Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Pathogens within the oomycete genus Phytophthora are among some of the most destructive plant pathogens globally, causing disease and significant losses in important agricultural and forestry crops, damaging the environment, as well as impeding attempts to mitigate climate change. What is of increasing interest is the involvement of Phytophthora effectors in regulating programed cell death (PCD)—in particular, the hypersensitive response.
- plant
- necrosis
- pathogens
1. Introduction
Pathogens within the oomycete genus Phytophthora are among some of the most destructive plant pathogens globally, causing disease and significant losses in important agricultural and forestry crops, damaging the environment, as well as impeding attempts to mitigate climate change [1][2][3][4]. One of the more well-known incidences of Phytophthora disease is the Irish Potato Famine in 1845. This incident was caused by Phytophthora infestans—the causal agent of late blight of potatoes. The disease resulted in the death of half of the potato crop that year and about three-quarters of the crop over the next seven years [3][5]. Other Phytophthora spp., which cause significant impact worldwide, include the causal agents of sudden oak death in California (Phytophthora ramorum), stem rot of soybean (Phytophthora sojae), black shank of tobacco Phytophthora nicotianae), phytophthora root rot of avocado and jarrah dieback of trees in the Jarrah Forest, both caused by Phytophthora cinnamomi [3][6][7]. Despite the economic and ecological relevance of P. cinnamomi, the mechanisms this pathogen utilizes to infect and successfully colonize its hosts are still largely unknown. P. cinnamomi is known to infect plants that are important for agriculture and forestry, with the most significant food losses occurring in avocados. There is little to no knowledge on how P. cinnamomi, a hemi-biotrophic pathogen, maintains a biotrophic lifestyle early in the infection and a necrotrophic lifestyle later in the infection.
Pathogenic lifestyles are centered around feeding on host tissue, where success is dependent on the pathogen’s ability to overcome host defenses. One host defense strategy Phytophthora spp. must evade to sustain their biotrophic phase is the hypersensitive response (HR). The HR is a form of programed cell death (PCD) and is generally the last resort in a host plant’s defense response against a pathogen. The response involves the localized death of cells surrounding the initial site of infection to inhibit the spread of the pathogen. Later in the infection, the HR is favored during the necrotrophic phase. Studies have shown that Phytophthora spp. manipulate the host plant’s cell death machinery to elicit a susceptible outcome [8][9].
Phytophthora spp. harbor a distinct set of genes involved in moderating host–pathogen interactions [10]. These genes encode effectors—small, secreted proteins—that interfere with host defense processes. There are two groups of effectors, cytoplasmic and apoplastic effectors, which are classified by where in the host cell they act. The most well-studied classes of Phytophthora cytoplasmic effectors are Crinklers (CRNs) and RxLRs (Arg-x-Leu-Arg, where x is any amino acid) [4]. Research into Phytophthora effectors has greatly expanded due to the availability of genomic and transcriptomic data, allowing for the prediction of putative effector homologs in Phytophthora spp. [11]. These valuable tools—followed by functional characterization techniques, such as transient transformation in model plants—allow for the identification of effectors that may play crucial roles during infection. However, little genomic research has been conducted on P. cinnamomi, which leaves a gap in knowledge on the mechanisms employed by this pathogen to successfully infect and cause disease in economically and ecologically important plants.
Due to their economic impact, Phytophthora spp. are some of the most studied among oomycetes [4][12]. There is, however, still limited knowledge on the mechanisms utilized to regulate cell death in host plants. It is likely these processes are determined by the delivery of functionally distinct pathogen effectors into the host cell [13].
2. Programed Cell Death in Plants
Plants are immobile organisms and have had to develop morphological, biochemical and physiological adaptations to survive in their environment. PCD is an important mechanism for plant development or defense and can be triggered by both abiotic and biotic stressors [9][14][15]. PCD is described as a genetically controlled process where selected cells are eliminated through a coordinated multi-step fashion [15]. This phenomenon is of considerable importance in agriculture because PCD can significantly affect plant health and subsequent yield [16][17]. Therefore, it is important to understand both the triggers and the pathways through which PCD is elucidated.
2.1. Classification of Programed Cell Death
There has been some confusion regarding how different forms of PCD should be classified and how terminology should be standardized. Cell death is classified based on the morphological characteristics and, as a result, two major classes of PCD are proposed to occur in plant biology [14]. Class one is vacuolar cell death, which involves the engulfment of the cytoplasm by lytic vacuoles, uptake and degradation of portions of the cytoplasm in the vacuolar lumen and, finally, rupture of the tonoplast followed by a massive release of vacuolar hydrolases. This results in the rapid destruction of the entire protoplast—a cell whose cell wall has been removed by enzymes—or, in some cases, even the entire cell, including the cell wall (Figure 1A). Class two is necrotic cell death, which is distinguished from vacuolar cell death by mitochondrial swelling, absence of the growing lytic vacuoles and early rupture of the plasma membrane, resulting in shrinkage of the protoplast (Figure 1B). Necrosis is regarded as an acute death response, which develops rapidly, taking anywhere from several minutes to a day to complete [14]. The use of morphology to classify PCD has allowed a better understanding of how cell death manifests. Although one limitation in this is that a well-known form of PCD, known as the HR, cannot be ascribed to either class, as its development displays characteristics of both vacuolar and necrotic cell death [14][18][19][20].
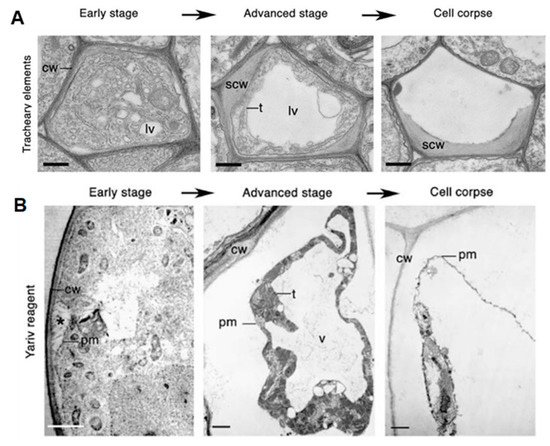
Figure 1. Classes of cell death. (A) Vacuolar cell death. Electron micrographs of programed cell death (PCD) in Arabidopsis tracheary elements. cw, cell wall; lv, lytic vacuole; n, nucleus; scw, secondary cell wall; t, tonoplast. Scale bars, 500 nm (tracheary elements). Manifests by a gradual decrease in cytoplasm volume and an increase in lytic vacuole volume. (B) Necrotic cell death. Electron micrographs of Yariv-reagent-induced death in the Arabidopsis cell culture. Asterisks denote the detachment of plasma membrane form the cell wall during early stages of cell death. c, chloroplast; cw, cell wall; pm, plasma membrane; t, tonoplast; v, vacuole. Scale bars, 2 µm. There is an absence of a growing lytic vacuole, and there is early rupture of the plasma membrane, which results in shrinkage of the protoplast. Pictures of Arabidopsis treachery elements were republished with authors’ permission from Avci, U.; Petzold, E.; Ismail, I.O.; Beers, E.P.; Haigler, C.H. Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J. 2008, 56, 303–315, https://doi.org/10.1111/j.1365-313X.2008.03592.x [21] and those of the Yariv-reagent-induced cell death were republished with authors’ permission from Gao, M.; Showalter, A.M.; Yariv reagent treatment induces PCD in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J. 1999, 19, 321–331, https://doi.org/10.1046/j.1365-313X.1999.00544.x [22].
The HR is a special form of PCD, involving rapid localized cell death at the point of pathogen penetration [16][23]. The host plant utilizes HR to limit biotrophic pathogen growth and generates long-range signals for systemic acquired resistance (SAR) [24]. Thus, another PCD classification system was developed to accommodate the placement of the HR. This system classifies forms of PCD based on what functions they play in the host plant, rather than by their morphology or pathways. Two classes were described: developmentally controlled PCD (dPCD) and pathogen-triggered PCD (pPCD). During vegetative and reproductive development, dPCD occurs and is often a final differentiation step for specific cell types [25]. Conversely, pPCD is elicited in the host plant by invading agents and can benefit either the plant or pathogen, depending on the studied plant–pathogen interaction [26]. An additional class has also been proposed to describe PCD resulting from environmental stress, termed ePCD [25]. The ePCD classification includes stresses, such as temperature or irradiation, or biotic aggressors, such as pathogens [15]. pPCD is specific to pathogen-triggered cell death, whereas ePCD includes all external stressors as PCD triggers. The use of ePCD as a classification may, however, be problematic, since different PCD pathways may be in play during both abiotic and biotic-triggered PCD.
2.2. Programed Cell Death in Host Plants
Plant PCD pathways are not as well understood as animal cell death. Animals have a core PCD machinery that is mainly regulated post-translationally [17][27], whereas it is not known whether the different forms of plant PCD share the same core machinery or whether the similarities they share were independently adopted to fulfill analogous roles for different pathways [9]. When looking at the two main plant PCD forms—dPCD and pPCD—there are marked differences as well as commonalities in their proposed pathways. For one, a vacuolar type of cell death is associated with dPCD, and features of both necrosis and vacuolar PCD are seen in pPCD [9]. There is also evidence of transcriptional regulation and signaling in both forms of plant PCD, but in different contexts. Unfortunately, there are still gaps in knowledge regarding pPCD. This is largely due to predominance of previous dPCD-centered investigations. In addition, variability has been seen in pPCD responses to a multitude of different abiotic and biotic factors, whereas dPCD is a relatively conserved process across all plant species. This section serves to summarize current knowledge on the transcriptional regulation, hormonal signaling and triggers involved in pPCD.
2.2.1. Transcriptional Regulation of pPCD
The stimulation and repression of cell death pathways by transcription regulators has been seen in animal PCD [28][29], and recent evidence indicates that some level of transcriptional control of PCD is also likely in plants [30][31][32][33][34]. Different classes of transcription factors (TFs), including members of NAC, ethylene-responsive element-binding factors (ERFs) and WRKY families, have been shown to play roles in cell fate regulation in response to different stresses. NAC TFs have been linked to the regulation of PCD triggered by both abiotic and biotic stresses [34]. One example is that of OsNAC4, which has been shown to be a key positive regulator of the HR by modulating the expression of almost 150 genes in rice, such as Copper Zinc Superoxide Dismutase 1 (CSD1) gene and BAX Inhibitor 1(BI-1) gene [35]. ERF TFs also play a role in the regulation of the HR, where the conditional expression of NbCD1—from Nicotiana benthamiana—in response to multiple HR elicitors is sufficient to induce the HR [30]. Numerous WRKY TFs are involved in the regulation of cell death, and they may play a role in the suppression of the HR during initial infection of the necrotrophic fungus, Botrytis cinerea, in Arabidopsis [36], through the activation or suppression of antagonistic signaling pathways, such as salicylic acid (SA), ethylene (ET) and jasmonic acid (JA) mediated pathways. Although there is a large body of research on TFs and their role in pPCD, there is still a lack of knowledge on Phytophthora pathogens and the involvement of TFs in eliciting or suppressing PCD during Phytophthora infection.
2.2.2. Phytohormone Signaling Pathways Involved in Pathogen-Triggered PCD (pPCD)
Different phytohormones play a role in dPCD, such as JA, auxin, strigolactones and ET—ET being the most characterized dPCD hormone [37][38][39]. Phytohormones control the dPCD processes via transcriptional regulation of genes, such as proteases and nucleases, to gradually build up dPCD competence during cellular differentiation. This contrasts with pPCD, where no preparation is required, and the cells are always ready to initiate an immune response upon pathogen attack [9]. The infection strategy of a plant pathogen—whether the pathogen adopts a biotrophic, necrotrophic or hemi-biotrophic lifestyle—determines the underlying mechanism for phytohormone-regulated pPCD in the host during plant–pathogen interactions [40][41]. It has been shown that SA plays an essential role in host defense response against biotrophic and the early stages of hemi-biotrophic pathogens, whereas JA and ET play an important role in the host defense response against necrotrophic and the later stages of hemi-biotrophic pathogens [41]. SA is the only phytohormone shown to play an essential role in the establishment of pPCD, allowing immunity toward biotrophic pathogens and susceptibility to necrotrophic pathogens. [42][43]. It has been found that some pathogens interfere with cellular SA biosynthesis or signaling through the delivery of effector proteins. [26]. For example, penetration-specific effector 1 (PSE1) from Phytophthora parasitica inhibits SA-mediated cell death and increased pathogen growth by promoting auxin accumulation at infection sites [44]. Due to the importance of phytohormones in the different trophic interactions, it would be of value to investigate their roles in the maintenance and switch from the biotrophic to necrotrophic stage in hemi-biotrophic pathogens, such as Phytophthora. This will shed light on how Phytophthora is able to successfully infect a host plant and avoid the hosts’ defense responses.
2.2.3. Triggers of Pathogen-Triggered PCD
dPCD requires preparation before PCD can be triggered/executed. Several cytoplasmic signals are implicated in dPCD triggering, such as calcium fluxes, accumulation of reactive oxygen species (ROS) and cytoplasmic acidification [45]. During the self-incompatibility (SI) response—the inability of a plant with functional pollen to set seeds when self-pollinated—in Papaver rhoeas, calcium influx triggers a signaling cascade, which results in rapid PCD of the incompatible pollen tubes [46]. In contrast, pPCD requires no preparation and is only triggered upon pathogen attack. The main pPCD trigger is cytoplasmic immune receptor-mediated recognition at the site of attack [47]. Calcium influxes, as well as accumulation of SA, ROS and nitric oxides (NO), are triggered upon pathogen perception during pPCD. SA signaling subsequently amplifies the ROS burst in a positive feedback loop, creating a toxic environment [48]. Some necrotrophic pathogens have been known to ‘hijack’ PCD machinery, where pathogens, such as Cochliobolus victoriae, secrete PCD triggering toxins [49][50]. Common triggers that are recognized by host receptors are effectors. Different Phytophthora effectors and their role in host PCD will be discussed in a later section.
3. Cell Death and Phytophthora Virulence
Different forms of pPCD will benefit either the plant or pathogen, depending on the type of plant–pathogen interaction and the trophic lifestyle of the pathogen [4][9][25]. Most Phytophthora spp. are hemi-biotrophic pathogens, meaning they feature a biotrophic life stage during early infection followed by a switch to necrotrophy during the later stages of host tissue colonization [4][51]. As the HR is generally considered most effective against biotrophic pathogens, while potentially benefiting necrotrophic pathogens, hemi-biotrophic pathogens—such as Phytophthora—are at a distinct advantage [52][53]. This response is a race between the host and pathogen, where the pathogen attempts to tip the balance toward suppression of host defense, and the host tries to launch an effective defense response to prevent infection [4].
Phytophthora spp. may have developed a strategy to ‘hijack’ a plant’s HR machinery, suppressing the HR during the biotrophic stage and inducing it during the necrotrophic stage [24][54][55]. This ‘hijack’ strategy is further supported by the production of haustoria that deliver defense-controlling pathogenicity factors and effectors, which function in keeping the host cell alive [56][57]. Conversely, the switch to necrotrophy, which involves the upregulation of specialized effector genes—such as Nep1-like proteins (NLPs)—aims to deliberately kill the host cell [58]. This is further supported by the similarities in metabolic enzyme expression between P. infestans during the necrotrophic stage and the necrotrophic pathogen Pythium ultimum [59]. This strategy increases the virulence of Phytophthora spp. through the differential expression and delivery of effectors at different stages of host plant infection and colonization [13][54][55][60][61][62].
References
- Kroon, L.P.N.M.; Brouwer, H.; De Cock, A.W.A.M.; Govers, F. The Phytophthora genus anno 2012. Phytopathology 2012, 102, 348–364.
- Lamour, K.H.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant Pathol. 2012, 13, 329–337.
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434.
- Boevink, P.C.; Birch, P.R.J.; Turnbull, D.; Whisson, S.C. Devastating intimacy: The cell biology of plant—Phytophthora interactions. New Phytol. 2020, 228, 445–458.
- Kinealy, C. This Great Calamity: The Irish Famine 1845-52; Gill & Macmillan Ltd.: Dublin, Ireland, 2006; pp. 10–98.
- Batini, F.E.; Hopkins, E.R. Phytophthora cinnamomi Rands—a root pathogen of the Jarrah Forest. Aust. For. 1972, 36, 57–68.
- Rani, G.D. Advances in Soil Borne Plant Diseases; New India Publishing: New Dehli, India, 2008; pp. 371–413.
- Mafurah, J.J.; Ma, H.; Zhang, M.; Xu, J.; He, F.; Ye, T.; Shen, D.; Chen, Y.; Rajput, N.A.; Dou, D. A virulence essential CRN effector of Phytophthora capsici suppresses host defense and induces cell death in plant nucleus. PLoS ONE 2015, 10, e0127965.
- Huysmans, M.; Coll, N.S.; Nowack, M.K. Dying two deaths—programmed cell death regulation in development and disease. Curr. Opin. Plant Biol. 2017, 35, 37–44.
- Tyler, B.M.; Tripathy, S.; Zhang, X.; Dehal, P.; Jiang, R.H.Y.; Aerts, A.; Arredondo, F.D.; Baxter, L.; Bensasson, D.; Beynon, J.L.; et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 2006, 313, 1261–1266.
- Engelbrecht, J.; Duong, T.A.; Prabhu, S.A.; Seedat, M.; Berg, N.V.D. Genome of the destructive oomycete Phytophthora cinnamomi provides insights into its pathogenicity and adaptive potential. BMC Genom. 2021, 22, 302.
- Hardham, A.R. Phytophthora cinnamomi. Mol. Plant Pathol. 2005, 6, 589–604.
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell. Microbiol. 2011, 13, 1849–1857.
- van Doorn, W.G.; Beers, E.P.; Dangl, J.L.; E Franklin-Tong, V.; Gallois, P.; Hara-Nishimura, I.; Jones, A.M.; Kawai-Yamada, M.; Lam, E.; Mundy, J.; et al. Morphological classification of plant cell deaths. Cell Death Differ. 2011, 18, 1241–1246.
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69.
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462.
- Burke, R.; Schwarze, J.; Sherwood, O.L.; Jnaid, Y.; McCabe, P.F.; Kacprzyk, J. Stressed to death: The role of transcription factors in plant programmed cell death induced by abiotic and biotic stimuli. Front. Plant Sci. 2020, 11, 1235.
- Kiraly, Z.; Barna, B.; Ersek, T. Hypersensitivity as a consequence, not the cause, of plant resistance to infection. Nature 1972, 239, 456–458.
- Hatsugai, N.; Kuroyanagi, M.; Yamada, K.; Meshi, T.; Tsuda, S.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 2004, 305, 855–858.
- Rojo, E.; Martín, R.; Carter, C.; Zouhar, J.; Pan, S.; Plotnikova, J.; Jin, H.; Paneque, M.; Serrano, J.J.S.; Baker, B.; et al. VPEγ exhibits a caspase-like activity that contributes to defense against pathogens. Curr. Biol. 2004, 14, 1897–1906.
- Avci, U.; Petzold, H.E.; Ismail, I.O.; Beers, E.P.; Haigler, C.H. Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J. 2008, 56, 303–315.
- Gao, M.; Showalter, A.M. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J. 1999, 19, 321–331.
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520.
- Zhang, M.; Li, Q.; Liu, T.; Liu, L.; Shen, D.; Zhu, Y.; Liu, P.; Zhou, J.-M.; Dou, D. Two cytoplasmic effectors of Phytophthora sojae regulate plant cell death via interactions with plant catalases. Plant Physiol. 2015, 167, 164–175.
- Daneva, A.; Gao, Z.; Van Durme, M.; Nowack, M.K. Functions and regulation of programmed cell death in plant development. Annu. Rev. Cell Dev. Biol. 2016, 32, 441–468.
- Mukhtar, M.S.; McCormack, M.E.; Argueso, C.T.; Pajerowska-Mukhtar, K.M. Pathogen tactics to manipulate plant cell death. Curr. Biol. 2016, 26, 608–619.
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758.
- Zhai, Z.; Ha, N.; Papagiannouli, F.; Hamacher-Brady, A.; Brady, N.; Sorge, S.; Bezdan, D.; Lohmann, I. Antagonistic regulation of apoptosis and differentiation by the Cut transcription factor represents a tumor-suppressing mechanism in Drosophila. PLoS Genet. 2012, 8, e1002582.
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113.
- Bin Nasir, K.H.; Takahashi, Y.; Ito, A.; Saitoh, H.; Matsumura, H.; Kanzaki, H.; Shimizu, T.; Ito, M.; Fujisawa, S.; Sharma, P.C.; et al. High-throughput in planta expression screening identifies a class II ethylene-responsive element binding factor-like protein that regulates plant cell death and non-host resistance. Plant J. 2005, 43, 491–505.
- Xie, H.-T.; Wan, Z.-Y.; Li, S.; Zhang, Y. Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023.
- Lee, M.H.; Jeon, H.S.; Kim, H.G.; Park, O.K. An Arabidopsis NAC transcription factor NAC4 promotes pathogen-induced cell death under negative regulation by microRNA164. New Phytol. 2017, 214, 343–360.
- Awwad, F.; Bertrand, G.; Grandbois, M.; Beaudoin, N. Auxin protects Arabidopsis thaliana cell suspension cultures from programmed cell death induced by the cellulose biosynthesis inhibitors thaxtomin A and isoxaben. BMC Plant Biol. 2019, 19, 512.
- Yuan, X.; Wang, H.; Cai, J.; Li, D.; Song, F. NAC transcription factors in plant immunity. Phytopathol. Res. 2019, 1, 3.
- Kaneda, T.; Taga, Y.; Takai, R.; Iwano, M.; Matsui, H.; Takayama, S.; Isogai, A.; Che, F.-S. The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J. 2009, 28, 926–936.
- Xu, X.; Chen, C.; Fan, B.; Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326.
- Yin, L.-L.; Xue, H.-W. The MADS29 transcription factor regulates the degradation of the nucellus and the nucellar projection during rice seed development. Plant Cell 2012, 24, 1049–1065.
- Qi, T.; Wang, J.; Huang, H.; Liu, B.; Gao, H.; Liu, Y.; Song, S.; Xie, D. Regulation of jasmonate-induced leaf senescence by antagonism between bHLH subgroup IIIe and IIId factors in Arabidopsis. Plant Cell 2015, 27, 1634–1649.
- Ueda, H.; Kusaba, M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol. 2015, 169, 138–147.
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227.
- Huang, S.; Zhang, X.; Fernando, W.G.D. Directing trophic divergence in plant-pathogen interactions: Antagonistic phytohormones with no doubt? Front. Plant Sci. 2020, 11, 600063.
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316.
- Birkenbihl, R.P.; Somssich, I.E. Transcriptional plant responses critical for resistance towards necrotrophic pathogens. Front. Plant Sci. 2011, 2, 76.
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309.
- Van Durme, M.; Nowack, M.K. Mechanisms of developmentally controlled cell death in plants. Curr. Opin. Plant Biol. 2016, 29, 29–37.
- Wilkins, K.A.; Bosch, M.; Haque, T.; Teng, N.; Poulter, N.S.; Franklin-Tong, V.E. Self-incompatibility-induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiol. 2015, 167, 766–779.
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1247–1256.
- Herrera-Vãsquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171.
- Lorang, J.; Kidarsa, T.; Bradford, C.S.; Gilbert, B.; Curtis, M.; Tzeng, S.-C.; Maier, C.S.; Wolpert, T.J. Tricking the guard: Exploiting plant defense for disease susceptibility. Science 2012, 338, 659–662.
- Lorang, J. Necrotrophic exploitation and subversion of plant defense: A lifestyle or just a phase, and implications in breeding resistance. Phytopathology 2019, 109, 332–346.
- Stam, R.; Jupe, J.; Howden, A.J.M.; Morris, J.A.; Boevink, P.C.; Hedley, P.E.; Huitema, E. Identification and characterisation CRN effectors in Phytophthora capsici shows modularity and functional diversity. PLoS ONE 2013, 8, e59517.
- Münch, S.; Lingner, U.; Floss, D.S.; Ludwig, N.; Sauer, N.; Deising, H.B. The hemibiotrophic lifestyle of Colletotrichum species. J. Plant Physiol. 2008, 165, 41–51.
- Jupe, J.; Stam, R.; Howden, A.J.; Morris, J.A.; Zhang, R.; Hedley, P.E.; Huitema, E. Phytophthora capsici-tomato interaction features dramatic shifts in gene expression associated with a hemi-biotrophic lifestyle. Genome Biol. 2013, 14, R63.
- Liu, T.; Ye, W.; Ru, Y.; Yang, X.; Gu, B.; Tao, K.; Lu, S.; Dong, S.; Zheng, X.; Shan, W.; et al. Two host cytoplasmic effectors are required for pathogenesis of Phytophthora sojae by suppression of host defenses. Plant Physiol. 2011, 155, 490–501.
- Maximo, H.J.; Dalio, R.O.; Litholdo, C.G.; Felizatti, H.L.; Machado, M.A. PpCRN7 and PpCRN20 of Phythophthora parasitica regulate plant cell death leading to enhancement of host susceptibility. BMC Plant Biol. 2019, 19, 544.
- Wang, S.; Welsh, L.; Thorpe, P.; Whisson, S.C.; Boevink, P.C.; Birch, P.R.J. The Phytophthora infestans haustorium is a site for secretion of diverse classes of infection-associated proteins. MBio 2018, 9, e01216-18.
- Judelson, H.S.; Ah-Fong, A.M.V. Exchanges at the plant-oomycete interface that influence disease. Plant Physiol. 2019, 179, 1198–1211.
- Dong, S.; Kong, G.; Qutob, D.; Yu, X.; Tang, J.; Kang, J.; Dai, T.; Wang, H.; Gijzen, M.; Wang, Y. The NLP toxin family in Phytophthora sojae includes rapidly evolving groups that lack necrosis-inducing activity. Mol. Plant Microbe Interact. 2012, 25, 896–909.
- Ah-Fong, A.M.V.; Shrivastava, J.; Judelson, H.S. Lifestyle, gene gain and loss, and transcriptional remodeling cause divergence in the transcriptomes of Phytophthora infestans and Pythium ultimum during potato tuber colonization. BMC Genom. 2017, 18, 764.
- Van Damme, M.; Bozkurt, T.O.; Cakir, C.; Schornack, S.; Sklenář, J.; Jones, A.M.E.; Kamoun, S. The Irish potato famine pathogen Phytophthora infestans translocates the CRN8 kinase into host plant cells. PLoS Pathog. 2012, 8, e1002875.
- Li, Q.; Ai, G.; Shen, D.; Zou, F.; Wang, J.; Bai, T.; Chen, Y.; Li, S.; Zhang, M.; Jing, M.; et al. A Phytophthora capsici effector targets ACD11 binding partners that regulate ROS-mediated defense response in Arabidopsis. Mol. Plant 2019, 12, 565–581.
- Toljamo, A.; Blande, D.; Munawar, M.; Kärenlampi, S.O.; Kokko, H. Expression of the GAF sensor, carbohydrate-active Enzymes, elicitins, and RXLRs differs markedly between two Phytophthora cactorum isolates. Phytopathology 2019, 109, 726–735.
More
