stresses, in contrast to the optimum conditions of culturing, the introduced population starts reducing quickly. Fluctuations and shifts in some environmental factors like temperature, pH, moisture, as well as toxic pollutant levels, and depletion of nutrients influence microbial growth. Goldstein et al. argued that in natural systems, unlike their potential in cultures, inoculant usually cannot degrade organic pollutants [77][38]. Problems regarding the adaptation of the inoculated microorganisms, the rivalry between introduced and indigenous biomass, insufficiency of the substrate, and the use of other organic substrates are the most important reasons for the failure of degradation in natural systems [71][32].
Table 2.
Microbial consortia in bioaugmentation.
| Pollutant Type | Microorganisms | Reference | |
|---|---|---|---|
| PAHs (fluorene, pyrene, phenanthrene) | Rhodococcus sp., Acinetobacter sp., Pseudomonas sp. | Aircraft Exhaust [56][17] | [78][39]Oil Tankers [57][18] |
| Industrial Sources | Coke Production/Burning [58][19] | Cement Manufacturing [59][20] | Tyre Manufacturing |
| Gasoline | Methylibium petroleiphilum LMG22953 | [79][40] | [58][19] |
| Domestic Sources | Coal Cooking [56][17] | Wood Burning [ | |
| Crude oil | Roseomonas sp., Bacillus marisflavi, Microbacterium oxydans | 55][16] | [80][41Cigarette/Tobacco Smoking [60][21] |
| ] | Agricultural Sources | Agricultural Wastes [55][16] | Pesticides [61][22] |
| Crude oil | Alcanivorax borkumensis, Thalassolituus oleivorans | [81][ | Fertilizers [61][22] |
| Natural Sources | Forest Fire [62][23] | Volcanic Eruptions [63][24] | Wild Fire [63][24] |
2. Bioremediation and Affecting Factors
The first modern bioremediation use refers to six decades ago when George Robinson recruited the microbes to consume oil spills on the coast of Santa Barbara, California [64][25]. However, this technology was not important for the removal of HCs and other hazardous compounds like potentially toxic metals until the early 1980s [64][25]. Generally, bioremediation is a process associated with microorganisms and their metabolites to remove the different contaminants from aquatic ecosystems and sediment. Particularly, indigenous microorganisms in sediment are the main players in bioremediation because they can transform organic compounds into simple inorganic compounds or into their constituent elements through a process called mineralization [65][26]. Microorganisms in the bioremediation process have the potential to eliminate, decrease and transform oil contaminants into natural compounds found in air, sediments, water, sludge, and soil. Often, bioremediation co-occurs with detoxification, when microorganisms remove or immobilize waste materials by altering mineralization, and transformation [66][27]
| 42 |
| ] | ||
| Crude oil | ||
| P. aeruginosa, Rhodococcus | sp. CE461, Rhodococcus sp. CT451 | [82][43] |
| Petroleum HCs | Rhizopus sp., Penicillium funiculosum, Aspergillus sydowii, Rhizobiales sp., Pseudomonas sp., Brucella sp., Bacillus sp., Rhodococcus sp., Microbacterium sp. | [83] |
| Crude petroleum oil hydrocarbon | ||
| B. subtilis | DM-04, P. aeruginosa M and NM | [87][48] |
3.2. Biostimulation
In many situations, environmental criteria can be altered to boost the process of biodegradation. In marine crude oil spills, it is challenging to extend the life span of populations of HCs-eating microbes, owing to the problematic use of nutrients, and several native species can be killed or weakened because of oil toxicity in the spill region [88][49]. This toxicity is an inhibitory factor for stimulating the remaining indigenous bacteria and fungi. In this situation, biostimulation—helping optimize the environmental factors—is an appropriate strategy for degrading the HCs when the indigenous microbes are retained, and toxicity concentration is decreased [88][49]. Biostimulation may be more beneficial than bioaugmentation, as it stimulates indigenous bacteria that were already more competitive. It has been reported that if microorganisms growth is stimulated in the spill area, the HCs degrading efficiency may reach 70% during 30 days of incubation [89][50].
Nutrients are vital components of an effective biostimulation of HCs, as some nutrients may become an inhibiting factor, negatively affecting the biodegradation process [88][49]. The most prevalent additives that promote bacterial growth in a community are phosphate salts and nitrate [90][51]. The main aim is to boost the metabolism of autochthonous HCs degraders through in situ addition of limiting factors, which results in a quicker rate of the HCs degradation. The application of biostimulation in HCs bioremediation has been widely reported [34][52]; however, this approach also has its limitations and challenges [91][53]. For example, in soils, the use of inorganic fertilizer is challenged by the large cost of bioremediation and the likely chance of eutrophication [92][54].
3.3. Biosurfactants
Biological active surface molecules produced by microorganisms with a huge range of applications owing to their specific characteristics, minute toxicity, and biological admissibility are called biosurfactants [93][55]. These products as additives aim to produce petrochemicals and organic chemicals that are more accessible. Biosurfactants are amphiphilic compounds and able to alter the cell surface of microorganisms and increase the hydrophobic substance surface area. Microorganisms thanks to their ability of using hydrocarbon waste as raw materials can produce biosurfactants. One of the unique properties of biosurfactants is tolerance to environmental extreme conditions like ionic strength, acidity, temperature, salt concentration, demulsifying-emulsifying ability, and anti-inflammatory potential due to surface and interface activity [94][56]. Recent investigations have reported that lichenysin biosurfactant produced by Bacillus licheniformis was hardly impacted by Ca, NaCl concentrations, or temperature (up to 50 °C) and pH (4.5–9.0) [95][57]. Additionally, lipopeptides produced by Bacillus subtilis are stable at −15 °C, and beyond autoclavable temperature (121 °C) and concentrations, greater than 15% of NaCl when stored for six months [95][57]. An ideal biosurfactant decreases the surface tension of water. For instance, biosurfactants like surfactin, rhamnolipid, and sophorolipids produced, respectively, by B. Subtilis, P. aeruginosa, and Candida bombicola lower the surface tension [96][58]. Biosurfactants are productive and efficient, and their critical micelle concentration (CMC) is ten to forty times lower than that of chemical surfactants. Hence, less biosurfactant is required to decrease the surface tension [97][59]. Biosurfactants also have antioxidant, antimicrobial, and anti-inflammatory activities. For instance, recent research suggested that the polyanionic surfactant named emulsan produced by Acinetobacter calcoaceticus has indicated lethal concentration 50 (LC50) against other microbes like Photobacterium phosphoreum, which is much less than Pseudomonas rhamnolipids [98][60]. Biodegradability and environmental toxic friendly nature are two other properties of biosurfactants, as most biosurfactants are easily degradable.
Various microorganisms produce biosurfactants involved in the bioremediation of HCs. Table 3 shows economic and prevalent biosurfactants produced by different microorganisms. Rhamnolipid in two forms of mono and di-rhamnolipid is a type of biosurfactant produced by P. aeruginosa [99][61]. A correlation exists between the type of surfactant and the type of HCs that are degraded. For example, rhamnolipids produced by P. aeruginosa specifically degrade hexadecane. Many studies were done on phenanthrene degradation by chemical surfactants, and findings have proven that increased phenanthrene degradation is along with bacterial isolates producing a non-ionic surfactant [100][62]. Based on a study, HCs degradation capacity was multiplied when it was mixed with a biosurfactant, like a combination of chemical surfactant ‘FinasolOSR-5′ with biosurfactant trehalose-5, 5′-dicorynomycolates [100][62]. It has been reported that PAHs are significantly degraded by bacteria producing sophorose lipids and glycolipids in less than a month because surface-active glycolipids increase the biodegradation of 2,4-dichlorophenolindophenol (DCPIP) when supplemented with HCs sites [101][63].
Table 3.
Economic and prevalent biosurfactants in the bioremediation process.
| Microorganisms | Biosurfactant | Economic Significance | References | |||
|---|---|---|---|---|---|---|
| P. aeruginosa | Rhamno lipids | Bioremediation | [102][64] | |||
| Acinetobacter calcoaceticus | Emulsan Glycolipopeptide | Enhanced oil recovery by microbes | [103][65] | |||
| Rhodococcuserythropolis | Trehalose lipids | Dissolution of HCs | [104][66] | |||
| Ustilagomaydis | Cellobiose lipids | Antifungal compounds | [105][67] | |||
| Microbacterium | Microbactan Glycolipopeptide | Emulsifier | [106][68] | |||
| [ | 44 | ] | ||||
| B. licheniformis | Lichenysin | Enhanced oil recovery by microbes | [107][69] | Petroleum HCs | Pseudomonas oleovorans, Ochrobactrum sp., Stenotrophomonas maltophila | [84][45] |
| C. bombicola | Sophoro lipids | Antimicrobial property | [108][70] | Mixture of PAHs (anthracene, naphthalene, phenanthrene, pyrene, dibenzo[a]anthracene |
Bacillus strains B1F, B5A and B3G, Chromobacterium sp. 4015, Enterobacter aglomerans sp. B1A | [85][46] |
| PAHs (anthracene, phenanthrene, pyrene) | ||||||
| B. subtilis | Surfactin | Antimicrobial property | [109][71] | Mycobacterium fortuitum, Bacillus cereus, Microbacterium sp., Gornodia, Polyisoprenivorans, Microbacteriaceae, Bacterium, Fusarium oxysporium | [86][47] |
3.4. Cell Immobilization Techniques for Increasing Bioremediation Efficiency
Several techniques are available for immobilization of the microbial cells to increase the bioremediation efficiency by microorganisms. A carrier allows supplying moisture and aeration, better access to nutrients, and physical support for biomass, which increases the survival of the microbes [110][72]. To increase the viability and efficiency of the microbial cells, entrapment and encapsulation are two main techniques that accelerate the biodegradation process compared to uncoated cells.
Entrapment is an irremeable immobilization strategy for capturing cells inside fibers, and has been extensively studied. The systems provide enough protection by creating barriers around the immobilized microbial cells, therefore ensuring sustainable viability of the cell when the microorganisms are surrounded by the polymer [111][73]. Synthetic polymers like photo-cross-linkable resins and polyester as well as natural polymers like gelatin, collagen, and alginates are the most significant accessible matrices for entrapment. This process is designed with microbial cells within a solid network to allow the penetration of the substrate when inhibiting the cells from scattering into the medium. Owing to moderate required conditions and easy application of alginate gel polymer, many studies have reported practical use of this polymer for entrapment [111][73]. High cost, leakage of the cells, low loading capacity for the conjugation of biocatalysts into the matrix, limitations in disseminations, and attrition of biocarriers caused by high mechanical power are the most significant demerits of this method [112][74]. Nonetheless, this method is the most prevalent technique.
Encapsulation can reduce the concentration of toxic substances, control the availability of the nutrients, decrease the cell membrane damage, and protects the microbial cells from predation and competition [112][74]. Gellan gum, polyurethane, alginate, polyvinyl alcohol gel, kappa-carrageenan, gelatin, agar, agarose, and acrylate copolymers have been the studied components for immobilization and encapsulation of the HCs-degrader bacteria [71][32]. A study conducted by Muslemy et al. [113][75] worked on one bacterial consortium encapsulation with gellan gum microbeads and showed that encapsulated cells revealed a shorter lag phase. Consequently, a higher HCs degradation rate was observed in comparison with normal form (Free cell) at equal microbial concentrations. Liu et al. (2009) investigated the ability of Sphingomonas sp. FG03 and Acinetobacter sp. XA05 strains for biodegradation of phenol in both free and encapsulated settings in marine ecosystems [114][76]. Findings showed that a mixture of two bacterial strains had better efficiency in phenol biodegradation than pure cultures, and had enhanced functions for the high concentration of phenols [114][76]. In a study, Yaohui and Mang [115][77] worked on the differences between biostimulation and bioaugmentation treatments in the bioremediation of HCs and encapsulated the microbes with peanut hull powder as a carrier and bulking agent. They reported that mass transfer rate of nutrients, oxygen, water, HCs, and nutrition for the microflora was accelerated [115][77]. Wang et al. argued that immobilization of the Mucor sp. F2 (MF) fungal consortium, Mycobacterium sp. B2 (MB) fungal consortium, and MB+MF with pyrene, respectively, increased by 159.1%, 59.9%, and 60.0% after incubation [116][78]. Dehydrogenase activity has also a considerable improvement when biocarriers are used. Keryn et al. [117][79] investigated the effectiveness of economically sustainable biocarriers (mussel shells, coir peat complex) for marine HCs remediation and reported an accelerated degradation in the immobilized forms compared with free cells.
Researchers have investigated different ways of combining biostimulation and bioaugmentation technologies for bioremediation. Researchers have assessed several combinations of consortium or single bacteria and fungi with biostimulators such as fertilizers, corn-steep-liquor, solid-waste-dates, and other materials containing N, K, and P and obtained satisfying results. The degradation efficiency reached 97% and 91% for 0.5% w/v crude oil in 4 weeks, respectively by solid-waste-dates and corn-steep-liquor as biostimulants and using single strain Pseudomonas [118][80]. The degradation efficiency is affected by the type of total petroleum HCs pollutant to be degraded as light crude oil is more degradable than heavy crude oil, and this process is faster. In a study, Arabian light crude oil (1000 ppm) was degraded within three weeks by a single strain of Alcanivorax borkumensis SK2, and the presence of biostimulators like 0.1 g/L NaNO3, 0.077 g/L KH2PO4, and 0.2 g/L NH4Cl [119][81]. Additionally, 94.4% efficiency was obtained for 10% v/v crude oil (Escravos light) in 8 weeks by Aspergillus niger and Pseudomonas aeruginosa, and the presence of K, N, P [119][81]. Another study treated water polluted with crude oil by a combination of biostimulation and bioaugmentation technologies by A. niger and P. aeruginosa. The researchers created four different conditions including (nutrient-free), A (nutrient N, P, K), B (nutrient-plus aeration), and C (nutrient-free, aeration, and agitation), and achieved efficiencies of 92.3%, 93.6%, and 94.4%, respectively, after 56 days, for total petroleum HCs degradation [120][82].
Cross-linking is another technique employing hydrogen bonds (Covalent) between microbial cells and inorganic support by agents. Chemical modification of the surface of support materials is the main element of this technique. Compared to the previous technique, cross-linking is less practical because covalent binding often cuts functional integrities in the microbial cell. However, this method has very high efficiency for the cell when the functional integrities remain without interruption. Many successes regarding this technique refer to the immobilization of yeast cells. For example, in two studies in this area, researchers reported a successful application of cross-linking in the immobilization of Saccharomyces carlsbergensis on porous silica beads [121][83] and Saccharomyces amurcea and Saccharomyces cerevisiae on zirconia ceramics [122][84].
References
- Passow, U.; Stout, S.A. Character and sedimentation of “lingering” Macondo oil to the deep-sea after the Deepwater Horizon oil spill. Mar. Chem. 2020, 218, 103733.
- Yang, T.; Nigro, L.M.; Gutierrez, T.; Joye, S.B.; Highsmith, R.; Teske, A. Pulsed blooms and persistent oil-degrading bacterial populations in the water column during and after the Deepwater Horizon blowout. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 282–291.
- Ullrich, S.R.; Poehlein, A.; Tischler, J.S.; González, C.; Ossandon, F.J.; Daniel, R.; Holmes, D.S.; Schlömann, M.; Mühling, M. Genome analysis of the biotechnologically relevant acidophilic iron oxidising strain JA12 indicates phylogenetic and metabolic diversity within the novel genus “Ferrovum”. PLoS ONE 2016, 11, e0146832.
- Warwick, R.; Clarke, K. Relearning the ABC: Taxonomic changes and abundance/biomass relationships in disturbed benthic communities. Mar. Biol. 1994, 118, 739–744.
- Sanyal, O.; Shinde, V.L.; Meena, R.M.; Damare, S.; Shenoy, B.D. The ITS-based phylogeny of fungi associated with tarballs. Mar. Pollut. Bull. 2016, 113, 277–281.
- Nazina, T.N.; Shestakova, N.M.; Semenova, E.M.; Korshunova, A.V.; Kostrukova, N.K.; Tourova, T.P.; Min, L.; Feng, Q.; Poltaraus, A.B. Diversity of metabolically active bacteria in water-flooded high-temperature heavy oil reservoir. Front. Microbiol. 2017, 8, 707.
- Calderoli, P.A.; Espínola, F.J.; Dionisi, H.M.; Gil, M.N.; Jansson, J.K.; Lozada, M. Predominance and high diversity of genes associated to denitrification in metagenomes of subantarctic coastal sediments exposed to urban pollution. PLoS ONE 2018, 13, e0207606.
- Daccò, C.; Girometta, C.; Asemoloye, M.; Carpani, G.; Picco, A.; Tosi, S. Key fungal degradation patterns, enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int. Biodeterior. Biodegrad. 2020, 147, 104866.
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83.
- Fatima, K.; Imran, A.; Naveed, M.; Afzal, M. Plant-bacteria synergism: An innovative approach for the remediation of crude oil-contaminated soils. Soil Environ. 2017, 36, 93–113.
- Bacosa, H.P.; Erdner, D.L.; Liu, Z. Differentiating the roles of photooxidation and biodegradation in the weathering of Light Louisiana Sweet crude oil in surface water from the Deepwater Horizon site. Mar. Pollut. Bull. 2015, 95, 265–272.
- Fathepure, B.Z. Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front. Microbiol. 2014, 5, 173.
- Ebadi, A.; Sima, N.A.K.; Olamaee, M.; Hashemi, M.; Nasrabadi, R.G. Effective bioremediation of a petroleum-polluted saline soil by a surfactant-producing Pseudomonas aeruginosa consortium. J. Adv. Res. 2017, 8, 627–633.
- Hidayat, A.; Tachibana, S. Biodegradation of aliphatic hydrocarbon in three types of crude oil by Fusarium sp. F 092 under stress with artificial sea water. J. Environ. Sci. Technol. 2012, 5, 64–73.
- AI-Jawhari, I.F.H. Ability of some soil fungi in biodegradation of petroleum hydrocarbon. J. Appl. Environ. Microbiol. 2014, 2, 46–52.
- Ravindran, A.; Sajayan, A.; Priyadharshini, G.B.; Selvin, J.; Kiran, G.S. Revealing the efficacy of thermostable biosurfactant in heavy metal bioremediation and surface treatment in vegetables. Front. Microbiol. 2020, 11, 222.
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195.
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123.
- Gupte, A.; Tripathi, A.; Patel, H.; Rudakiya, D.; Gupte, S. Bioremediation of polycyclic aromatic hydrocarbon (PAHs): A perspective. Open Biotechnol. J. 2016, 10, 363–378.
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971.
- St. Helen, G.; Goniewicz, M.L.; Dempsey, D.; Wilson, M.; Jacob, P., III; Benowitz, N.L. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem. Res. Toxicol. 2012, 25, 952–964.
- Yuan, H.; Liu, E.; Zhang, E.; Luo, W.; Chen, L.; Wang, C.; Lin, Q. Historical records and sources of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in sediment from a representative plateau lake, China. Chemosphere 2017, 173, 78–88.
- Johnsen, A.R.; Karlson, U. Diffuse PAH contamination of surface soils: Environmental occurrence, bioavailability, and microbial degradation. Appl. Microbiol. Biotechnol. 2007, 76, 533–543.
- Kozak, K.; Ruman, M.; Kosek, K.; Karasiński, G.; Stachnik, Ł.; Polkowska, Ż. Impact of volcanic eruptions on the occurrence of PAHs compounds in the aquatic ecosystem of the southern part of West Spitsbergen (Hornsund Fjord, Svalbard). Water 2017, 9, 42.
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmention: A review. Int. J. Environ. Bioremediation Biodegrad. 2015, 3, 28–39.
- Vogt, C.; Richnow, H.H. Bioremediation via in situ microbial degradation of organic pollutants. Geobiotechnology II 2013, 142, 123–146.
- Kumar, V.; Shahi, S.; Singh, S. Bioremediation: An eco-sustainable approach for restoration of contaminated sites. In Microbial Bioprospecting for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 123–146.
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896–4906.
- Militon, C.; Jézéquel, R.; Gilbert, F.; Corsellis, Y.; Sylvi, L.; Cravo-Laureau, C.; Duran, R.; Cuny, P. Dynamics of bacterial assemblages and removal of polycyclic aromatic hydrocarbons in oil-contaminated coastal marine sediments subjected to contrasted oxygen regimes. Environ. Sci. Pollut. Res. 2015, 22, 15260–15272.
- Mukherjee, A.K.; Das, K. Correlation between diverse cyclic lipopeptides production and regulation of growth and substrate utilization by Bacillus subtilis strains in a particular habitat. FEMS Microbiol. Ecol. 2005, 54, 479–489.
- Sayed, K.; Baloo, L.; Sharma, N.K. Bioremediation of total petroleum hydrocarbons (TPH) by bioaugmentation and biostimulation in water with floating oil spill containment booms as bioreactor basin. Int. J. Environ. Res. Public Health 2021, 18, 2226.
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241.
- El Fantroussi, S.; Agathos, S.N. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol. 2005, 8, 268–275.
- Hazra, C.; Kundu, D.; Chaudhari, A. Biosurfactant-assisted bioaugmentation in bioremediation. In Microorganisms in Environmental Management; Satyanarayana, T., Johri, B., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 631–664.
- Alisi, C.; Musella, R.; Tasso, F.; Ubaldi, C.; Manzo, S.; Cremisini, C.; Sprocati, A.R. Bioremediation of diesel oil in a co-contaminated soil by bioaugmentation with a microbial formula tailored with native strains selected for heavy metals resistance. Sci. Total Environ. 2009, 407, 3024–3032.
- Rahman, K.; Banat, I.; Thahira, J.; Thayumanavan, T.; Lakshmanaperumalsamy, P. Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant. Bioresour. Technol. 2002, 81, 25–32.
- Li, X.; Lin, X.; Li, P.; Liu, W.; Wang, L.; Ma, F.; Chukwuka, K. Biodegradation of the low concentration of polycyclic aromatic hydrocarbons in soil by microbial consortium during incubation. J. Hazard. Mater. 2009, 172, 601–605.
- Goldstein, R.M.; Mallory, L.M.; Alexander, M. Reasons for possible failure of inoculation to enhance biodegradation. Appl. Environ. Microbiol. 1985, 50, 977–983.
- Yu, S.; Ke, L.; Wong, Y.; Tam, N. Degradation of polycyclic aromatic hydrocarbons by a bacterial consortium enriched from mangrove sediments. Environ. Int. 2005, 31, 149–154.
- Daghio, M.; Tatangelo, V.; Franzetti, A.; Gandolfi, I.; Papacchini, M.; Careghini, A.; Sezenna, E.; Saponaro, S.; Bestetti, G. Hydrocarbon degrading microbial communities in bench scale aerobic biobarriers for gasoline contaminated groundwater treatment. Chemosphere 2015, 130, 34–39.
- Zhao, D.; Liu, C.; Liu, L.; Zhang, Y.; Liu, Q.; Wu, W.-M. Selection of functional consortium for crude oil-contaminated soil remediation. Int. Biodeterior. Biodegrad. 2011, 65, 1244–1248.
- Wittich, R.-M.; Wolff, P. Growth of the genetically engineered strain Cupriavidus necator RW112 with chlorobenzoates and technical chlorobiphenyls. Microbiology 2007, 153, 186–195.
- Varjani, S.J.; Rana, D.P.; Jain, A.K.; Bateja, S.; Upasani, V.N. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124.
- Mancera-López, M.; Esparza-García, F.; Chávez-Gómez, B.; Rodríguez-Vázquez, R.; Saucedo-Castaneda, G.; Barrera-Cortés, J. Bioremediation of an aged hydrocarbon-contaminated soil by a combined system of biostimulation–bioaugmentation with filamentous fungi. Int. Biodeterior. Biodegrad. 2008, 61, 151–160.
- Supaphol, S.; Panichsakpatana, S.; Trakulnaleamsai, S.; Tungkananuruk, N.; Roughjanajirapa, P.; O’Donnell, A.G. The selection of mixed microbial inocula in environmental biotechnology: Example using petroleum contaminated tropical soils. J. Microbiol. Methods 2006, 65, 432–441.
- Silva, Í.S.; dos Santos, E.d.C.; de Menezes, C.R.; de Faria, A.F.; Franciscon, E.; Grossman, M.; Durrant, L.R. Bioremediation of a polyaromatic hydrocarbon contaminated soil by native soil microbiota and bioaugmentation with isolated microbial consortia. Bioresour. Technol. 2009, 100, 4669–4675.
- Jacques, R.J.; Okeke, B.C.; Bento, F.M.; Teixeira, A.S.; Peralba, M.C.; Camargo, F.A. Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour. Technol. 2008, 99, 2637–2643.
- Das, K.; Mukherjee, A.K. Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour. Technol. 2007, 98, 1339–1345.
- Brzeszcz, J.; Kapusta, P.; Steliga, T.; Turkiewicz, A. Hydrocarbon removal by two differently developed microbial inoculants and comparing their actions with biostimulation treatment. Molecules 2020, 25, 661.
- Hassanshahian, M.; Yakimov, M.M.; Denaro, R.; Genovese, M.; Cappello, S. Using Real-Time PCR to assess changes in the crude oil degrading microbial community in contaminated seawater mesocosms. Int. Biodeterior. Biodegrad. 2014, 93, 241–248.
- Al-Hawash, A.B.; Dragh, M.A.; Li, S.; Alhujaily, A.; Abbood, H.A.; Zhang, X.; Ma, F. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt. J. Aquat. Res. 2018, 44, 71–76.
- Nikolopoulou, M.; Pasadakis, N.; Kalogerakis, N. Evaluation of autochthonous bioaugmentation and biostimulation during microcosm-simulated oil spills. Mar. Pollut. Bull. 2013, 72, 165–173.
- Orji, F.A.; Ibiene, A.A.; Dike, E.N. Laboratory scale bioremediation of petroleum hydrocarbon–polluted mangrove swamps in the Niger Delta using cow dung. Malays. J. Microbiol. 2012, 8, 219–228.
- Ponsin, V.; Coulomb, B.; Guelorget, Y.; Maier, J.; Höhener, P. In situ biostimulation of petroleum hydrocarbon degradation by nitrate and phosphate injection using a dipole well configuration. J. Contam. Hydrol. 2014, 171, 22–31.
- Shivlata, L.; Tulasi, S. Thermophilic and alkaliphilic Actinobacteria: Biology and potential applications. Front. Microbiol. 2015, 6, 1014.
- Rodrigues, L.R. Microbial surfactants: Fundamentals and applicability in the formulation of nano-sized drug delivery vectors. J. Colloid Interface Sci. 2015, 449, 304–316.
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102.
- Kim, L.H.; Jung, Y.; Yu, H.-W.; Chae, K.-J.; Kim, I.S. Physicochemical interactions between rhamnolipids and Pseudomonas aeruginosa biofilm layers. Environ. Sci. Technol. 2015, 49, 3718–3726.
- Anjum, F.; Gautam, G.; Edgard, G.; Negi, S. Biosurfactant production through Bacillus sp. MTCC 5877 and its multifarious applications in food industry. Bioresour. Technol. 2016, 213, 262–269.
- Gregorich, E.; Gillespie, A.; Beare, M.; Curtin, D.; Sanei, H.; Yanni, S. Evaluating biodegradability of soil organic matter by its thermal stability and chemical composition. Soil Biol. Biochem. 2015, 91, 182–191.
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558.
- Itrich, N.R.; McDonough, K.M.; van Ginkel, C.G.; Bisinger, E.C.; LePage, J.N.; Schaefer, E.C.; Menzies, J.Z.; Casteel, K.D.; Federle, T.W. Widespread microbial adaptation to l-glutamate-N, N-diacetate (L-GLDA) following its market introduction in a consumer cleaning product. Environ. Sci. Technol. 2015, 49, 13314–13321.
- Joy, S.; Rahman, P.K.; Sharma, S. Biosurfactant production and concomitant hydrocarbon degradation potentials of bacteria isolated from extreme and hydrocarbon contaminated environments. Chem. Eng. J. 2017, 317, 232–241.
- Amani, H.; Müller, M.M.; Syldatk, C.; Hausmann, R. Production of microbial rhamnolipid by Pseudomonas aeruginosa MM1011 for ex situ enhanced oil recovery. Appl. Biochem. Biotechnol. 2013, 170, 1080–1093.
- Goldman, S.; Shabtai, Y.; Rubinovitz, C.; Rosenberg, E.; Gutnick, D. Emulsan in Acinetobacter calcoaceticus RAG-1: Distribution of cell-free and cell-associated cross-reacting material. Appl. Environ. Microbiol. 1982, 44, 165–170.
- Urum, K.; Pekdemir, T. Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 2004, 57, 1139–1150.
- Morita, T.; Konishi, M.; Fukuoka, T.; Imura, T.; Kitamoto, D. Microbial conversion of glycerol into glycolipid biosurfactants, mannosylerythritol lipids, by a basidiomycete yeast, Pseudozyma antarctica JCM 10317T. J. Biosci. Bioeng. 2007, 104, 78–81.
- Camacho-Chab, J.C.; Guézennec, J.; Chan-Bacab, M.J.; Ríos-Leal, E.; Sinquin, C.; Muñiz-Salazar, R.; Rosa-García, S.D.C.D.L.; Reyes-Estebanez, M.; Ortega-Morales, B.O. Emulsifying activity and stability of a non-toxic bioemulsifier synthesized by Microbacterium sp. MC3B-10. Int. J. Mol. Sci. 2013, 14, 18959–18972.
- Qiu, Y.; Xiao, F.; Wei, X.; Wen, Z.; Chen, S. Improvement of lichenysin production in Bacillus licheniformis by replacement of native promoter of lichenysin biosynthesis operon and medium optimization. Appl. Microbiol. Biotechnol. 2014, 98, 8895–8903.
- Solaiman, D.K.; Ashby, R.D.; Zerkowski, J.A.; Foglia, T.A. Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnol. Lett. 2007, 29, 1341–1347.
- Lee, B.-S.; Kim, E.-K. Lipopeptide production from Bacillus sp. GB16 using a novel oxygenation method. Enzym. Microb. Technol. 2004, 35, 639–647.
- Rahmati, F. Microencapsulation of Lactobacillus acidophilus and Lactobacillus plantarum in Eudragit S100 and alginate chitosan under gastrointestinal and normal conditions. Appl. Nanosci. 2020, 10, 391–399.
- Nwankwegu, A.S.; Onwosi, C.O. Microbial cell immobilization: A renaissance to bioaugmentation inadequacies. A review. Environ. Technol. Rev. 2017, 6, 186–198.
- Rahmati, F. Impact of microencapsulation on two probiotic strains in alginate chitosan and Eudragit S100 under gastrointestinal and normal conditions. Open Biotechnol. J. 2019, 13, 59–67.
- Moslemy, P.; Neufeld, R.J.; Guiot, S.R. Biodegradation of gasoline by gellan gum-encapsulated bacterial cells. Biotechnol. Bioeng. 2002, 80, 175–184.
- Liu, Y.; Zhang, A.; Wang, X. Biodegradation of phenol by using free and immobilized cells of Acinetobacter sp. XA05 and Sphingomonas sp. FG03. Biochem. Eng. J. 2009, 44, 187–192.
- Xu, Y.; Lu, M. Bioremediation of crude oil-contaminated soil: Comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010, 183, 395–401.
- Wang, S.; Li, X.; Liu, W.; Li, P.; Kong, L.; Ren, W.; Wu, H.; Tu, Y. Degradation of pyrene by immobilized microorganisms in saline-alkaline soil. J. Environ. Sci. 2012, 24, 1662–1669.
- Simons, K.L.; Ansar, A.; Kadali, K.; Bueti, A.; Adetutu, E.M.; Ball, A.S. Investigating the effectiveness of economically sustainable carrier material complexes for marine oil remediation. Bioresour. Technol. 2012, 126, 202–207.
- El Mahdi, A.M.; Aziz, H.A.; Abu Amr, S.S.; El-Gendy, N.S.; Nassar, H.N. Isolation and characterization of Pseudomonas sp. NAF1 and its application in biodegradation of crude oil. Environ. Earth Sci. 2016, 75, 380.
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial degradation of hydrocarbons—basic principles for bioremediation: A review. Molecules 2020, 25, 856.
- Amenaghawon, A.N.; Osunbor, O.; Obahiagbon, K.O. Impact of nutrients, aeration and agitation on the bioremediation of crude oil polluted water using mixed microbial culture. Int. J. Sci. Res. Environ. Sci. 2014, 2, 43.
- Martins, S.C.S.; Martins, C.M.; Fiúza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418.
- Messing, R.; Oppermann, R.; Kolot, F. Pore dimensions for accumulating biomass. II. Microbes that form spores and exhibit mycelial growth. Biotechnol. Bioeng. 1979, 21, 59–67.
More
