Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Fiammetta Verni and Version 2 by Catherine Yang.
Vitamin B6 is a cofactor for approximately 150 reactions that regulate the metabolism of glucose, lipids, amino acids, DNA, and neurotransmitters. In addition, it plays the role of antioxidant by counteracting the formation of reactive oxygen species (ROS) and advanced glycation end-products (AGEs). Epidemiological and experimental studies indicated an evident inverse association between vitamin B6 levels and diabetes, as well as a clear protective effect of vitamin B6 on diabetic complications.
- vitamin B6
- diabetes
- AGEs
1. Vitamin B6: Roles and Synthesis
Vitamin B6 is a very important compound for general cellular metabolism [1]. The term vitamin B6 refers to six common forms or vitamers, namely pyridoxine (PN), pyridoxal (PL), pyridoxamine (PM), and their related 5′-phosphate derivatives (PNP, PLP, and PMP). The biologically active form, the pyridoxal 5′-phosphate (PLP), acts as coenzyme in about 150 distinct enzymatic activities that catalyze crucial metabolic reactions, such as synthesis, transformation, and degradation of amines and amino acids, supply of one carbon units, transsulfuration, synthesis of tetrapyrrolic compounds (including heme) and polyamines, biosynthesis, and degradation of neurotransmitters [2][3][2,3]. Although vitamin B6 is not classified as a classical antioxidant compound, it is able to quench oxygen reactive species (ROS) [4] and counteract the formation of advanced glycation end products (AGEs), genotoxic compounds associated with senescence, and diabetes [5]. Furthermore, PLP works as a modulator of transcription factors, has a role in enzyme folding, and can bind to steroid receptors, playing a role in membrane transport [6].
Mammals, different from microorganisms, are not able to synthesize PLP but recycle it through a salvage pathway from B6 vitamers as pyridoxal (PL), pyridoxamine (PM), and pyridoxine (PN) contained in food [7]. In the cytoplasm PL, PM, and PN are converted into the 5′-phosphorylated vitamers through pyridoxal kinase (PDXK), while the FMN-dependent pyridoxine 5′-phosphate oxidase (PNPO) converts PNP and PMP into PLP (Figure 1). Once ingested, PLP, PNP, and PMP are dephosphorylated by the tissue-non-specific alkaline phosphatase (TNSALP), which is anchored to the cell membrane. Then, PM, PN, and PL are absorbed from the upper small intestine by a carrier-mediated system and is delivered to the liver. Here, they are converted to PLP, thanks to the combined action of PDXK and PNPO. From the liver, PLP bound to albumin, along with dephosphorylated B6 vitamers, reach the peripheral tissues through the blood stream. In order to enter cells, PLP needs to be dephosphorylated again by TNSALP [8]. In the cytosol, a ubiquitous PLP phosphatase is instead required for vitamin B6 catabolism [9].
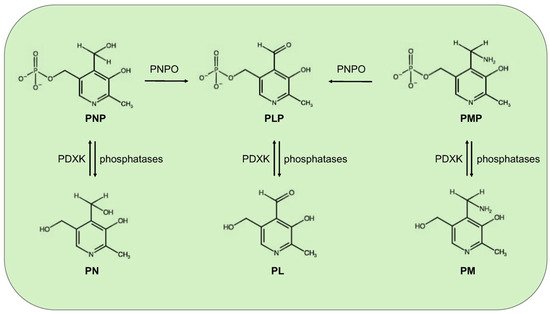
Figure 1. Schematic vitamin B6 metabolism in humans. The diagram corresponds to the pyridoxal 5′-phosphate salvage pathway. PLP, pyridoxal 5′-phosphate; PNP, pyridoxine 5′-phosphate; PMP, pyridoxamine 5′-phosphate; PL, pyridoxal; PN, pyridoxine; PM, pyridoxamine; PDXK, pyridoxal kinase; and PNPO, pyridoxine 5′-phosphate oxidase.
Vitamin B6 recommended dietary allowance is 1.3 mg day-1 for adults; it is present in several foods including meat, fish, poultry, vegetables, and fruits, thus, a severe deficiency of this vitamin is uncommon in developed countries, within any diet. However, PLP concentrations appear to be reduced in certain contexts, as for example, alcoholism [10], obesity [11], and pregnancy [12]. Moreover, some pathological conditions such as end-stage renal diseases, chronic renal insufficiency, and other kidney diseases can lower the vitamin B6 levels [12]. In addition, vitamin B6 deficiency has been correlated to malabsorption syndromes, such as celiac disease and inflammatory bowel diseases [12][13][14][12,13,14]. Even the intake of certain medications, including isoniazid [15], penicillamine [16], and cycloserine [17], as well as oral contraceptives, can reduce PLP availability [18]. PLP levels can also be decreased by the inflammation conditions and stress hormones [19][20][19,20].
2. Vitamin B6 and Diabetes
By considering the plethora of reactions in which vitamin B6 is involved, it is not surprising that its deficiency has been implicated in several clinically relevant diseases, including autism, schizophrenia, Alzheimer, Parkinson, epilepsy, Down’s syndrome, diabetes, and cancer; however, the underlying mechanisms remain unknown in most cases [21][22][23][21,22,23].
Diabetes mellitus (DM) represents a global health problem, touching more than 400 million people and consists of a group of metabolic disorders characterized by persistent hyperglycemia arising from impaired insulin secretion, insulin action, or both [24]. DM is a multifactorial disease caused by the concerted action of genetic and environmental factors, and on the basis of its etiology, it can be classified into three major types—type1 (T1D), type2 (T2D), and gestational diabetes (GDM). T1D is an autoimmune disorder that leads to the destruction of pancreatic beta-cells and accounts for only 5–10% of all diabetes. T2D, the more frequent form (90–95%), is mainly caused by insulin resistance consisting of a diminished tissue response to insulin that leads glucose to accumulate in blood. Consequently, the rate of insulin secretion increases to meet the body’s needs, but this overload, in the long-term, compromises pancreas functionality. GDM is a common pregnancy complication that affects approximately 14% of pregnancies worldwide. It is associated with insulin resistance, in turn generated by a combined action of pregnancy hormones and other factors [25].
Substantial evidence correlates vitamin B6 to diabetes and its complications. Some population screenings have been carried out to compare PLP levels in diabetic groups vs. healthy subjects; in addition, several studies focused on the impact of vitamin B6 on diabetic complications and others on the effectiveness of vitamin B6 as a preventive treatment. Vitamin B6 levels are commonly assessed by measuring plasma pyridoxal 5′-phosphate (PLP) concentration and an inadequate vitamin B6 status is generally associated with a concentration, under the cut-off level of 30 nmol/L. Other methods include the measurement of plasma pyridoxal or total vitamin B6 and urinary 4-pyridoxic acid, as well the ratio between PLP and PL [26]. By examining the studies reported in literature, an inverse relation between vitamin B6 levels and diabetes emerges. Satyanarayana and coworkers [27] in a cross-sectional case-control study found that the mean plasma PLP levels were significantly lower in T2D subjects, compared to the healthy controls. By comparing the results obtained in a Korean study by Ahn and coworkers [28] to those obtained by Nix and collaborators [29] in a German cohort, vitamin B6 levels appeared to be inversely related to the progression of diabetes. Ahn and collaborators [28], in fact, examined diabetic people with an early status of the disease, finding a mean plasma PLP level reduction to be relevant but not statistically significant, with respect to controls; in contrasts, the diabetic group examined by Nix [29], being composed of people with advanced clinical stage, exhibited median plasma concentrations of PLP, PN, and PL that were significantly decreased in a diabetic group compared to the controls. Interestingly, median plasma levels of the PM, PMP, and pyridoxic acid were significantly higher in the diabetes groups than in the controls; this finding led Nix and collaborators to advance the hypothesis that T2D might be associated with an altered activity of the enzymes involved in the interconversion of B6 vitamers [29]. In another study, based on the evidence of increased urinary clearance of vitamin B6, it was hypothesized that decreased vitamin B6 levels in T2D subjects could derive from an impaired reabsorption processes [30]. The same inverse relationship between B6 levels and diabetes was observed in experimental animals [31][32][31,32]. Roger was the first to describe decreased PLP levels in streptozotocin-diabetic rats accompanied by less storage in the liver of the mitochondrial PLP [31].
Decreased PLP levels have also be associated with GDM. In a study performed in a group of women affected by GDM, Bennink and Schreurs [33] found that 13 out of 14 displayed reduced PLP levels. Moreover, pyridoxine administration ameliorated oral glucose tolerance. Analogous results were obtained by Spellacy and coworkers [34], which found a clear blood glucose decrease and a normalization of insulin secretion following pyridoxine therapy in GDM women, indicating that vitamin B6 might ameliorate plasma insulin biological activity.
Other intervention studies reported that pyridoxine supplementation is capable of lowering blood glucose levels in streptozotocin-treated rats [35], as well as glycosylated hemoglobin levels in T2D patients [36]. Moreover, Kim and collaborators [37] showed that vitamin B6 can reduce postprandial blood glucose levels following sucrose and starch ingestion, by inhibiting the activity of small-intestinal α-glucosidases.
