1. Introduction
Cancer has been a global problem and there is an urgent need to develop effective anti-cancer drugs. About 97% of oncology preclinical trials has failed to achieve the FDA approval as 2D and 3D in vitro and animal in vivo platforms has not been able to mimic the tumor microenvironment. Microfluidic platform offers numerous capabilities that mimic the TME conditions such as dynamic fluid motion, tissue–tissue interaction, spatio-temporal nutrient diffusion, inter- and intracell interactions and others which can assist in understanding the cancer cell biology and therapeutic testing.
Many factors should be considered when designing a microfluidic platform for the screening of anti-cancer drugs. Firstly, the biocompatible materials should be used for the fabrication of the microfluidic chip. Silicon or glass materials used for the fabrication have their drawbacks on high cost, opacity (of silicon) and poor permeability which are overcome PDMS-based chip due to its transparency, low cost, gas permeability and tunable mechanical properties. Still, PDMS has its issue on hydrophobicity which can bias the results in drug screening. Hydrogel has also been used to recreate the TME due to its hydrophilic nature and permeability and has a huge potential for an anti-cancer drug screening platform. Next, considerations should be made to mimic the TME on the chip. The most recent technology of tumor-on-a-chip has emerged as a new tool which provides a unique approach for understanding cancer biology and allowed cost-effective drug discovery platforms. The tumor-on-a-chip platform can accurately mimic the complex TME which consists of a mixture of many cell types such as tumor cells, immune cells, stromal cells and ECM matrix and vascularization components.
The advent of microfluidic technology has linked the pharmaceutical research by providing a novel culturing platform that can be continuously perfused and mimic the physiological functions of the tissues and organs. With the use of a lower reagent volume, it can generate mechanical stimuli such as shear stress and a well-controlled concentration gradient of molecules. Additionally, its miniature size allows for parallelization, which is essential for high-throughput drug screening.
2. Drug Response Studies Using Droplet Microfluidics
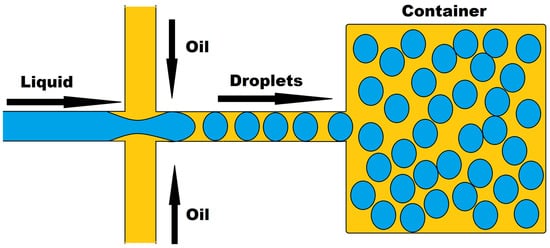
Droplet microfluidics, a new platform that allows for the precise confinement of single cells or molecules within microdroplets for high-throughput analysis, has a lot of promise in drug development and genomics. Two immiscible liquids in a channel use confinement and capillary forces to generate these droplets, as shown in
Figure 1. Thousands of cell-based assays can be performed in a single experiment with this technique, making it an excellent tool for biomedical research. Microdroplets with volumes ranging from μL to fL can be produced at very high frequencies ranging from Hz to KHz, encapsulating biomaterials such as cells, DNA, mRNA, and bacteria. Microdroplets can also be handled for mixing, combining, diluting, dividing, and sorting. It uses a smaller number of cells per device and per experiment, which may be acceptable for tumor samples with a limited number of cells. Many recent improvements in oncology have been made by combining droplet microfluidics with nucleic acid analysis
[1][2][96,97], protein analysis
[3][98], and drug discovery
[4][99].
Figure 1.
Droplet-based microfluidics.
Droplet microfluidics, a potent technology, has been used to conduct research on single-cell-based high-throughput drug resistance investigations and drug screening for therapeutic purposes. These droplets can be utilized to encapsulate anti-cancer drugs as well as other molecules such as antibodies, proteins, growth factors, indicators, and macrophages for a variety of bio-applications, and they can be released by active or passive processes. These droplets can be created using microfluidic techniques, allowing for programmable drug absorption, confinement, and controlled release
[5][100].
Brouzes et al.
[6][57] demonstrated a droplet-based microfluidic system that allowed for the encapsulation of a single cell and reagent in separate aqueous microdroplets that were individually labeled with an optical label. The droplets were then combined and tested for cytotoxicity against U937 cells. Yu et al.
[7][58] exploited the benefits of a droplet-based microfluidic system to make alginate beads with entrapped breast tumor cells for drug testing utilizing tumor spheroids rather than single cells, and found that tumor spheroids responded to doxorubicin in a dose-dependent manner. Wang et al.
[8][59] created a microfluidic droplet-based approach for forming multicellular tumor spheroids utilizing alginate and Matrigel mixed hydrogel beads while containing HeLa cells and observing a dose-dependent response to vincristine. Sabachandani et al.
[9][60] devised a microfluidic device to produce 1000 individual 3D spheroids for the sequential treatment of doxorubicin and paclitaxel.
The platform of droplet microfluidic technologies has been proven to be ideal for anti-cancer drug screening because to its advantages of low drug quantities, high-throughput data collection, and low cell numbers in this research. Furthermore, the elimination or reduction in cell processing stages prior to application to the droplet microfluidic device transforms it into an entirely automated high-throughput platform
[10][101].
3. Organ-on-a-Chip Platform
Drug development has always been a field which requires novel, innovative, and insightful methods for the testing of the new drugs or molecular compounds to find the beneficial effects against many diseases. Before testing it on humans, researcher must conduct much preclinical research
in vitro or
in vivo to provide the detailed information about dosing and toxicity levels. The costly animal testing methods of drug testing have been there for many decades and is still existing. Animal models have also immensely contributed to
ou
r understanding of physiology and drug response; however, the efficacy and toxicity in animals and humans might not be in accordance and might not be as predictive as one might think
[11][12][102,103]. Before conducting the animal testing, all the testing compounds should be studied using 2D or 3D cell culture models. In 2D cell culture systems, cells are grown on a flat dish which carries some inherent flaws of cell–cell signaling. Three-dimensional cell cultures have shown improvements in the studies of morphology, cell number monitoring, proliferation, response to stimuli, differentiation, drug metabolism, and protein synthesis and have been a more accurate representation of the
in vivo scenario
[13][104]. However, 3D cell cultures techniques fail to recreate the crucial features of the living organs such as tissue–tissue interface, concentration gradients and mechanical simulations of the microenvironment. Therefore, despite the development of cell culture techniques, currently more than 80% of drugs fails on clinical testing
[14][105].
To address these drawbacks of the
in vitro approaches, the microfluidic platform of “Organ-on-a-chip” prevailed to imitate the activities, mechanics and physiological response of the organ and provided a bridge between
in vivo and
in vitro practices. Organ-on-a-chip is a biomimetic, microengineered device that mimics essential physiological organ properties such as concentration gradient, shear force, cell patterning, tissue boundaries, and tissue organ interactions
[15][106]. A single-cell type is lined in a microchannel or planted in a single chamber while being perfused with media in the simplest model of organ-on-a-chip. Donald Ingber created the first organ-on-a-chip to mimic the human breathing lung, which included alveolar epithelial cells of the lung interacting with endothelial cells within a microfluidic channel that mirrored the structure and function of tissue-vasculature structure of the alveoli of the lung
[16][107].
The development of cytology, tissue engineering, and microengineering as an alternative to understand the physiology and drug development process has resulted in organ-on-a-chip development. The microfluidic platform offers a significant advantage in terms of precise fluid control via external valves and the creation of concentration gradients to mimic the key structure and functions of tissues and organs. This technology is also more cost-effective since it uses a less time-consuming in vitro procedure and reduces fabrication expenses. The fundamental benefit of adopting a microfluidic platform is the laminar fluid flow, which prevents nearby streams from mixing. This unique property is being used to investigate cell motility in response to chemical stimuli, as well as other sophisticated cell activities.
Three-dimensional arrangements of tissues on platforms, the incorporation of multiple types of cells to represent a more physiological balance of cells (such as stromal, vascular and immune cells), and the existence of mechanical influences (such as shear stress) appropriate to the tissue being designed are all defining characteristics of organ-on-a-chips
[11][102]. To effectively mimic the biological milieu in the chip, one must be mindful of the flow dynamics that microfluidic technology can produce. Depending on the flow conditions, the cells in the body are subjected to continual mechanical forces or shear forces. To simulate the microsystem, the oxygen rate, nutrition rate, and blood flow rate must all be precisely controlled.
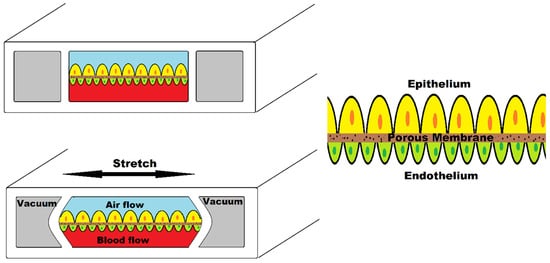
Figure 2 illustrates human lung-on-a-chip device simulating breathing mechanism. This device consists of two central chambers and two side chambers. The central chamber is separated by a porous membrane; the upper part is seeded with alveolar epithelial cells and the lower part contains vascular endothelial cells. Two side chambers simulate the breathing mechanism when vacuum is applied by stretching the membrane.
Figure 2.
Human lung-on-a-chip device.
Hassell et al. designed such cell culture technology injecting non-small cell lung cancer (NSCLC) cell line within primary alveolus to recapitulate the tumor growth, tumor dormancy using mechanical breathing actuation
[17][108]. They also observed the tumor response to tyrosine kinase inhibitor (TRI) therapy. Such mechanical forces to replicate the tumor microenvironment have been used by Strelez et al. who developed colorectal-cancer-on-chip device to study early invasive stage of cancer
[18][109]. Peristalsis-like cyclic stretching mechanical forces and fluid flow are incorporated in a chip consisting of endothelial and epithelial compartments separated by porous membrane.
Many brain, lung, heart, kidney, artery, skin, bone, and cartilage organ-on-a-chip models have been presented that can imitate the activities and physiological responses of the full organ. The basic physiological requirements for the specific organ, as well as critical elements such as cell kinds, structures, and the organ’s specific physiochemical environment, should be examined, and a cell culture system should be devised based on these qualities.
The bioprinting technology relies on layer-by-layer printing, which has been used in organ-on-a-chip fabrication, wherein multiple bio functional components and cell types can be printed onto a surface of cell-compatible biomaterials to create a 3D complex structure with high spatial resolution and reproducibility
[19][110]. Three-dimensional bioprinting on organ-on-a-chip allows for a multiscale setup of cells or biological molecules that is similar to their original microenvironment, allowing for physiological interactions under diverse conditions which better capture tissue environmental factors
[20][111]. The bioprinting approach has a great deal of potential for organ-on-a-chip devices since it can increase tissue and function by organizing precise cell arrangements and speed up medical research
[21][112].
4. Organ-on-a-Chip in Cancer Research
The convergence of numerous fields such as biology, physics, and engineering has resulted in new advancements in replicating physiological systems of the body, which have opened new opportunities for researching various aspects of human physiology and the drug development process
[15][106]. Organ-on-a-chip is a cutting-edge technology for simulating organ function at the cellular level. Such platforms can be utilized to accurately imitate the microenvironment and model different disorders of the neurological system, respiratory system, digestive system, cancer, and others by incorporating biophysiochemical variables. Microfluidics and the organ-on-a-chip platform in the pharmaceutical industry can be regarded a paradigm shift in the drug development process for disease modelling and drug testing and show a great potential for revolutionizing the drug development process
[22][113].
Microfluidics and organ-on-a-chip technology can be extremely beneficial in cancer research. The fundamental problem is a lack of understanding of the tumor microenvironment’s diversity and heterogeneity. Traditional in vitro systems have made a significant contribution to our present knowledge of the disease and treatments. However, due to a lack of structural, mechanical, and biological cues, these lack the genuine intricacy of cell-ECM and cell–cell interactions. Mechanical factors such as fluid shear stress, hydrostatic pressure, and tissue deformation were missing in complexity in the 3D tissue structure of living organs. They were also not perfused with nutrient-rich media running via an endothelium-lined vascular, resulting in a lack of understanding of tissue–tissue interactions, the involvement of circulating immune cells, and therapeutic drug physiological dosage. Organ-on-a-chip is a new platform for cancer research that is based on the microenvironment of a specific organ in which key molecular, biophysical, cellular, and tissue elements can be differed in a controlled fashion to better understand the pathophysiology of cancer in a target organ.
The organ-on-a-chip platform have a lot of potential in cancer research since they can replicate the physiological function and 3D microstructure of a human organ and simulate the organ’s complexity. They offer a cost-effective high-throughput test option with good replicability. Tumor-on-a-chip is an appealing promise of organ-on-a-chip for studying cancer biology and expedited therapy possibilities for cancer cells.
5. Replication of Tumor Microenvironment on Chip
Cancer cells, stromal cells, immune cells, and vascular cells are all lodged in the tumor tissue. Dense extracellular matrix (ECM) components are also present. An effect of cancer stromal interaction on carcinogenesis, angiogenesis, tumor invasion, metastasis, and treatment resistance has been discovered. Tumor growth is influenced by molecules released by malignant and stromal cells. Immune cells, too, have a role in tumor growth. The three-dimensional nature of solid tumors has a significant impact on tumor dynamics. As a result, cancer can be defined as a collection of complex diseases, necessitating the development of tumor models based on their three-dimensional nature and structural and dynamical complexity.
Only by considering the features of the actual tumor, including biophysical and metabolic factors, can a realistic tumor be represented. Cancer initiation, progression, and treatment effects are all influenced by the tumor microenvironment. TME’s cellular and noncellular components communicate bi-directionally to control cancer cell proliferation, function, and death. The release of soluble substances in the interstitial fluid, cell–cell or cell-ECM adhesion, and mechanical aspects all play a role in cell signaling. Shear stress, fluid forces, interstitial flow, ECM organization, composition, and stiffness are all mechanical forces.
ECM components play a key role in all cellular activities, including cell signaling and cell migration, as well as providing mechanical stability in the microenvironment. Hypoxia inducible factor (HIF-1), a transcription factor that responds to a decrease in available oxygen in cells, is one of the chemical alterations found within the tumor niche. Because the tumor lacks access to blood vessels, cells enter a condition of hypoxia, and HIF-1 accumulates quickly, driving angiogenesis by allowing vessels to develop in the TME. Alterations in DNA, such as the mutation in the p53 transcription factor, can potentially cause physiologic changes. p53 is a tumor-suppressor protein that can block cell cycle progression in G1 phase to allow damaged DNA to be repaired before DNA replication. p53 can also induce apoptosis in cells if their damaged DNA cannot be fixed. A loss of p53 gene function will allow cells with damaged DNA to be replicated, which can promote tumor formation
[23][114].
Advanced microfabrication and tissue engineering techniques can be applied to microphysiological systems to attain physiology in the microscale, allowing clinical adaptations of preclinical findings in vitro to be facilitated. Microfluidic tissue chips are frequently made using lithographic techniques and replica molding. These chips can help imitate physiological flow, shear stress, nutrient delivery, and medication exposure by manipulating fluid. The fluid flow dynamics may be easily simulated mathematically thanks to laminar flow of fluid at the microscale, allowing theoretical predictions of complicated biological events.
Although a full regeneration of TME can be challenging, researchers have recapitulated some key features of TME over the years including vasculature, co-culture, shear stress, pressure, and chemical and oxygen gradients. A study was recently conducted by Bhattacharya et al. on the role of oxygen in tumor–immune interactions in which breast cancer cell were grown in 3D scaffolds to generate physio- and pathophysiological oxygen levels
[24][115]. Tumor-on-a-chip platforms can very well recreate human TME and hold a great promise for cost-effective and high-throughput drug screening platforms.
Regarding tumor–stromal interactions, it plays a pivotal role in tumor growth progression along with metastasis and therapeutic intervention. These nonmalignant stromal cells consist of ECM components, fibroblasts, macrophages, and endothelial cells. These cells communicate with the cancer cells by secreting a range of molecules to influence tumor behavior via several signaling pathways. These interactions modulate tumor growth, metastasis, angiogenesis, and therapeutic resistance. Several microfluidic co-culture platforms have been designed to study and investigate these interactions. Menon et al.
[25][61] developed a microfluidic co-culture device where two cell models, bone marrow stromal cells (HS5) and liver tumor cells (HuH7) were cultured in different hydrophilic compartments where the hydrophobic compartment placed in between acted as a barrier which was later removed after cells reached confluence by introducing liquid, giving a medium for cell migration and interaction. The findings of this
respape
archr revealed tumor-induced stromal cell death, which could be triggered by the paracrine signaling of reactive oxygen species (ROS) generated by tumor cells in the main local microenvironment homeostasis. However, when compared to monoculture, tumor cell movement appeared to be aided in co-culture. Gioella et al.
[26][62] modelled breast cancer in a microfluidic chip with both epithelial and stromal tissues for the
in vitro mimicking of stromal activation in tumor epithelial invasion as the activation and morphological changes in tumor stroma has its role in tumor progression.
In addition, the ECM components embedded in the
in vitro microenvironment create a 3D structural framework to provide biochemical and structural support to the cells. The ECM has been found to simulate the development of cancer cells
in vitro; hence, their interaction plays an important role in TME. Synthetic scaffold-based culture methods are widely used to recreate the ECM on chip as they closely resemble natural ECM
[27][116]. Rijal et al.
[28][63] developed a reconstitute tissue matrix scaffold system which was fabricated using native tissue ECM whose structural and compositional feature promote cell survival, proliferation, migration, and invasion in culture along with vascularized tumors.
6. Modelling Angiogenesis
The formation of new blood vessels from existing vasculature for the supply of oxygen and nutrients to the tumor cells is known as angiogenesis. Being one of the prominent landmarks of TME, it indeed needs to be engineered within the tumor for recapitulation of tumor physiology especially when tumor exceeds the diameter greater than 200 μm as when the critical diameter of tumor exceeds this value, hypoxia develops and complex metastatic cascade signals the start of vascularization
[29][117].
The co-culture of cancer and endothelial cells has been a way of enabling vascularization. Nashimoto et al.
[30][64] constructed such a vascular network on tumor-on-a-chip platform by culturing tumor spheroid where human umbilical vein endothelial cells (HUVEC), human lung fibroblast (hLF) and human breast cancer cell (MCF-7) were grown. Here, the tumor spheroid’s fibroblasts triggered angiogenesis and formed a perfusable vascular network in the spheroid. Furthermore, the findings of the literature highlighted the significance of flow in the vascular network for assessing tumor activity in drug screening platform.
7. Modelling Vascularized Tumor Models
One of the diverse factors of the TME is the vascular constructs within it. The microvascular engineering method has made it easier to rebuild tumor vasculature for network analysis and anti-cancer medication testing using patient-derived tumor cellular activities. This self-organization mechanism has also been used to mimic metastatic cascades, such as tumor cell extravasation
[31][118].
Recent development provides the progress towards bioprinted vascular networks as well. Cao et al.
[32][65] developed such a device in microfluidic chip pairing blood and lymph vessel which were separately bioprinted with individual permeability equivalent to their native profiles. They investigated anti-cancer drugs in different combinations of blood and lymphatic vessels to analyze their dynamics and interactions in anti-cancer medication administration.
The example of engineered vascular network includes the work done by Mannino et al.
[33][66] who created a lymphoma-on-chip model to recapitulate the interaction between immune cell, cancer cell and endothelial cell in TME. They used stainless steel wire for creating a vascular network having an average channel diameter of 32 ± 51 μm and the platform building was done using common laboratory materials and easy techniques for fabrication.
For various reasons, the integration of vascularization in tumor chip is a game changer including recreating the structure and function of vascular tumor mass, simulating crucial phages of metastasis of interaction between tumor, endothelial cells and stromal cells, building physiologically selective barriers to nutrition and medication delivery to tumor and such setup can be used to directly evaluate medicines with anti-angiogenic and anti-metastatic properties
[34][119]. However, the development of biomaterials to replicate the human vasculature more closely is still a problem.
8. Metastatic Cascade in Organ-on-a-Chip
Cancer cells or CTCs spreading through blood or the lymphatic system to develop new tumors in distant parts of the body define the phenomenon of metastasis. This is characterized by a wide range of sequential mechanisms including invasion, intravasation, circulation and extravasation at appropriate metastatic locations. Being a leading cause of cancer-related death, the phenomenon of metastasis is subjected to a lot of research but still our understanding of disease and its path to metastasis remains limited and its linkage to the drug resistance mechanism is unclear.
Several initiatives are underway to construct a metastatic model to aid in oncology studies and the development of novel therapeutics. Years of development of microfluidic devices provided a novel organ-on-a-chip platform, a biomimetic device to recreate the essence of human tissues and organ in vitro. These devices provide realistic models for better understanding of the process of cellular dissemination and mimic TME. As a result, these are the ideal models for the pathological study of cancer as it enables researchers to investigate the tumor development, proliferation, vascularization, and sequential events of metastasis by recreating essential aspects of cell–cell and cell–ECM interactions, physical and chemical gradients and spatio-temporal hydrodynamic properties.
Skardal et al.
[35][67] presented a device for the real-time monitoring of colon cancer cells moving from hydrogel-fabricated gut to liver constructs in a circulatory fluidic channel to study the attachment and invasion of liver, marking the first microfluidic model of metastasis to recreate the movement from 3D originating tissue to 3D target tissue. Zervantokis et al.
[36][68] developed a 3D microfluidic platform modelling the tumor–vascular interface to link invasion with endothelial permeability relating to cytokine-induced endothelial cell activation and paracrine signaling loops involving macrophages and tumor cells. The findings reported that endothelial permeability increased by macrophage signaling or TNF-α stimulation was linked to an increased intravasation rate and blockage of TNF-α produced by macrophages reduced intravasation and restore endothelial barrier integrity. Xu et al.
[37][69] constructed a multi-organ-on-a-chip model to study lung cancer metastasis. In this device, to simulate the lung cancer cell metastasized to the brain, bone, and liver, the bronchial epithelial, lung cancer, microvascular endothelial, mononuclear and fibroblast cells were grown on the lung chamber and astrocytes, osteocytes and hepatocytes were grown in faraway chambers. After the formation of lung tumor mass upon culture, it demonstrated EMT and invasive potential, thus producing a tool to study cell–cell interactions during cancer metastasis and replicate the in vivo milieu of cancer metastasis. Wang et al.
[38][70] developed a model of metastasis-on-a-chip to predict therapeutic effect and dose responses of anti-cancer medications in physiologically appropriate hepatic microenvironment by simulating the growth of kidney cancer cells in the liver. This cost-effective model has proved to be feasible for screening therapeutic compounds and assessing treatment potency.
So, organ-on-a-chip devices provides a revolutionary in vitro platform to better elucidate the complex cascade of metastasis by recreating the physiochemical complexity of the disease to understand the progression of the tumor cells and the formation of the secondary colony. Furthermore, the development of personalized models of metastasis-on-a-chip will create an exact pharmaceutical screening for personalized medicine tailored to each patient.