The incidence of obesity and colorectal cancer (CRC) has risen rapidly in recent decades. More than 650 million obese and 2 billion overweight individuals are currently living in the world. CRC is the third most common cancer. Obesity is regarded as one of the key environmental risk factors for the pathogenesis of CRC. In the present review, we mainly focus on the epidemiology of obesity and CRC in the world, the United States, and China. We also summarize the molecular mechanisms linking obesity to CRC in different aspects, including nutriology, adipokines and hormones, inflammation, gut microbiota, and bile acids. The unmet medical needs for obesity-related CRC are still remarkable. Understanding the molecular basis of these associations will help develop novel therapeutic targets and approaches for the treatment of obesity-related CRC.
- obesity
- colorectal cancer
- epidemiology
- hormones
- inflammation
- gut microbiota
- bile acids
- Introduction
1. Introduction
Obesity is associated with various metabolic disorders [1], such as diabetes, non-alcoholic fatty liver diseases, cardiovascular diseases, hypertension, and obstructive sleep apnea syndrome, as well as with some cancers [2–4][2][3][4], including esophageal adenocarcinoma, multiple myeloma, cardia cancer, colorectal cancer (CRC), cholangiocarcinoma, pancreatic cancer, breast cancer, endometrial cancer, ovarian cancer, and renal cancer. Obesity is closely related to increased incidence and progression of these cancers, and it is estimated to cause about 20% cancer-associated deaths [5,6][5][6].
- The Epidemiology of Obesity and CRC
2. The Epidemiology of Obesity and CRC
2.1. The Epidemiology of Obesity
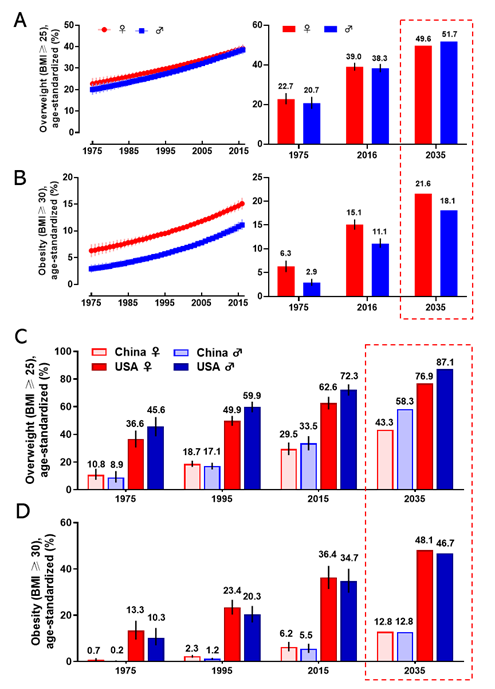
Obesity has become a worldwide health burden. Body mass index (BMI) is a typical value derived from the weight and height to define overweight (25 ≥ BMI < 30) and obesity (BMI ≥ 30) in adult men and women. According to the World Health Organization (WHO) reports, the rate of obesity has nearly tripled globally since 1975. In 2016, about 2 billion adults were overweight, and more than 650 million of them were obese. The worldwide prevalence of overweight was 22.7% in women, and 20.7% in men in 1975; it was markedly increasing to 39.0% and 38.3% in 2016 and it will arrive at 49.6% and 51.7% in women and men respectively in 2035 (Figure 1A). The global prevalence of obesity was 6.3% for women, and 2.9% for men in 1975; this proportion rose to 15.1% (women) and 11.1% (men) in 2016 and will reach 21.6% (women) and 18.1% (men) in 2035 (Figure 1B). The regions with the highest prevalence of obesity are American and European [7]. With an estimated 89.6 million obese, China has the largest population of obese in the world [8]. Since 1975, the prevalence of overweight and obesity in men and women every two decades in China and the United States is shown in Figure 1C,D. If the current trends continue, as predicted, the prevalence of overweight and obesity in the USA will reach 76.9% (women) and 87.1% (men), and 48.1% (women) and 46.7% (men) in 2035, respectively. The prevalence of overweight and obesity in China will reach 43.3% (women) and 58.3% (men), and 12.8% both in women and men in 2035, respectively. Obesity has been a serious threat to human health and a heavy financial burden of health insurance, which affects the normal physiological function of humans.
Figure 1. The prevalence of overweight and obesity in women and men. The global prevalence of overweight (A) and obesity (B) in women and men from 1975 to 2016 (left), and the value in 1975 and 2016, and the prediction in 2035 (right). The prevalence of overweight (C) and obesity (D) for women and men in 1975, 1995, 2015, and the prediction in 2035 in China and the United States. The predicted values were boxed with the dashed line. Data are from the WHO website.
2.2. The Epidemiology of Colorectal Cancer
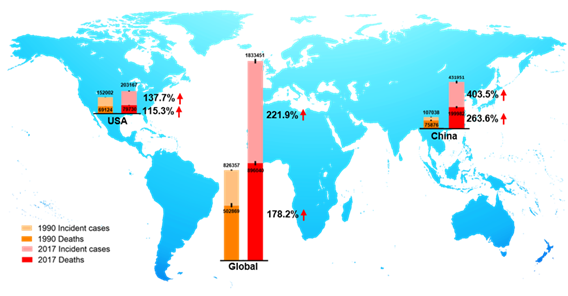
CRC is the third most prevalent cancer and is also the third leading cause of cancer-associated death globally in both men and women from the 1980s [9,10][9][10]. In 2018, there were 1.8 million new CRC cases, causing 0.86 million deaths worldwide, according to global cancer statistics [11]. Currently, there are more than 1 million CRC survivors in America. Based on American Cancer Society statistics 2020, the estimated numbers of new CRC cases and deaths in the United States are approximately 150,000 and 54,000, respectively [9]. Global Burden of Disease Study 2017 (GBD 2017) reported the numbers of incident cases and deaths of CRC globally, in the USA, and China from 1990 to 2017, as shown in Figure 2. We observed that in 1990, incident cases and deaths of CRC are about 107,000 and 76,000 in China, and about 432,000 and 200,000 in 2017, respectively [12]. Over the past 27 years, the incidence cases of CRC have doubled worldwide, and been increased three times in China. The unmet medical needs of CRC have been a growing public health issue.
Figure 2. The incident cases and deaths of colorectal cancer (CRC) from 1990 to 2017 in the world, the USA, and China. Data are from Global Burden of Disease Study 2017 (GBD 2017).
Growing epidemiological data indicated a strong positive correlation between obesity and colorectal carcinogenesis [13–15][13][14][15]. General obesity causes a higher risk of colon cancer in males compared to females, and it has a stronger association with colon cancer than rectal cancer in both genders [16,17][16][17]. Dose-response meta-analysis reported that body weight gain of 10 kg was accompanied by approximately 8% increased risk of CRC [18,19][18][19]. Early-life obese individuals are at greater risk of developing CRC in adulthood [13,18,20][20]. As expected, body weight loss by bariatric surgery reduces about 27% risk of CRC [21,22][21][22]. Understanding the association between body weight and the risk of CRC is essential to guide body weight management for CRC patients.
- The Mechanistic Insights Linking Obesity with CRC
3. The Mechanistic Insights Linking Obesity with CRC
Although increasing evidence suggests the positive correlation between obesity and CRC, the underlying molecular mechanisms are still not fully understood. Obesity-induced abnormal lipid metabolism, adipokines and hormones, chronic inflammation, gut microbiota dysbiosis, and disrupted bile acid homeostasis may play important roles in the complex metabolic regulation of CRC tumorigenesis.
3.1. Nutriology
Obesity is excess body adiposity, especially ectopic deposition of white adipose tissues. Mature adipocytes (white adipocytes) act as an energy bank to store and release energy [23]. Systemic and local energy metabolic homeostasis is primarily controlled by adipocytes [24,25][24][25]. Tumor cell growth requires a lot of energy. Understanding whether and how tumor cells get energy directly from the adipocytes helps develop new therapeutic strategies.
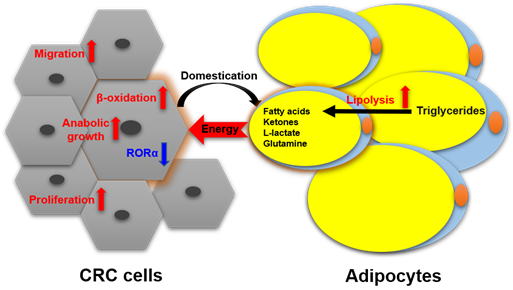
Nieman et al. reported [26] [26] that intra-abdominal tumors are more likely home to and proliferate in the omentum majus, which is an organ mainly composed of white adipocytes. Adipocyte-tumor cell coculture induces lipolysis in adipocytes and β-oxidation in tumor cells, resulting in the rapid proliferation of tumor cells. An emerging concept in cancer metabolism is that the adipocytes surrounding tumors provide energy or nutrients for the anabolic growth of cancer cells [27–29][27][28][29]. We validated this concept by observing more adipocytes surrounding colorectal tumor tissues than normal tissues in clinical pathological sections [30]. In in vitro experiments, we found adipocyte-conditioned medium promotes proliferation and migration of colon cancer cells (SW480 and C26) through retinoic acid-related orphan α (RORα), which is a lipid metabolism-associated nuclear receptor [30]. Sadahiro et al. reported that primary adipocytes, preadipocytes, and adipose tissues enhanced the growth of colon cancer cells (CACO-2, T84, and HT29) in the cocultured system [31]. Adipocytes are part of tumor microenvironment. It is domesticated to produce and transfer energy-rich metabolites to tumor cells, including free fatty acids, glutamine, ketones, and L-lactate, and promote the growth and migration of tumors [29]. The summarized crosstalk between CRC cells and adipocytes in nutriology is shown in Figure 3. CRC cells domesticate adipocytes which supply energy or nutrients to cancer cells for further rapid growth.
Figure 3. The crosstalk between CRC cells and adipocytes in nutriology.
Cancer cachexia (CC), also known as wasting syndrome, is characterized by weight loss in cancer patients. It is caused by tumor factors and regulated by catabolic metabolism [32]. This complex multifactorial metabolic syndrome often accompanies increased lipolysis in adipose tissues. A total of 54% of colon cancer patients suffer from CC that causes about 20% of cancer-associated deaths [33,34][33][34]. It might be a piece of evidence that adipose tissues provide nutrients for tumor growth in systemic nutriology.
Understanding the role of adipocytes in tumor microenvironment is critical to the discovery of new strategies. Targeted blocking energy transfers might be novel therapies for the treatment of CRC.
3.2. Adipokines and Hormones
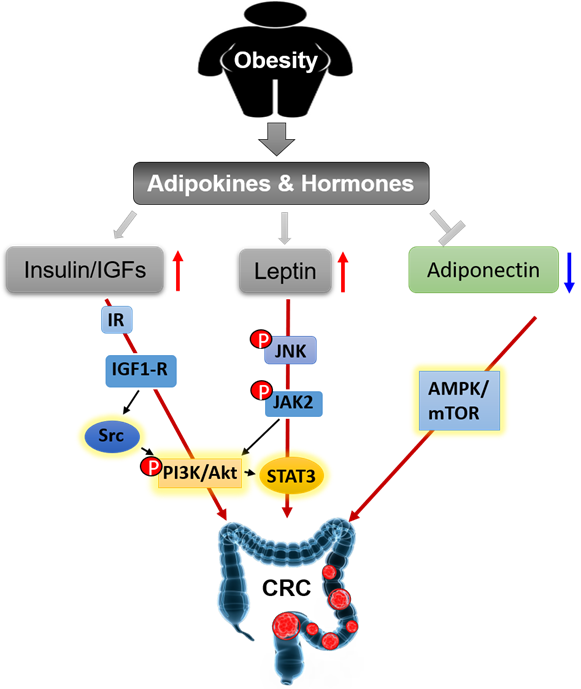
Adipose tissues have long been thought to be energy storage tissues as the body accumulates excess nutrients and to resist cold temperature [35]. It is currently regarded as a highly active endocrine or metabolic organ [36]. It liberates more than twenty kinds of hormones and adipokines, such as estrogens, insulin, insulin-like growth factors (IGFs), leptin, adiponectin, apelin, visfatin, resistin, chemerin, omentin, nesfatin, vaspin, inflammatory cytokines (e.g., tumor necrosis factor-alpha (TNF-α), chemokine (C-C motif) ligand 2 (CCL2), plasminogen activator inhibitor-1(PAI-1), and the interleukin families (e.g., IL-1β, IL-6, IL-8, IL-10, IL-27, and IL-31). The related adipokines and hormones and their functions in the development and progression of CRC are introduced below.
3.2.1. Insulin and IGFs
The insulin/IGFs system is a major driver in the pathogenesis of CRC. This system consists of insulin, insulin receptor (IR), IGF-1 and -2, IGF-1 receptor (IGF-1R), IGF-binding protein (IGFBP)-1 and -2, and IR substrates (IRS) 1 and 2 [37]. Overweight generally increases the levels of insulin and IGF-I and decreases the levels of IGFBP-1 and IGFBP-2 in serum [38]. Insulin and IGFs have been reported to promote the proliferation of HCT116 and HT29 colon cancer cell lines through activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway [39–41][39][40][41]. PI3K/Akt signal pathway is an important therapeutic target for treating colon cancer [42,43][42][43]. Tyrosine-protein kinase Src is a non-receptor tyrosine kinase encoded by the SRC gene in humans [44]. It regulates PI3K/Akt pathway through phosphorylation of PI3K. Src also plays a critical role in the transformation and growth of CRC cells. Knockdown or inhibition of Src inhibited cell metastasis and proliferation in human cancer cells SW480 and HT29 [45,46][45][46]. Phosphorylated IR (pIR) was highly expressed in low-grade colorectal adenocarcinoma, which indicated activation of IR is an early event in CRC tumorigenesis [47]. The expression levels of IGF1 and IGF-1R were increased in colorectal carcinomas, compared with normal colonic mucosa. Overexpression and activation of IGF1-R can activate Src, leading to elevated proliferation and migration of colon cancer in vitro [48]. Renehan et al. reported that IGF-2 SD scores (SDS) were slightly increased in CRC patients compared to healthy controls, and it showed a more dramatic increase in advanced colonic carcinomas compared with earlier stages, but the scores dropped down immediately after curative resection [37]. Taken together, the insulin and IGFs system plays an important role in the pathogenesis and prognosis of CRC through independent or joint signaling networks.
3.2.2. Leptin and Adiponectin
Leptin, a peptide hormone encoded by Ob gene, is mainly secreted by adipose tissues, which informs the brain that the energy runs out in the liver through binding to leptin receptors [49–51][49][50][51]. Obese individuals have high levels of circulating leptin, because of leptin resistance [52]. Leptin is a risk factor for CRC [53,54][53][54]. The expression of leptin is increased in human colorectal tumors and is associated with tumor progression and clinic pathological parameters [55]. Soluble leptin receptor (sOB-R) is a potential marker of leptin resistance. European Prospective Investigation into Cancer and Nutrition (EPIC) cohort also showed circulating sOB-R inversely correlated with the risk of CRC [56,57][56][57]. In azoxymethane (AOM) induced murine colon cancer model, Leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice showed inhibited tumor growth through Wnt signaling pathway [54]. Leptin increases cell proliferation and prevents apoptosis in HT29 cells through phosphorylation of c-Jun NH2-terminal kinase (JNK). JNK phosphorylation stimulates a cascade of downstream protein phosphorylation, including Janus kinase 2 (JAK2) and PI3K/Akt, then activates signal transducer and activator of transcription (STAT3) and activator protein 1 (AP-1) [58]. Leptin promotes cell migration and lamellipodial extension in human CRC cell lines LS174T and HM7 through activation of Rho family of GTPases, including ras homolog family member A (RhoA), cell division control protein 42 (Cdc42), and ras-related C3 botulinum toxin substrate 1 (Rac1) [59]. Adipose tissues secreted leptin inhibits mitochondrial respiration rate in HCT116 cells [60,61][60][61]. Leptin provides a link between obesity and the risk of CRC, it is a sensitive marker of obesity-induced hormonal aberrations and may be directly involved in CRC tumorigenesis.
Adiponectin is a protein hormone encoded by ADIPOQ gene in humans [62]. It is one of the most abundant hormones released from adipose tissues and performs an essential function in obesity-associated cancers. The expression and circulating levels of adiponectin are reduced in most obese individuals and animal models of obesity [63–65][63][64][65]. Epidemiology studies showed that decreased plasma adiponectin levels are inversely correlated with the risk of colon cancer [66,67][66][67]. Adiponectin knockout (APNKO) mice exhibited more tumor numbers and areas in dextran sodium sulfate (DSS) and 1,2-dimethylhydrazine (DMH) induced colon cancer model through increasing the differentiation from epithelial cells to goblet cells and inhibiting goblet cell apoptosis. It indicated that adiponectin protected against chronic inflammation-induced colon cancer [68]. High-fat diet treated mice had more and larger colorectal tumors than chow-diet mice. Adiponectin administration decreased tumor growth through inhibiting angiogenesis [69,70][69][70]. In vitro experiments, adiponectin inhibits colon cancer cell growth in adiponectin receptor (AdipoR1- and -R2) positive HCT116, HT29, and LoVo cells through the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) signaling pathway [71,72][71][72]. Moon et al. demonstrated that adiponectin directly regulated cell proliferation, migration, adhesion, and colon formation through regulation of metabolism, inflammation, and cell cycle in MCA38, HT29, HCT116, and LoVo cells [69]. These results indicate the potential inhibitory effect of adiponectin on the development of CRC. Together, leptin and adiponectin generally show opposite molecular effects on obesity and cellular behaviors. They are relevant but reverse players in obesity-related CRC.
3.2.3. Estrogens
It is well established that estrogen contributes to obesity-associated hormone-responsive cancers, especially breast cancer [73,74][73][74]. The role of estrogen in obesity-associated CRC is complicated. First, estrogens have been found to reduce the risk of CRC [75]. Hormone replacement therapy confers protection against CRC, especially for lean women, as indicated by epidemiological data [76]. Estrogen replacement therapy in postmenopausal women reduces CRC-related mortality [77]. These cohort studies indicated estrogens may play a protective role in the pathogenesis of CRC. Interestingly, adipose tissues are also partial source of estrogen in addition to ovaries. Plasma estrogen levels are increased in obese men and postmenopausal women, because adipose tissue aromatase transforms androgenic precursors to estrogens [78]. However, several studies have shown that high BMI increased the risk of CRC in men and premenopausal women, but not postmenopausal women [79,80][79][80]. Adiposity also positively correlates with blood insulin, leading to increased IGF-1. The inducible effect of insulin/IGF-1 axis on CRC appears to be compromised by estrogen released from adiposity in postmenopausal women. In premenopausal women, the primary source of estrogen is ovary compared to adiposity. Thus, more hormone supplement cannot provide more benefits [79,81][81]. This concept has been suggested by several cohort studies showing a positive correlation between BMI and CRC risk in younger women (<55-year-old) but not in older women [79,80,82][80][82]. This association was further confirmed by the study subjected between BMI and CRC risk in premenopausal and postmenopausal women. The risk of CRC in postmenopausal women is independent of BMI [83]. Although the relationship among hormones, obesity, and CRC is not fully understood, these observations and reasonable speculation emphasize the same importance of weight control in both genders.
The effect of estrogen is mediated by its receptors, estrogen receptor (ER)-α and ER-β. The expression of ER-α is very low in normal colorectal tissues. However, the ER-α expression is increased with the development of colon cancer, and it positively correlates with CRC stages and worse survival [75]. ER-β is enriched in colon tissues [84]. The expression of ER-β is lower in colon tumor tissues compared with normal tissues and inversely correlates with the progression of CRC [85,86][85][86]. ER-β overexpression induced cell-cycle arrest and inhibited cell proliferation and tumor growth in SW480 cells and mouse xenografts model [87]. In the ApcMin/+ mouse model, estrogen treatment protected against CRC and increased the ratio of ER-β to ER-α [88]. Ablation of ER-β in ApcMin/+ mice significantly increased tumor formation, and treatment with estrogen could not prevent this phenotype [89]. These results indicate that ER-β is responsible for the protective effect of estrogens on colon tumorigenesis.
We summarize the signaling pathways of obesity-secreted adipokines and hormones in the pathogenesis of CRC (Figure 4).
Figure 4. Obesity secreted adipokines and hormones contributing to pathogenesis of CRC.
References
- Cho, E.J.; Kim, S.M. Explantation of Adjustable Gastric Bands: An Observation Study of 10 Years of Experience at a Tertiary Center. Yonsei Med. J. 2019, 60, 782–790.
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477.
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working, G. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798.
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46.
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154.
- Xu, Y.X.Z.; Mishra, S. Obesity-Linked Cancers: Current Knowledge, Challenges and Limitations in Mechanistic Studies and Rodent Models. Cancers 2018, 10, 523.
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab.olism 2019, 92, 6–10.
- Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Li, S.; Peppelenbosch, M.P.; Smits, R. Bacterial biofilms as a potential contributor to mucinous colorectal cancer formation. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 74–79.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Collaborators, G.B.D.C.C. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933.
- Garcia, H.; Song, M. Early-life obesity and adulthood colorectal cancer risk: A meta-analysis. Rev. Panam. Salud Publica 2019, 43, e3.
- Dong, Y.; Zhou, J.; Zhu, Y.; Luo, L.; He, T.; Hu, H.; Liu, H.; Zhang, Y.; Luo, D.; Xu, S.; et al. Abdominal obesity and colorectal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37, doi:10.1042/BSR20170945.
- Lee, J.; Meyerhardt, J.A.; Giovannucci, E.; Jeon, J.Y. Association between body mass index and prognosis of colorectal cancer: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0120706.
- Jochem, C.; Leitzmann, M. Obesity and Colorectal Cancer. Recent Results Cancer Res. 2016, 208, 17–41.
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947.
- Schlesinger, S.; Aleksandrova, K.; Abar, L.; Vieria, A.R.; Vingeliene, S.; Polemiti, E.; Stevens, C.A.T.; Greenwood, D.C.; Chan, D.S.M.; Aune, D.; et al. Adult weight gain and colorectal adenomas-a systematic review and meta-analysis. Ann. Oncol. 2017, 28, 1217–1229.
- Chen, Q.; Wang, J.; Yang, J.; Jin, Z.; Shi, W.; Qin, Y.; Yu, F.; He, J. Association between adult weight gain and colorectal cancer: A dose-response meta-analysis of observational studies. Int. J. Cancer 2015, 136, 2880–2889.
- Schlesinger, S.; Lieb, W.; Koch, M.; Fedirko, V.; Dahm, C.C.; Pischon, T.; Nothlings, U.; Boeing, H.; Aleksandrova, K. Body weight gain and risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Obes. Rev. 2015, 16, 607–619.
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014, 149, 275–287.
- Afshar, S.; Kelly, S.B.; Seymour, K.; Lara, J.; Woodcock, S.; Mathers, J.C. The effects of bariatric surgery on colorectal cancer risk: Systematic review and meta-analysis. Obes. Surg. 2014, 24, 1793–1799.
- Xu, P.; Li, J.; Liu, J.; Wang, J.; Wu, Z.; Zhang, X.; Zhai, Y. Mature adipocytes observed to undergo reproliferation and polyploidy. FEBS Open Bio 2017, 7, 652–658.
- Xu, P.; Dai, S.; Wang, J.; Zhang, J.; Liu, J.; Wang, F.; Zhai, Y. Preventive obesity agent montmorillonite adsorbs dietary lipids and enhances lipid excretion from the digestive tract. Sci. Rep. 2016, 6, 19659.
- Xu, P.; Zhai, Y.; Wang, J. The Role of PPAR and Its Cross-Talk with CAR and LXR in Obesity and Atherosclerosis. Int. J. Mol. Sci. 2018, 19, 1260.
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–503.
- Villanueva, M.T. Gynecological cancer: Home is where the fat is. Nat. Rev. Clin. Oncol. 2011, 9, 6.
- Martin-Padura, I.; Gregato, G.; Marighetti, P.; Mancuso, P.; Calleri, A.; Corsini, C.; Pruneri, G.; Manzotti, M.; Lohsiriwat, V.; Rietjens, M.; et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res. 2012, 72, 325–334.
- Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Power surge: Supporting cells “fuel” cancer cell mitochondria. Cell Metab. 2012, 15, 4–5.
- Xiao, L.; Wang, J.; Li, J.; Chen, X.; Xu, P.; Sun, S.; He, D.; Cong, Y.; Zhai, Y. RORalpha inhibits adipocyte-conditioned medium-induced colorectal cancer cell proliferation and migration and chick embryo chorioallantoic membrane angiopoiesis. Am. J. Physiol. Cell Physiol. 2015, 308, C385–C396.
- Amemori, S.; Ootani, A.; Aoki, S.; Fujise, T.; Shimoda, R.; Kakimoto, T.; Shiraishi, R.; Sakata, Y.; Tsunada, S.; Iwakiri, R.; et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G923–G929.
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29.
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495.
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179.
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190.
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74.
- Vigneri, P.G.; Tirro, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230.
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol. Metab. 2006, 17, 328–336.
- Chen, J.; Katsifis, A.; Hu, C.; Huang, X.F. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr. Drug Discov. Technol. 2011, 8, 119–125.
- Huang, X.F.; Chen, J.Z. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009, 10, 610–616.
- Watkins, L.F.; Lewis, L.R.; Levine, A.E. Characterization of the synergistic effect of insulin and transferrin and the regulation of their receptors on a human colon carcinoma cell line. Int. J. Cancer 1990, 45, 372–375.
- Garcia-Echeverria, C.; Sellers, W.R. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008, 27, 5511–5526.
- Chen, J. Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 306–307.
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25.
- Zhu, S.; Bjorge, J.D.; Fujita, D.J. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007, 67, 10129–10137.
- Nam, J.S.; Ino, Y.; Sakamoto, M.; Hirohashi, S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin. Cancer Res. 2002, 8, 2430–2436.
- Abbruzzese, C.; Diodoro, M.G.; Sperduti, I.; Mileo, A.M.; Pattaro, G.; De Salvo, L.; Cosimelli, M.; Perrotti, N.; Paggi, M.G. Detection of phosphorylated insulin receptor in colorectal adenoma and adenocarcinoma: Implications for prognosis and clinical outcome. J. Cell Physiol. 2015, 230, 562–567.
- Sekharam, M.; Nasir, A.; Kaiser, H.E.; Coppola, D. Insulin-like growth factor 1 receptor activates c-SRC and modifies transformation and motility of colon cancer in vitro. Anticancer Res. 2003, 23, 1517–1524.
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and Metab.olic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124.
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352.
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metab.olism. Molecules 2019, 24, 2545.
- Engin, A. Diet-Induced Obesity and the Mechanism of Leptin Resistance. Adv. Exp. Med. Biol. 2017, 960, 381–397.
- Modzelewska, P.; Chludzinska, S.; Lewko, J.; Reszec, J. The influence of leptin on the process of carcinogenesis. Contemp. Oncol. 2019, 23, 63–68.
- Endo, H.; Hosono, K.; Uchiyama, T.; Sakai, E.; Sugiyama, M.; Takahashi, H.; Nakajima, N.; Wada, K.; Takeda, K.; Nakagama, H.; Nakajima, A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011, 60, 1363–1371.
- Koda, M.; Sulkowska, M.; Kanczuga-Koda, L.; Surmacz, E.; Sulkowski, S. Overexpression of the obesity hormone leptin in human colorectal cancer. J. Clin. Pathol. 2007, 60, 902–906.
- Aleksandrova, K.; Schlesinger, S.; Fedirko, V.; Jenab, M.; Bueno-de-Mesquita, B.; Freisling, H.; Romieu, I.; Pischon, T.; Kaaks, R.; Gunter, M.J.; et al. Metab.olic Mediators of the Association Between Adult Weight Gain and Colorectal Cancer: Data From the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Am. J. Epidemiol. 2017, 185, 751–764.
- Aleksandrova, K.; Boeing, H.; Jenab, M.; Bueno-de-Mesquita, H.B.; Jansen, E.; van Duijnhoven, F.J.; Rinaldi, S.; Fedirko, V.; Romieu, I.; Riboli, E.; et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 2012, 72, 5328–5337.
- Ogunwobi, O.O.; Beales, I.L. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int. J. Colorectal. Dis. 2007, 22, 401–409.
- Jaffe, T.; Schwartz, B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int. J. Cancer 2008, 123, 2543–2556.
- Yehuda-Shnaidman, E.; Nimri, L.; Tarnovscki, T.; Kirshtein, B.; Rudich, A.; Schwartz, B. Secreted human adipose leptin decreases mitochondrial respiration in HCT116 colon cancer cells. PLoS ONE 2013, 8, e74843.
- Ristic, B.; Bhutia, Y.D.; Ganapathy, V. Cell-surface G-protein-coupled receptors for tumor-associated Metab.olites: A direct link to mitochondrial dysfunction in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 246–257.
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321.
- Polito, R.; Nigro, E.; Elce, A.; Monaco, M.L.; Iacotucci, P.; Carnovale, V.; Comegna, M.; Gelzo, M.; Zarrilli, F.; Corso, G.; et al. Adiponectin Expression Is Modulated by Long-Term Physical Activity in Adult Patients Affected by Cystic Fibrosis. Mediators Inflamm. 2019, 2019, 2153934.
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913.
- De Rosa, A.; Monaco, M.L.; Capasso, M.; Forestieri, P.; Pilone, V.; Nardelli, C.; Buono, P.; Daniele, A. Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. Eur. J. Endocrinol. 2013, 169, 37–43.
- Wei, E.K.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Mantzoros, C.S. Low plasma adiponectin levels and risk of colorectal cancer in men: A prospective study. J. Natl. Cancer Inst. 2005, 97, 1688–1694.
- Kelesidis, I.; Kelesidis, T.; Mantzoros, C.S. Adiponectin and cancer: A systematic review. Br. J. Cancer 2006, 94, 1221–1225.
- Saxena, A.; Baliga, M.S.; Ponemone, V.; Kaur, K.; Larsen, B.; Fletcher, E.; Greene, J.; Fayad, R. Mucus and adiponectin deficiency: Role in chronic inflammation-induced colon cancer. Int. J. Colorectal. Dis. 2013, 28, 1267–1279.
- Moon, H.S.; Liu, X.; Nagel, J.M.; Chamberland, J.P.; Diakopoulos, K.N.; Brinkoetter, M.T.; Hatziapostolou, M.; Wu, Y.; Robson, S.C.; Iliopoulos, D.; et al. Salutary effects of adiponectin on colon cancer: In vivo and in vitro studies in mice. Gut 2013, 62, 561–570.
- La Cava, A. Adiponectin: A relevant player in obesity-related colorectal cancer? Gut 2013, 62, 483–484.
- Sugiyama, M.; Takahashi, H.; Hosono, K.; Endo, H.; Kato, S.; Yoneda, K.; Nozaki, Y.; Fujita, K.; Yoneda, M.; Wada, K.; et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 2009, 34, 339–344.
- Kim, A.Y.; Lee, Y.S.; Kim, K.H.; Lee, J.H.; Lee, H.K.; Jang, S.H.; Kim, S.E.; Lee, G.Y.; Lee, J.W.; Jung, S.A.; et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 2010, 24, 1441–1452.
- Cleary, M.P.; Grossmann, M.E. Minireview: Obesity and breast cancer: The estrogen connection. Endocrinol.ogy 2009, 150, 2537–2542.
- Ando, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62.
- Chen, J.; Iverson, D. Estrogen in obesity-associated colon cancer: Friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control. 2012, 23, 1767–1773.
- Potter, J.D.; Bostick, R.M.; Grandits, G.A.; Fosdick, L.; Elmer, P.; Wood, J.; Grambsch, P.; Louis, T.A. Hormone replacement therapy is associated with lower risk of adenomatous polyps of the large bowel: The Minnesota Cancer Prevention Research Unit Case-Control Study. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 779–784.
- Al-Azzawi, F.; Wahab, M. Estrogen and colon cancer: Current issues. Climacteric 2002, 5, 3–14.
- Percik, R.; Stumvoll, M. Obesity and cancer. Exp. Clin. Endocrinol. Diabetes 2009, 117, 563–566.
- Terry, P.D.; Miller, A.B.; Rohan, T.E. Obesity and colorectal cancer risk in women. Gut 2002, 51, 191–194.
- Liu, P.H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37–44.
- Bernstein, L.; Ross, R.K. Endogenous hormones and breast cancer risk. Epidemiol. Rev. 1993, 15, 48–65.
- Terry, P.; Giovannucci, E.; Bergkvist, L.; Holmberg, L.; Wolk, A. Body weight and colorectal cancer risk in a cohort of Swedish women: Relation varies by age and cancer site. Br. J. Cancer 2001, 85, 346–349.
- Slattery, M.L.; Ballard-Barbash, R.; Edwards, S.; Caan, B.J.; Potter, J.D. Body mass index and colon cancer: An evaluation of the modifying effects of estrogen (United States). Cancer Causes Control. 2003, 14, 75–84.
- Barzi, A.; Lenz, A.M.; Labonte, M.J.; Lenz, H.J. Molecular pathways: Estrogen pathway in colorectal cancer. Clin. Cancer Res. 2013, 19, 5842–5848.
- Papaxoinis, K.; Triantafyllou, K.; Sasco, A.J.; Nicolopoulou-Stamati, P.; Ladas, S.D. Subsite-specific differences of estrogen receptor beta expression in the normal colonic epithelium: Implications for carcinogenesis and colorectal cancer epidemiology. Eur. J. Gastroenterol. Hepatol. 2010, 22, 614–619.
- Castiglione, F.; Taddei, A.; Rossi Degl’Innocenti, D.; Buccoliero, A.M.; Bechi, P.; Garbini, F.; Chiara, F.G.; Moncini, D.; Cavallina, G.; Marascio, L.; et al. Expression of estrogen receptor beta in colon cancer progression. Diagn. Mol. Pathol. 2008, 17, 231–236.
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Williams, C.; Strom, A.; Gustafsson, J.A. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009, 69, 6100–6106.
- Weyant, M.J.; Carothers, A.M.; Mahmoud, N.N.; Bradlow, H.L.; Remotti, H.; Bilinski, R.T.; Bertagnolli, M.M. Reciprocal expression of ERalpha and ERbeta is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res. 2001, 61, 2547–2551.
- Giroux, V.; Lemay, F.; Bernatchez, G.; Robitaille, Y.; Carrier, J.C. Estrogen receptor beta deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int. J. Cancer 2008, 123, 303–311.