THyperspectral-based plant disOn the detection, quantification, diagnosis, anbasis of simply describing the types of pathogens and host–pathogen interaction processes, this entry expounds the great advantages of hyperspectral technologies in plant
disease identification of plantdetection. Then, in the process of describing the hyperspectral diseases is particularly crucial for precision agriculture. Recently, tra analysis steps, the articles, algorithms, and methods from disease detection to qualitative and quantitative evaluation are mainly summarizing. Additional visual assessmentlly, according to the discussion of the current major problems in plant disease detection with hyperspectral technology has not been able to meet the needs of precision agricultural informatization development, anies, we propose that different pathogens’ identification, biotic and abiotic stresses discrimination, plant disease early warning, and satellite‐based hyperspectral technology, as a typical type of non-invasive technology, has received increasing attention. Dis are the primary challenges and pave the way for a targeted response.ease detection technologies have became more and more significant on plant science.
- pathogens, hyperspectral, plant disease detection
1. Introduction
With the changes in world climate and the accelerated development of global trade, the distributions, host ranges, and impacts of plant diseases have expanded continuously, and many of these diseases can still spread or break out after having been under control. In Bangladesh in 2016, the outbreak of wheat blast caused total crop failure with an impact range reaching nearly 15,000 ha
1. Introduction
[1]. In addition, the report of the Food and Agriculture Organization of United Nations (FAO) shows that the occurrence of only Xylella fastidiosa could cost nearly $104 million a year in wine losses in California alone [2]. Hence, plant diseases are now among the most basic, important, and noteworthy issues in agriculture management.
Whilst the economic losses caused by plant diseases are on the one hand, on the other hand, considering population and food imbalance, it is more serious that the diseases cause food losses. FAO statistics show that to fulfill the food requirements of 9.1 billion population by 2050, a 70% steady increase in agricultural production is needed [3]. However, between 20% and 40% of global crops have been lost annually to pests and diseases in the last 45 years. Furthermore, according to Carvajal-Yepes et al. [4], the average worldwide yield losses caused by pests and diseases in wheat, rich, maize, potatoes, and soybeans are estimated to be 21.5%, 30.0%, 22.6%, 17.2%, and 21.4%, respectively. In addition, diseases spread to large areas upon infection and cause large-scale production reduction, also resulting in a decrease in the quality of agricultural products or even endangering life. Fusarium head blight (FHB) is a prominent example. From 1998 to 2000 alone, the cumulative direct and secondary economic losses from FHB in primary food crops were estimated to be $2.67 billion [5]. In addition, once a plant disease breaks out on a large scale, the damage to the environment is considerable. According to FAO statistics, the consumption of pesticides increased from 3.05 million tonnes in 2000 to 4.09 million tonnes in 2016. Plant diseases are considered as risks because they constantly contribute to significant yield, economic, and environment losses worldwide [6]. Therefore, the early and accurate detection, monitoring, and assessment of plant diseases is important and necessary for farmers, managers, and decision makers.
2. Plant Disease Detection Techniques
Artificial visible investigation, as the most basic direct method in practice, is still being used. However, this approach requires professional knowledge of the relevant plant phenotype and plant pathology. Researchers have developed various computer vision-based diagnostic systems with the help of computer technology to diagnose various crop diseases. These systems feed by the RGB images of the plants or their parts (leaves, fruits, roots, etc.) and then the machine learning algorithms or deep learning models decide whether the image shows a healthy or unhealthy plant [7][8].
Another mainstream, direct plant disease detection technique can be called the biological molecular method [9][10][11]. Biological molecular techniques require detailed sampling and have complex processing methods. Compared with artificial investigation methods, these approaches are more professional and cyclical. These two techniques are basic, significant, efficient and always involve the use of manual plant disease monitoring and detection methods. The processes of all of these manual methods are expensive, time-consuming, and labor-intensive [12]. These shortcomings limit the development and application of artificial methods in large-scale farms. In particular, it is necessary to note that these direct plant disease detection methods are usually performed in the middle to later stages of the infection, of which visible symptoms are typically manifest [13]. In the environment of modern facility agriculture and precision agriculture, the demand for real-time and large-scale detection of plant diseases is becoming increasingly prominent. This time-lag is inevitable and not conducive to early detection.
In the last decade, a number of non-invasive techniques have been developed, which are sensitive, consistent, standard, high throughput, rapid, and cost-effective [13]. Spectroscopy-based, imaging-based, and relevant remote sensing (RS) methods provide reliable and precise technical support for real-time and large-scale plant disease detection and monitoring. There are some links among these non-invasive approaches, which exist side by side and interact. The International Standards Committee has formally accepted methods developed using spectroscopy [14]. The commonly used spectroscopy techniques in plant diseases are mainly focused on visible–near infrared (VIS–NIR), electric impedance, and fluorescence spectroscopy [14][15][16][17]. Thus, imaging-based and RS techniques have attracted considerable attention because they can provide accurate geographic information [12]. Many different imaging sensors, such as digital [18][19], fluorescence [20], thermal [21][22], and multispectral or hyperspectral sensors [23][24][25] have been studied for the detection of symptomatic and asymptomatic plant diseases [10]. The applications of these techniques have been steadily developing from sensor development, image acquisition, and system construction to image segmentation and classification algorithm analysis [26][27].
Hyperspectral technology can sometimes be considered as a part of spectroscopy. The electromagnetic spectrum ranges of hyperspectral sensors mainly concentrate on VIS–NIR (400–1000 nm) and sometimes contain a short wave infrared range (SWIR, 1000–2500 nm). These sensors could acquire spectral information from hundreds of narrow spectral bands [28]. These narrow wavebands have high sensitivity to the subtle plant changes caused by diseases and make it possible to distinguish different disease types and perform early asymptomatic detection. Among many non-invasive plant disease monitoring methods, including both hyperspectral non-imaging and imaging techniques, hyperspectral RS has developed rapidly and has outstanding effects in agriculture research [29]. Except for the common advantages of non-invasive RS techniques, hyperspectral imaging can be implemented in automated systems as an objective method, resulting in a considerably reduced workload
With the changes in world climate and the accelerated development of global trade, the distributions, host ranges, and impacts of plant diseases have expanded continuously, and many of these diseases can still spread or break out after having been under control. In Bangladesh in 2016, the outbreak of wheat blast caused total crop failure with an impact range reaching nearly 15,000 ha [1]. In addition, the report of the Food and Agriculture Organization of United Nations (FAO) shows that the occurrence of only Xylella fastidiosa could cost nearly $104 million a year in wine losses in California alone [2]. Hence, plant diseases are now among the most basic, important, and noteworthy issues in agriculture management.
Whilst the economic losses caused by plant diseases are on the one hand, on the other hand, considering population and food imbalance, it is more serious that the diseases cause food losses. FAO statistics show that to fulfill the food requirements of 9.1 billion population by 2050, a 70% steady increase in agricultural production is needed [3]. However, between 20% and 40% of global crops have been lost annually to pests and diseases in the last 45 years. Furthermore, according to Carvajal-Yepes et al. [4], the average worldwide yield losses caused by pests and diseases in wheat, rich, maize, potatoes, and soybeans are estimated to be 21.5%, 30.0%, 22.6%, 17.2%, and 21.4%, respectively. In addition, diseases spread to large areas upon infection and cause large-scale production reduction, also resulting in a decrease in the quality of agricultural products or even endangering life. Fusarium head blight (FHB) is a prominent example. From 1998 to 2000 alone, the cumulative direct and secondary economic losses from FHB in primary food crops were estimated to be $2.67 billion [5]. In addition, once a plant disease breaks out on a large scale, the damage to the environment is considerable. According to FAO statistics, the consumption of pesticides increased from 3.05 million tonnes in 2000 to 4.09 million tonnes in 2016. Plant diseases are considered as risks because they constantly contribute to significant yield, economic, and environment losses worldwide [6]. Therefore, the early and accurate detection, monitoring, and assessment of plant diseases is important and necessary for farmers, managers, and decision makers.
2. Plant Disease Detection Techniques
Artificial visible investigation, as the most basic direct method in practice, is still being used. However, this approach requires professional knowledge of the relevant plant phenotype and plant pathology. Another mainstream, direct plant disease detection technique can be called the biological molecular method [7][8][9]. Biological molecular techniques require detailed sampling and have complex processing methods. Compared with artificial investigation methods, these approaches are more professional and cyclical. These two techniques are basic, significant, efficient and always involve the use of manual plant disease monitoring and detection methods. The processes of all of these manual methods are expensive, time-consuming, and labor-intensive [10]. These shortcomings limit the development and application of artificial methods in large-scale farms. In particular, it is necessary to note that these direct plant disease detection methods are usually performed in the middle to later stages of the infection, of which visible symptoms are typically manifest [11]. In the environment of modern facility agriculture and precision agriculture, the demand for real-time and large-scale detection of plant diseases is becoming increasingly prominent. This time-lag is inevitable and not conducive to early detection.
In the last decade, a number of non-invasive techniques have been developed, which are sensitive, consistent, standard, high throughput, rapid, and cost-effective [11]. Spectroscopy-based, imaging-based, and relevant remote sensing (RS) methods provide reliable and precise technical support for real-time and large-scale plant disease detection and monitoring. There are some links among these non-invasive approaches, which exist side by side and interact. The International Standards Committee has formally accepted methods developed using spectroscopy [12]. The commonly used spectroscopy techniques in plant diseases are mainly focused on visible–near infrared (VIS–NIR), electric impedance, and fluorescence spectroscopy [12][13][14][15]. Thus, imaging-based and RS techniques have attracted considerable attention because they can provide accurate geographic information [10]. Many different imaging sensors, such as digital [16][17], fluorescence [18], thermal [19][20], and multispectral or hyperspectral sensors [21][22][23] have been studied for the detection of symptomatic and asymptomatic plant diseases [8]. The applications of these techniques have been steadily developing from sensor development, image acquisition, and system construction to image segmentation and classification algorithm analysis [24][25].
Hyperspectral technology can sometimes be considered as a part of spectroscopy. The electromagnetic spectrum ranges of hyperspectral sensors mainly concentrate on VIS–NIR (400–1000 nm) and sometimes contain a short wave infrared range (SWIR, 1000–2500 nm). These sensors could acquire spectral information from hundreds of narrow spectral bands
[26]
. These narrow wavebands have high sensitivity to the subtle plant changes caused by diseases and make it possible to distinguish different disease types and perform early asymptomatic detection. Among many non-invasive plant disease monitoring methods, including both hyperspectral non-imaging and imaging techniques, hyperspectral RS has developed rapidly and has outstanding effects in agriculture research
. Except for the common advantages of non-invasive RS techniques, hyperspectral imaging can be implemented in automated systems as an objective method, resulting in a considerably reduced workload [24][28][3129]. The applications have been classified from the level of satellite images to the macroscopic or molecular level. It can be seen that hyperspectral technologies have exhibited superiority in plant disease monitoring.
3. Conclusions
Plant diseases contribute to significant economic and post-harvest losses in the agricultural production sector worldwide, especially under the influences of the climate changes in recent years. In the continuous research, many effective methods for plant disease detection, monitoring and assessment have been accumulated. Professional visual interpretation, biochemical analysis, and pathological analysis have been well developed. Non-invasive technologies have been paid more attention in recent years, and hyperspectral technology is particularly prominent.
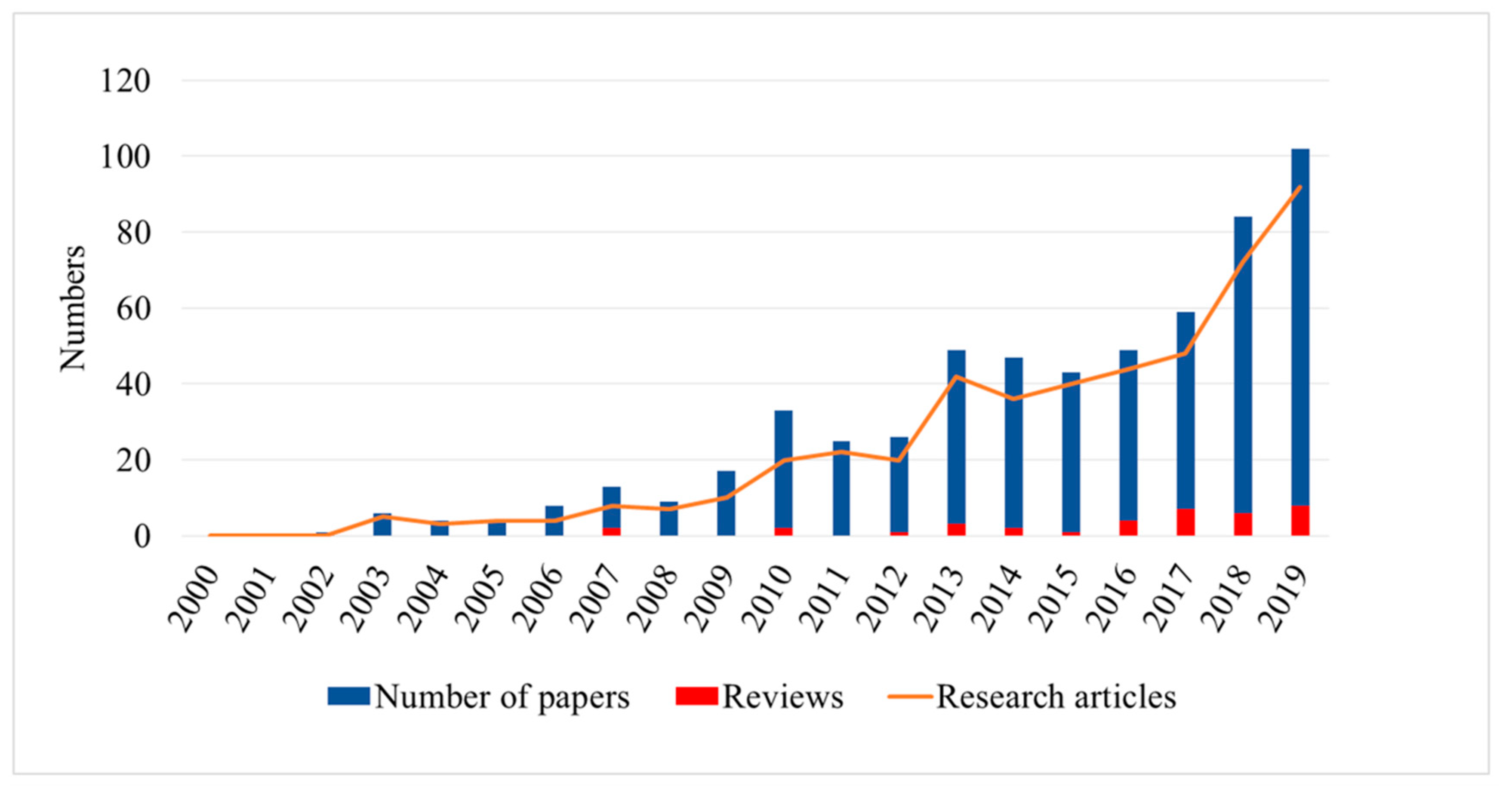
- Hyperspectral technology-based plant disease detection is drawing increasing attention. As shown in Figure 1, hyperspectral-based plant disease analysis technology emerged in 2002 and has been developing rapidly in the following 10 years. It has been developing continuously with the maturity of related technologies in the past 10 years. These developments provide many methods and ideas for future research and analysis, as well as reliable support for plant protection.
- The mainstream technologies are focused on small scales, and satellite payloads require further development and attention. In the past three decades, almost 86% of hyperspectral imaging research has been focused on field and laboratory environments and more concern has been placed on the leaf and canopy scales. However, large-scale accurate analysis is necessary for practical applications. Thus, scale transformation methods for both the spatial and spectral scales require more attention. Although the algorithms for hyperspectral data analysis on small scales can provide technical support for regional or larger scales, it is difficult to achieve large-scale monitoring without the assistance of satellite payload.
- Close attention should be paid to the information integration analysis of satellite scales. After the implementation of targeted hyperspectral satellite missions, big data collection, pre-processing, and analysis will be the priorities. The real-time dynamic monitoring of plant disease at the regional, national, and global scales can be realized only if large-scale data integration analysis is achieved. With the development of multi-source RS data, the fusion of multi-source data may be a development trend in the future.
References
- Moshou, D.; Pantazi, X.E.; Kateris, D.; Gravalos, I. Water stress detection based on optical multisensor fusion with a least squares support vector machine classifier. Biosyst. Eng. 2014, 117, 15–22.
- FAO. New Standards to Curb the Global Spread of Plant Pests and Diseases. Available online: http://www.fao.org/news/story/en/item/1187738/icode/ (accessed on 3 April 2019).
- FAO. Declaration of the World Summit on Food Security; Food and Agriculture Organization: Rome, Italy, 2009.
- Carvajal-Yepes, M.; Cardwell, K.; Nelson, A.; Garrett, K.A.; Giovani, B.; Saunders, D.G.O.; Kamoun, S.; Legg, J.P.; Verdier, V.; Lessel, J.; et al. A global surveillance system for crop diseases. Science 2019, 364, 1237–1239.
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ. 2004, 26, 332–347.
- Barbedo, J.G.A. A review on the main challenges in automatic plant disease identification based on visible range images. Biosyst. Eng. 2016, 144, 52–60.
- Ahmad Almadhor; Hafiz Rauf; Muhammad Lali; Robertas Damaševičius; Bader Alouffi; Abdullah Alharbi; AI-Driven Framework for Recognition of Guava Plant Diseases through Machine Learning from DSLR Camera Sensor Based High Resolution Imagery. Sensors 2021, 21, 3830, 10.3390/s21113830.Ruiz-Ruiz, S.; Ambrós, S.; Vives, M.D.C.; Navarro, L.; Moreno, P.; Guerri, J. Detection and quantitation of Citrus leaf blotch virus by TaqMan real-time RT-PCR. J. Virol. Methods 2009, 160, 57–62. [PubMed]
- Olusola Oluwakemi Abayomi‐Alli; Robertas Damaševičius; Sanjay Misra; Rytis Maskeliūnas; Cassava disease recognition from low‐quality images using enhanced data augmentation model and deep learning. Expert Systems 2021, 38, e12746, 10.1111/exsy.12746.Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–12.
- Ruiz-Ruiz, S.; Ambrós, S.; Vives, M.D.C.; Navarro, L.; Moreno, P.; Guerri, J. Detection and quantitation of Citrus leaf blotch virus by TaqMan real-time RT-PCR. J. Virol. Methods 2009, 160, 57–62. [PubMed]Yvon, M.; Thébaud, G.; Alary, R.; Labonne, G. Specific detection and quantification of the phytopathogenic agent ‘Candidatus Phytoplasma prunorum’. Mol. Cell. Probes 2009, 23, 227–234.
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–12.Ali, M.M.; Bachik, N.A.; Muhadi, N.A.; Yusof, T.N.T.; Gomes, C. Non-destructive techniques of detecting plant diseases: A review. Physiol. Mol. Plant Pathol. 2019, 108, 101426–101437.
- Yvon, M.; Thébaud, G.; Alary, R.; Labonne, G. Specific detection and quantification of the phytopathogenic agent ‘Candidatus Phytoplasma prunorum’. Mol. Cell. Probes 2009, 23, 227–234.Golhani, K.; Balasundram, S.K.; Vadamalai, G.; Pradhan, B. A review of neural networks in plant disease detection using hyperspectral data. Inf. Process. Agric. 2018, 5, 354–371.
- Ali, M.M.; Bachik, N.A.; Muhadi, N.A.; Yusof, T.N.T.; Gomes, C. Non-destructive techniques of detecting plant diseases: A review. Physiol. Mol. Plant Pathol. 2019, 108, 101426–101437.Purcell, D.E.; O’Shea, M.G.; Johnson, R.A.; Kokot, S. Near-infrared spectroscopy for the prediction of disease ratings for Fiji leaf gall in sugarcane clones. Appl. Spectrosc. 2009, 63, 450–457.
- Golhani, K.; Balasundram, S.K.; Vadamalai, G.; Pradhan, B. A review of neural networks in plant disease detection using hyperspectral data. Inf. Process. Agric. 2018, 5, 354–371.Shrestha, S.; Deleuran, L.C.; Gislum, R. Classification of different tomato seed cultivars by multispectral visible-near infrared spectroscopy and chemometrics. J. Spectr. Imaging 2016, 5, 1–8.
- Purcell, D.E.; O’Shea, M.G.; Johnson, R.A.; Kokot, S. Near-infrared spectroscopy for the prediction of disease ratings for Fiji leaf gall in sugarcane clones. Appl. Spectrosc. 2009, 63, 450–457.Borges, E.; Matos, A.P.; Cardoso, J.M.; Correia, C.; Vasconcelos, T.; Gomes, N. Early detection and monitoring of plant diseases by Bioelectric Impedance Spectroscopy. In Proceedings of the 2012 IEEE 2nd Portuguese Meeting in Bioengineering (ENBENG), Coimbra, Portugal, 23–25 February 2012; pp. 1–4.
- Shrestha, S.; Deleuran, L.C.; Gislum, R. Classification of different tomato seed cultivars by multispectral visible-near infrared spectroscopy and chemometrics. J. Spectr. Imaging 2016, 5, 1–8.Belasque, J., Jr.; Gasparoto, M.C.G.; Marcassa, L.G. Detection of mechanical and disease stresses in citrus plants by fluorescence spectroscopy. Appl. Opt. 2008, 47, 1922–1926. [PubMed]
- Borges, E.; Matos, A.P.; Cardoso, J.M.; Correia, C.; Vasconcelos, T.; Gomes, N. Early detection and monitoring of plant diseases by Bioelectric Impedance Spectroscopy. In Proceedings of the 2012 IEEE 2nd Portuguese Meeting in Bioengineering (ENBENG), Coimbra, Portugal, 23–25 February 2012; pp. 1–4.Dhingra, G.; Kumar, V.; Joshi, H.D. Study of digital image processing techniques for leaf disease detection and classification. Multimed. Tools Appl. 2017, 77, 19951–20000.
- Belasque, J., Jr.; Gasparoto, M.C.G.; Marcassa, L.G. Detection of mechanical and disease stresses in citrus plants by fluorescence spectroscopy. Appl. Opt. 2008, 47, 1922–1926. [PubMed]Barbedo, J.G.A. Digital image processing techniques for detecting, quantifying and classifying plant diseases. Springerplus 2013, 2, 660–671.
- Dhingra, G.; Kumar, V.; Joshi, H.D. Study of digital image processing techniques for leaf disease detection and classification. Multimed. Tools Appl. 2017, 77, 19951–20000.Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus Huanglongbing. Front. Plant Sci. 2017, 8, 1509–1519.
- Barbedo, J.G.A. Digital image processing techniques for detecting, quantifying and classifying plant diseases. Springerplus 2013, 2, 660–671.Raza, S.-e.-A.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 2015, 10, e0123262–e0123281.
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus Huanglongbing. Front. Plant Sci. 2017, 8, 1509–1519.Sankaran, S.; Maja, J.M.; Buchanon, S.; Ehsani, R. Huanglongbing (citrus greening) detection using visible, near infrared and thermal imaging techniques. Sensors 2013, 13, 2117–2130.
- Raza, S.-e.-A.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 2015, 10, e0123262–e0123281.Söderström, M.; Börjesson, T.; Roland, B.; Stadig, H. Modelling within-field variations in deoxynivalenol (DON) content in oats using proximal and remote sensing. Precis. Agric. 2014, 16, 1–14.
- Sankaran, S.; Maja, J.M.; Buchanon, S.; Ehsani, R. Huanglongbing (citrus greening) detection using visible, near infrared and thermal imaging techniques. Sensors 2013, 13, 2117–2130.Wahabzada, M.; Mahlein, A.-K.; Bauckhage, C.; Steiner, U.; Oerke, E.-C.; Kersting, K. Plant phenotyping using probabilistic topic models: Uncovering the hyperspectral language of plants. Sci. Rep. 2016, 6, 22482–22492.
- Söderström, M.; Börjesson, T.; Roland, B.; Stadig, H. Modelling within-field variations in deoxynivalenol (DON) content in oats using proximal and remote sensing. Precis. Agric. 2014, 16, 1–14.López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276.
- Wahabzada, M.; Mahlein, A.-K.; Bauckhage, C.; Steiner, U.; Oerke, E.-C.; Kersting, K. Plant phenotyping using probabilistic topic models: Uncovering the hyperspectral language of plants. Sci. Rep. 2016, 6, 22482–22492.Mahlein, A.-K.; Hammersley, S.; Oerke, E.-C.; Dehne, H.-W.; Goldbach, H.; Grieve, B. Supplemental blue LED lighting array to improve the signal quality in hyperspectral imaging of plants. Sensors 2015, 15, 12834–12840.
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276.Al-Saddik, H.; Simon, J.-C.; Cointault, F. Development of spectral disease indices for ‘Flavescence Doree’ grapevine disease identification. Sensors 2017, 17, 2772. [PubMed]
- Mahlein, A.-K.; Hammersley, S.; Oerke, E.-C.; Dehne, H.-W.; Goldbach, H.; Grieve, B. Supplemental blue LED lighting array to improve the signal quality in hyperspectral imaging of plants. Sensors 2015, 15, 12834–12840.Ghamisi, P.; Plaza, J.; Chen, Y.; Li, J.; Plaza, A. Advanced Supervised Spectral Classifiers for Hyperspectral Images A review. IEEE Geosci. Remote Sens. Mag. 2017, 5, 8–32.
- Al-Saddik, H.; Simon, J.-C.; Cointault, F. Development of spectral disease indices for ‘Flavescence Doree’ grapevine disease identification. Sensors 2017, 17, 2772. [PubMed]Zhou, W.; Zhang, J.; Zou, M.; Liu, X.; Du, X.; Wang, Q.; Liu, Y.; Liu, Y.; Li, J. Prediction of cadmium concentration in brown rice before harvest by hyperspectral remote sensing. Environ. Sci. Pollut. Res. Int. 2019, 26, 1848–1856. [PubMed]
- Ghamisi, P.; Plaza, J.; Chen, Y.; Li, J.; Plaza, A. Advanced Supervised Spectral Classifiers for Hyperspectral Images A review. IEEE Geosci. Remote Sens. Mag. 2017, 5, 8–32.Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2017, 125, 5–20.
- Zhou, W.; Zhang, J.; Zou, M.; Liu, X.; Du, X.; Wang, Q.; Liu, Y.; Liu, Y.; Li, J. Prediction of cadmium concentration in brown rice before harvest by hyperspectral remote sensing. Environ. Sci. Pollut. Res. Int. 2019, 26, 1848–1856. [PubMed]Virlet, N.; Sabermanesh, K.; Sadeghi-Tehran, P.; Hawkesford, M.J. Field Scanalyzer: An automated robotic field phenotyping platform for detailed crop monitoring. Funct. Plant Biol. 2017, 44, 143–153. [PubMed]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2017, 125, 5–20.
- Virlet, N.; Sabermanesh, K.; Sadeghi-Tehran, P.; Hawkesford, M.J. Field Scanalyzer: An automated robotic field phenotyping platform for detailed crop monitoring. Funct. Plant Biol. 2017, 44, 143–153. [PubMed]
