Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yuqing Ren and Version 2 by Sirius Huang.
Emulsion gels are emulsion with a gel network structure and stable mechanical properties. Emulsion gel formation is considered a strategy for oil stabilization and structuring, presenting advantages such as transporting functional components and improving the sensory and physical product properties.
- emulsion gels
- protein emulsion
- colloidal material
1. A Basic Introduction of Emulsion Gel
An emulsion is a colloidal dispersion generated by a liquid in the form of small droplets distributed in another insoluble liquid [1]. Emulsion gels, soft solid materials, are emulsion with a gel network structure and stable mechanical properties [2], while the emulsified droplets are embedded in the gel matrix, making it a complex colloidal material that can exist in both emulsion and gel states [3]. The properties of emulsion gels result from complex interactions between their components [4]. In oil-in-water (O/W) emulsion gels, this colloidal structure can be formed either via the dispersion of the emulsion droplets in a continuous gel matrix or by the aggregation of the dispersed droplets in the particle gels [5]. Compared with standard emulsions, emulsion gels display better storage stability and the potential to prolong intestinal drug release [6]. They also exhibit excellent stability, which is often used for embedding flavor substances. Furthermore, emulsion gels present a soft solid texture and can exhibit physical behavior similar to fat in emulsified meat products [5], which is especially suitable for designing and developing healthy, functional foods while showing significant potential for utilization as fat substitutes to produce low-fat meat products. These gels can replace animal fat while maintaining the physical and chemical properties (especially stiffness and water-holding properties) of the products [6]. The use of plant oil rich in PUFAs for preparing solid-structured oils and fats for replacing animal fats has attracted increasing attention. Emulsion gels are widely used in meat products [7].
Emulsion gels typically use a stable protein emulsion as a matrix, while the excellent emulsification characteristics of proteins allow them to be adsorbed on the oil–water interface to stabilize the oil droplets [8]. In emulsion-droplet-filled gels, the continuous phase of the stable emulsion forms a gel network structure in certain induced conditions, while the dispersed phase fills the gel network to yield a soft solid material and create an emulsion gel. (Figure 1).
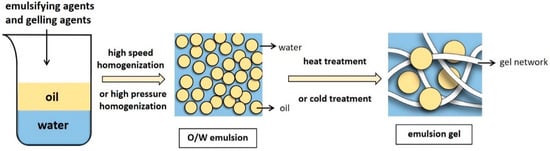
Figure 1. The preparation process of O/W emulsion gels.
2. Preparation of the Emulsion Gel
The emulsion gel preparation process mainly consists of two steps: stable emulsion preparation and gel formation. After adding an emulsifier, water and oil are mixed via high-pressure homogenization or high-speed mixing [9] to form stable emulsions. The oil to water ratio and protein content are critical. Sufficient protein coverage stabilizes the oil–water interface, while too little protein may lead to the adsorption of multiple droplets on one protein molecule (bridging flocculation), facilitating emulsion creaming. However, an excess may cause the protein covering the droplet surface to be ejected from the surface junction zone (depletion flocculation), promoting droplet coalescence [10].
Obtaining a solid-like emulsion gel involves continuous phase gelation (emulsion-droplet-filled gels) or droplet aggregation (emulsion-droplet-aggregated gels). The second step involves inducing the stable emulsion to an emulsion gel [9], which is achieved in two ways. One requires heat induction using traditional heat treatment methods, while the other uses gelation agents to obtain a cold-set gel. Cold-induced gelation can be broadly divided into acid-, salt-, and enzyme-induced methods depending on the added gelation agents. Furthermore, regulating environmental and processing factors (including temperatures, pH, or ionic strength) is also essential for preparing emulsion gels [11].
Heat treatment (>65 °C) is a simple, rapid method commonly used for converting protein-stabilized emulsions into gels. During the heat process, the folded structures of the protein molecules open due to denaturation and aggregation, allowing the unfolded protein to form new intra- and interchain disulfide bonds. In appropriate conditions, the gel networks aggregate to form three-dimensional (3D) structures via the interaction between chemical forces, such as disulfide bonds, hydrogen bonds, and the hydrophobic interaction between molecules [12]. Extending the heating time deforms the protein in the gel network, increasing the gel strength and elasticity. However, the degree of denaturation and aggregation can also affect the physical properties of the final gel. Therefore, many factors must be considered when preparing gels via heat treatment, such as protein concentration, temperature, and heating time [13].
Cold-set gel preparation involves adding gelation agents, such as salt, acid, and enzymes, to an emulsion to create a gel structure. This molding method usually requires preheating treatments. Unlike thermal induction, the gelation process does not proceed during the preheating stage but only after salt, acid, or enzyme addition. The unfolded protein remains soluble [14], while preheating enables the denatured unfolding of the protein in response to heat. Furthermore, it is not easy to deactivate the thermally unstable bioactive substances in the emulsion in the pre-heating process, causing loss of function [15]. During acid-induced gelation, the acidification caused by glucono-δ-lactone addition decreases the pH and neutralizes the surface charges of the protein aggregates, leading to gel structure formation via Van der Waals forces and hydrophobic interaction [6]. Salt induction usually comprises the addition of Ca2+ from CaCl2, which changes the pH and ionic strength to reduce the electrostatic repulsion between the proteins. This process promotes ionic cross-linking, allowing protein aggregates to form gel networks, increasing the ionic strength, and accelerating protein aggregation to generate larger aggregates [16]. Enzyme induction involves the addition of enzymes to facilitate the covalent cross-linking of proteins [17]. This process typically uses microbially derived transglutaminase (TG) to catalyze acyl transfer reactions, deamidation, and cross-linking between intra- or interchain protein glutamine and lysine peptide residues [18]. Previous studies have shown that the gel obtained using TG as a gelation agent exhibits a delicate, robust texture and higher mechanical strength than when using other methods [19]. Enzyme induction presents various advantages, such as mild and controllable gelation conditions and no by-products [20]. Furthermore, research has shown that ethanol is feasible in inducing the gelation of emulsion condensation [15], presenting a novel method for preparing a cold-set gel while providing insight into the development of new functional foods.
As rigid, polydisperse, hydrophilic macromolecules, polysaccharides can be used for thickening and gelling aqueous media to obtain polysaccharide-based emulsion gels. Non-starch polysaccharides can usually be gelatinized by cooling, heating, or adding calcium ions [9]. In experimental and practical applications, polysaccharide-based cold-gel agents, such as sodium alginate, xanthan gum, and carrageenan, have often been chosen to prepare polysaccharide-based emulsion gels or improve the properties of protein gels. These macromolecular species can facilitate polymer interaction to form continuous networks with emulsion gel functionality [4]. In practical situations, the specific mode of preparation depends on the matrix of the emulsion gel and its application in food.
References
- Yuqing, Z.; Xing, C.; McClements, D.J.; Liqiang, Z.; Wei, L. pH-, ion- and temperature-dependent emulsion gels: Fabricated by addition of whey protein to gliadin-nanoparticle coated lipid droplets. Food Hydrocoll. 2018, 77, 870–878.
- Dickinson, E. Colloid science of mixed ingredients. Soft Matter 2006, 2, 642–652.
- Dickinson, E. Food colloids research: Historical perspective and outlook. Adv. Colloid Interface Sci. 2011, 165, 7–13.
- Pintado, T.; Ruiz-Capillas, C.; Jimenez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478.
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241.
- Lin, D.Q.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137.
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188.
- Dickinson, E.; Evison, J.; Gramshaw, J.W.; Schwope, D. Flavour release from a protein-stabilized water-in-oil emulsion. Food Hydrocoll. 1994, 8, 63–67.
- Dickinson, E. Stabilising emulsion-based colloidal structures with mixed food ingredients. J. Sci. Food Agric. 2013, 93, 710–721.
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloid Surf. B-Biointerfaces 2010, 81, 130–140.
- Dickinson, E.; Radford, S.J.; Golding, M. Stability and rheology of emulsions containing sodium caseinate: Combined effects of ionic calcium and non-ionic surfactant. Food Hydrocoll. 2003, 17, 211–220.
- Dickinson, E.; Chen, J.S. Heat-set whey protein emulsion gels: Role of active and inactive filler particles. J. Dispers. Sci. Technol. 1999, 20, 197–213.
- Aiqian, Y.; Taylor, S. Characterization of cold-set gels produced from heated emulsions stabilized by whey protein. Int. Dairy J. 2009, 19, 721–727.
- Mao, L.K.; Roos, Y.H.; Miao, S. Study on the Rheological Properties and Volatile Release of Cold-Set Emulsion-Filled Protein Gels. J. Agric. Food Chem. 2014, 62, 11420–11428.
- Xi, Z.W.; Liu, W.; McClements, D.J.; Zou, L.Q. Rheological, structural, and microstructural properties of ethanol induced cold-set whey protein emulsion gels: Effect of oil content. Food Chem. 2019, 291, 22–29.
- Jie, Y.; Yong, W.; Dong, L.; Li-jun, W. Freeze-thaw stability and rheological properties of soy protein isolate emulsion gels induced by NaCl. Food Hydrocoll. 2022, 123, 107113.
- Zhu, Q.M.; Wu, F.F.; Saito, M.; Tatsumi, E.; Yin, L.J. Effect of magnesium salt concentration in water-in-oil emulsions on the physical properties and microstructure of tofu. Food Chem. 2016, 201, 197–204.
- Luisa, A.; Gaspar, C.; de Goes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322.
- Mao, Y.; Fu, L.; Chuan-He, T. Properties and microstructure of transglutaminase-set soy protein-stabilized emulsion gels. Food Res. Int. 2013, 52, 409–418.
- Tang, C.H.; Luo, L.J.; Liu, F.; Chen, Z. Transglutaminase-set soy globulin-stabilized emulsion gels: Influence of soy beta-conglycinin/glycinin ratio on properties, microstructure and gelling mechanism. Food Res. Int. 2013, 51, 804–812.
More
