Metabolic syndrome (MS) is a multifactorial disease entity and is not fully understood. Growing evidence suggests that early exposure to bisphenol A (BPA) is a significant risk factor for the development of metabolic diseases. BPA is a monomer used in the manufacturing of polycarbonate plastics, thermal receipt paper, and epoxy resins. Owing to its widespread use, BPA has been detected in human fluids and tissues, including blood, placental and breast milk, and follicular fluid.
- bisphenol A
- endocrine disruption
- metabolic disorder
1. Introduction
2. The Impact Effects of BPA Exposure on Human Health
3. Sources of BPA Exposure and BPA Metabolism
BPA is widely used in the manufacturing of many consumer products. This results in consumer dietary exposure to BPA. Thus, humans can easily be exposed, including mothers and infants. However, BPA exposure in humans can also occur via water, air, and soil. Nevertheless, the majority of exposure comes from the consumption of BPA-contaminated foods and beverages. Food, particularly canned food, is often regarded as the most significant source of BPA. Food contamination with BPA is typically caused by contact with food packaging products containing epoxy resins and polycarbonate monomers [29]. The predominant source of BPA is polycarbonate, which includes food-contact materials such as baby bottles, food containers, and epoxy resins that are used as cover linings for canned beverages and food [30][31]. Dietary and non-dietary exposure to BPA sources is summarised in Figure 1. Based on the available data from previous studies, it is apparent that exposure to BPA from dietary sources is higher than that from non-dietary items. Food was shown to contribute to more than 90% of the total exposure to BPA, while exposure from other sources accounted for less than 5% for all age groups [30].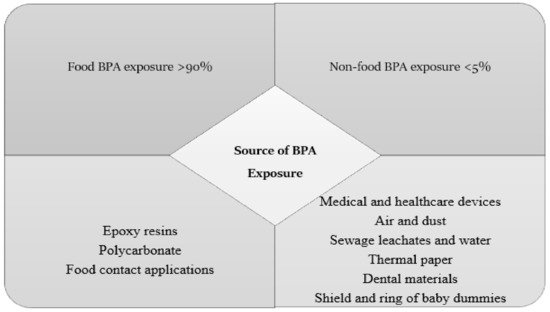
4. Different Doses of BPA Exposure
The detection and toxicological analysis of various chemicals are critical for understanding environmental pollutants and their risks, as well as how these pollutants may affect public health at multiple levels. Practical experimental research is crucial for discovering the lowest doses that pose a health risk, which is known as the lowest observed effect level (LOAEL), and the doses that do not pose a health risk, known as the no observed adverse effect level. Despite the fact that a large amount of research on the toxic effects and hormonal activity of BPA in animal models has been published, there have been significant differences in the outcomes of these studies in terms of both the nature of the effects observed and the levels at which they occur. Several studies have demonstrated that exposure to BPA, even at low doses, can result in adverse health effects. Infants, children, and pregnant women are those most harmed by exposure to BPA. There is inconsistency in the results of studies on whether in utero exposure to BPA leads to the remodelling and alteration of the metabolisms of children. There is also controversy about the dose that causes defects in metabolic parameters in children. According to the U.S. Environmental Protection Agency (EPA), the reference dose for tolerable daily BPA exposure for the human population without any considerable risk of deleterious effects during life is 50 µg/kg/d, according to rodent studies. In 2015, the European Food Safety Authority reduced the toxicological threshold for BPA from 50 to 4 µg/kg/d [35].5. Metabolic Effect of Prenatal Exposure
Several studies have shown that prenatal BPA exposure affects pancreatic β-cells, insulin secretion, and glucose metabolism. Some also suggest that BPA has an obesogenic and diabetogenic action, especially pronounced when exposure occurs in the early stage of development in humans and animals, which is a critical period of growth and differentiation of metabolically active tissues. It was confirmed by different experiments that BPA induced metabolic disorders in human and animal models of prenatal exposure.
5.1 Metabolic Effect of Prenatal BPA Exposure in Animal Studies
Long et al. suggested that gestational exposure from gestational day (GD) 7.5 until GD 16.5 to a low BPA dose (1 µg/kg/day) in C57BL/6J mice was associated with sex-dependent glucose and lipid metabolic dysfunction. The study’s findings showed that exposure in adult males (14 weeks), but not females, to low doses of BPA caused increased hepatic TG and glycogen levels, in addition to significantly increased levels of fasting blood glucose, insulin, IPGTT, and IPITT. Interestingly, BPA levels were found to be increased in the serum of male mice exposed to 1 μg/kg/day BPA, but no significant difference was observed in female mice relative to non-BPA-exposed (control) mice [36]. Another study conducted by Diamnte et al., (2021) did not show any effect on plasma insulin levels or glucose tolerance in female offspring at 10 or 21 weeks. Male exposure to low gestational BPA significantly decreased BW at 4 weeks and induced a faster glucose clearance based on IPGTT results at 10 and 21 weeks [37]. The study was conducted to investigate developmental exposure to a very low dose of BPA; interestingly, in Fischer 344 rat offspring, it was demonstrated that exposure to a very low BPA dose (0.5 µg /BPA/kg) correlated with insulin hypersecretion, while 50 µg BPA/kg was associated with reduced insulin secretion in both rat offspring and dams (5- and 52-week-old mice) [38]. Recently, a study was conducted to examine the impact of prenatal exposure to a very low dose of BPA (2.5 µg/kg/day) on hepatic lipid metabolism in male and female SD rat foetuses. They assessed the effect of very low BPA exposure on lipid metabolism parameters in pregnant rats. The findings of the study showed no significant differences in serum lipid profiles between BPA-exposed animals and vehicle control animals. In addition, the authors measured the effects of prenatal exposure to 2.5 µg/kg/day of BPA, and the results showed no changes in hepatic cholesterol or triglyceride content in rat foetuses when comparing the exposed and non-exposed groups [37]. In a study conducted in C57BL/6J mice to investigate gestational exposure to 1, 10, 100, and 1000 µg/kg/day of BPA, the study demonstrated that oral exposure to low (1 µg/kg/day) and high (1000 µg/kg/day) doses of BPA significantly increased the hepatic TG content in both male and female offspring. In the same study investigating the effect of gestational exposure to a low dose of BPA (1 µg/kg/day) on hepatic lipid accumulation in 14-week offspring, it was shown that gestational exposure to a low dose of BPA in adult male offspring caused increased hepatic TG and glycogen levels [36]. In summary, animal studies have shown that prenatal BPA exposure, even at very low concentrations (<4 μg/kg/day), may decrease birth weight and body weight in children. Moreover, it may cause the dysregulation of the glucose and lipid metabolism in offspring. Moreover, its effect was clear in adulthood, as many studies showed an increase in offspring BW and body fat composition, as well as increased lipid parameters, including hepatic TG and FFA.5.2 Metabolic Effect of Prenatal BPA Exposure in Children (Epidemiological Studies)
SIn the literature, studies have shown conflicting findings on the outcomes of BPA exposure. ResearchersWe found six epidemiological studies that investigated the effect of prenatal BPA exposure on metabolic parameters in children.
A prospective cohort study was conducted at a maternity and child health hospital in Shanghai, China. The results showed that a moderate maternal prenatal BPA level (1.14 ng/mL) was associated with higher plasma glucose levels in boys. In contrast, in girls, the plasma glucose level was lower (0.26 mmol/L) with moderate prenatal BPA levels; however, the difference was not significant. The study also showed no associations between prenatal BPA exposure and children’s body weight, BMI, skinfold thickness, serum lipid levels, or insulin levels in children aged two years old, either for girls or boys [39]. In 2017, a cross-sectional study was conducted on 250 Mexican mother–child pairs to investigate the relationship between prenatal and childhood exposure to BPA and phthalates on BMI z-score, waist circumference, and the sum of tricep and subscapular skinfold thicknesses in Mexican children. Spot urine was collected from mothers in the third trimester and at 8–14 years old from children. The findings showed that increased BPA exposure was positively associated with the sum of skinfold thicknesses and BMI z-scores in girls but not in boys [40]. Vafeiadi et al. analysed BPA levels in spot urine samples collected from pregnant Greek women in the first trimester of pregnancy and their children at two-and-a-half and four years of age using an Olympus 2700 immunoassay system. TIn this study, the LOD was equal to 0.01 ng/mL, and the average %>LOD was >99% in mothers and children. This study showed that urinary BPA concentrations were lower in pregnant women than in their children. Higher prenatal BPA concentrations were associated with BMI and adiposity measures that were lower in girls and higher in boys aged 1–4 years. However, there was no substantial evidence that BMI was different among children with high prenatal BPA concentrations compared to those with low prenatal BPA concentrations, based on the 80th percentile [41]. Notably, the association between prenatal exposure to BPA and the function of metabolic markers appeared to be modified by sex. Martin et al. found an inverse relationship between maternal urinary BPA and ADP levels in men. In contrast, female infants were shown to have higher leptin levels than males[42]. A birth cohort study conducted on 537 mother–child pairs of Mexican-American origin (LOD = 0.4ng/mL) showed that late-pregnancy urinary BPA levels (26 weeks) were associated with increased leptin levels in boys, while early-pregnancy BPA levels (13 weeks) were positively related to ADP levels in 9-year-old girls [42]. Remarkably, these cohort studies determined the relationship between prenatal exposure to BPA and its effect on metabolism in children. However, most of these studies were not designed to investigate the relationship between prenatal BPA exposure and biochemical parameters. Some biochemical parameters were determined, such as birth weight, waist circumference, lipid profile, glucose and insulin levels, and adipokine levels. Briefly, the results of these studies showed that prenatal BPA exposure affected the metabolic parameters of children in some manner. Nevertheless, exposure to BPA during pregnancy differed in its effect on children in terms of whether the exposure period was during the early or late period of pregnancy, as well as in terms of sex.References
- Talsness, Chris E.; Andrade, Anderson JM; Kuriyama, Sergio N.; Taylor, Julia A.; and Vom Saal, Frederick S.; Components of plastic: Experimental studies in animals and relevance for human health. Philosophical Transactions of the Royal Society B: Biological Sciences 2009, 364, 2079–2096, 10.1098/rstb.2008.0281.
- Kloukos, Dimitrios; Pandis, Nikolaos; and Eliades, Theodore; In vivo bisphenol-A release from dental pit and fissure sealants: A systematic review. Journal of Dentistry 2013, 41, 659-667, 10.1016/j.jdent.2013.04.012.
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P.; Bisphenol A environmental exposure and the detrimental effects on human metabolic health: is it necessary to revise the risk assessment in vulnerable population?. Journal of Endocrinological Investigation 2015, 39, 259-263, 10.1007/s40618-015-0336-1.
- Lorber, Matthew, Arnold Schecter, Olaf Paepke, William Shropshire, Krista Christensen, and Linda Birnbaum.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environment international 2015, 77, 55-62, https://doi.org/10.1016/j.envint.2015.01.008.
- D.J.P. Barker; The Developmental Origins of Adult Disease. Journal of the American College of Nutrition 2004, 23, 588S-595S, 10.1080/07315724.2004.10719428.
- Farahani, Masoumeh; Mostafa Rezaei‐Tavirani; Babak Arjmand; A systematic review of microRNA expression studies with exposure to bisphenol A. Journal of Applied Toxicology 2020, 41, 4-19, 10.1002/jat.4025.
- Almeida, Susana, António Raposo, Maira Almeida‐González, and Conrado Carrascosa; Bisphenol A: Food Exposure and Impact on Human Health. Comprehensive Reviews in Food Science and Food Safety 2018, 17, 1503-1517, 10.1111/1541-4337.12388.
- Lejonklou, Margareta H.; Linda Dunder; Emelie Bladin; Vendela Pettersson; Monika Rönn; Lars Lind; Tomas B. Waldén; and P. Monica Lind; Effects of Low-Dose Developmental Bisphenol A Exposure on Metabolic Parameters and Gene Expression in Male and Female Fischer 344 Rat Offspring. Environmental Health Perspectives 2017, 125, 067018, 10.1289/ehp505.
- Rochester, Johanna R.; Bisphenol A and human health: A review of the literature. Reproductive Toxicology 2013, 42, 132-155, 10.1016/j.reprotox.2013.08.008.
- Catherine A. Richter; Linda S. Birnbaum; Francesca Farabollini; Retha R. Newbold; Beverly S. Rubin; Chris E. Talsness; John G. Vandenbergh; Debby R. Walser-Kuntz; Frederick S. Vom Saal; In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology 2007, 24, 199-224, 10.1016/j.reprotox.2007.06.004.
- Bo Zhang; Yuan He; Hongkai Zhu; Xiongfei Huang; Xueyuan Bai; Kurunthachalam Kannan; Tao Zhang; Concentrations of bisphenol A and its alternatives in paired maternal–fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environment International 2020, 136, 105407, 10.1016/j.envint.2019.105407.
- Han, Changwoo; and Yun-Chul Hong.; ypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Current hypertension reports 2016, 18, 11.
- Moon, Shinje; Sung Hoon Yu; Chang Beom Lee; Young Joo Park; Hyung Joon Yoo; and Dong Sun Kim; Science of the Total Environment Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Science of the Total Environment 2020, 763, 142941, 10.1016/j.scitotenv.2020.142941.
- Samuel, Legeay; and Sébastien Faure.; Is bisphenol A an environmental obesogen?. Fundamental & clinical pharmacology 2017, 31, 594-609, https://doi.org/10.1111/fcp.12300.
- Catherine A. Richter; Linda S. Birnbaum; Francesca Farabollini; Retha R. Newbold; Beverly S. Rubin; Chris E. Talsness; John G. Vandenbergh; Debby R. Walser-Kuntz; Frederick S. Vom Saal; In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology 2007, 24, 199-224, 10.1016/j.reprotox.2007.06.004.
- Bo Zhang; Yuan He; Hongkai Zhu; Xiongfei Huang; Xueyuan Bai; Kurunthachalam Kannan; Tao Zhang; Concentrations of bisphenol A and its alternatives in paired maternal–fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environment International 2020, 136, 105407, 10.1016/j.envint.2019.105407.
- Han, Changwoo; and Yun-Chul Hong.; ypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Current hypertension reports 2016, 18, 11.
- Moon, Shinje; Sung Hoon Yu; Chang Beom Lee; Young Joo Park; Hyung Joon Yoo; and Dong Sun Kim; Science of the Total Environment Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Science of the Total Environment 2020, 763, 142941, 10.1016/j.scitotenv.2020.142941.
- Farahani, Masoumeh; Mostafa Rezaei‐Tavirani; Babak Arjmand; A systematic review of microRNA expression studies with exposure to bisphenol A. Journal of Applied Toxicology 2020, 41, 4-19, 10.1002/jat.4025.
- Samuel, Legeay; and Sébastien Faure.; Is bisphenol A an environmental obesogen?. Fundamental & clinical pharmacology 2017, 31, 594-609, https://doi.org/10.1111/fcp.12300.
- Pim Wassenaar; Leonardo Trasande; Juliette Legler; Systematic Review and Meta-Analysis of Early-Life Exposure to Bisphenol A and Obesity-Related Outcomes in Rodents. Environmental Health Perspectives 2017, 125, 106001, 10.1289/ehp1233.
- Tang-Péronard, J. L.; H. R. Andersen; T. K. Jensen; B. L. Heitmann; Endocrine-disrupting chemicals and obesity development in humans: A review. Obesity Reviews 2011, 12, 622-636, 10.1111/j.1467-789x.2011.00871.x.
- Vom Saal, Frederick S.; Susan C. Nagel; Benjamin L. Coe; Brittany M. Angle; Julia A. Taylor; The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Molecular and Cellular Endocrinology 2012, 354, 74-84, 10.1016/j.mce.2012.01.001.
- Boudalia, Sofiane; Aissam Bousbia; Boualem Boumaaza; Malha Oudir; Marie Chantal Canivenc Lavier; Relationship between endocrine disruptors and obesity with a focus on bisphenol A: a narrative review. BioImpacts 2020, 11, 289-300, 10.34172/bi.2021.33.
- Zulkifli, Sarah; Amirah Abdul Rahman; Siti Hamimah Sheikh Abdul Kadir; Noor Shafina Mohd Nor; Bisphenol A and its effects on the systemic organs of children. European Journal of Pediatrics 2021, 180, 3111-3127, 10.1007/s00431-021-04085-0.
- Mackay, Harry; Zachary R. Patterson; Rim Khazall; Shoyeb Patel; Dina Tsirlin; Alfonso Abizaid; Organizational Effects of Perinatal Exposure to Bisphenol-A and Diethylstilbestrol on Arcuate Nucleus Circuitry Controlling Food Intake and Energy Expenditure in Male and Female CD-1 Mice. Endocrinology 2013, 154, 1465-1475, 10.1210/en.2012-2044.
- Mouneimne, Youssef; Mona Nasrallah; Nathalie Khoueiry-Zgheib; Lara Nasreddine; Nancy Nakhoul; Hussein Ismail; Mohamad Abiad; Lynn Koleilat; Hani Tamim; Bisphenol A urinary level, its correlates, and association with cardiometabolic risks in Lebanese urban adults. Environmental Monitoring and Assessment 2017, 189, 517, 10.1007/s10661-017-6216-8.
- Bertoli, Simona; Alessandro Leone; Alberto Battezzati; Human Bisphenol A Exposure and the “Diabesity Phenotype”. Dose-Response 2015, 13, 26858585, 10.1177/1559325815599173.
- Chooi, Yu Chung; Cherlyn Ding; Faidon Magkos; The epidemiology of obesity. Metabolism 2019, 92, 6-10, https://doi.org/10.1016/j.metabol.2018.09.005.
- Tinne, Geens; Dominique Aerts; Carl Berthot; Jean-Pierre Bourguignon; Leo Goeyens; Philippe Lecomte; Guy Maghuin-Rogister et al.; A review of dietary and non-dietary exposure to bisphenol-A. Food and Chemical Toxicology 2012, 50, 3725-3740, 10.1016/j.fct.2012.07.059.
- Laura N. Vandenberg; Russ Hauser; Michele Marcus; Nicolas Olea; Wade V. Welshons; Human exposure to bisphenol A (BPA). Reproductive Toxicology 2007, 24, 139-177, 10.1016/j.reprotox.2007.07.010.
- Nishikawa, Miyu; Hidetomo Iwano; Risa Yanagisawa; Nanako Koike; Hiroki Inoue; Hiroshi Yokota; Placental Transfer of Conjugated Bisphenol A and Subsequent Reactivation in the Rat Fetus. Environmental Health Perspectives 2010, 118, 1196-1203, 10.1289/ehp.0901575.
- Balakrishnan, Biju; Kimiora Henare; Eric B. Thorstensen; Anna P. Ponnampalam; Murray D. Mitchell; Transfer of bisphenol A across the human placenta. American Journal of Obstetrics and Gynecology 2010, 202, 393.e1-393.e7, 10.1016/j.ajog.2010.01.025.
- Lee, Jangwoo; Kyungho Choi; Jeongim Park; Hyo-Bang Moon; Gyuyeon Choi; Jeong Jae Lee; Eunsook Suh; Hai-Joong Kim; So-Hee Eun; Gun-Ha Kim; et al.Geum Joon ChoSung Koo KimSungjoo KimSu Young KimSeunghyo KimSoyong EomSooran ChoiYoung Don KimSungkyoon Kim Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother–neonate pairs. Science of The Total Environment 2017, 626, 1494-1501, 10.1016/j.scitotenv.2017.10.042.
- E. F. S. A.; Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal 2015, 13, 3978, 10.2903/j.efsa.2015.3978.
- Long, Zi; Junshu Fan; Guangyuan Wu; Xiyu Liu; Hao Wu; Jiangzheng Liu; Yao Chen; Shuhao Su; Xiaodong Cheng; Zhongrui Xu; et al.Hongfei SuMeng CaoChunping ZhangChunxu HaiXin Wang Gestational bisphenol A exposure induces fatty liver development in male offspring mice through the inhibition of HNF1b and upregulation of PPARγ. Cell Biology and Toxicology 2020, 37, 65-84, 10.1007/s10565-020-09535-3.
- Diamante, Graciel; Ingrid Cely; Zacary Zamora; Jessica Ding; Montgomery Blencowe; Jennifer Lang; Abigail Bline; Maya Singh; Aldons J. Lusis; Xia Yang; et al. Systems toxicogenomics of prenatal low-dose BPA exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environment International 2020, 146, 106260, 10.1016/j.envint.2020.106260.
- Manukyan, Levon; Linda Dunder; P. Monica Lind; Peter Bergsten; Margareta Halin Lejonklou; Developmental exposure to a very low dose of bisphenol A induces persistent islet insulin hypersecretion in Fischer 344 rat offspring. Environmental Research 2019, 172, 127-136, 10.1016/j.envres.2019.02.009.
- Ouyang, Fengxiu; Guang-Hui Zhang; Kun Du; Lixiao Shen; Rui Ma; Xia Wang; Xiaobin Wang; Jun Zhang; Maternal prenatal urinary bisphenol A level and child cardio-metabolic risk factors: A prospective cohort study. Environmental Pollution 2020, 265, 115008, 10.1016/j.envpol.2020.115008.
- Yang, Tiffany; Karen E. Peterson; John Meeker; Brisa N. Sánchez; Zhenzhen Zhang; Alejandra Cantoral; Maritsa Solano; Martha Téllez Rojo; Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environmental Research 2017, 156, 326-333, 10.1016/j.envres.2017.03.038.
- Vafeiadi, Marina; Theano Roumeliotaki; Antonis Myridakis; Georgia Chalkiadaki; Eleni Fthenou; Eirini Dermitzaki; Marianna Karachaliou; Katerina Sarri; Maria Vassilaki; Euripides G. Stephanou; et al.Manolis KogevinasLeda Chatzi Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environmental Research 2016, 146, 379-387, 10.1016/j.envres.2016.01.017.
- Jillian Ashley-Martin; Linda Dodds; Tye E Arbuckle; Adrienne S Ettinger; Gabriel D Shapiro; Mandy Fisher; Anne-Sophie Morisset; Shayne Taback; Maryse F Bouchard; Patricia Monnier; et al.Renee DallaireWilliam D Fraser A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environmental Health 2014, 13, 13-84, 10.1186/1476-069x-13-84.
