Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Saad Saeed Alghamdi and Version 2 by Vivi Li.
Camel milk (CM) constitutes an important dietary source in the hot and arid regions of the world. CM is a colloidal mixture of nutritional components (proteins, carbohydrates, lipids, vitamins, and minerals) and non-nutritional components (hormones, growth factors, cytokines, immunoglobulins, and exosomes).
- bioactive peptides
- camel milk
- cancer
- diabetes
- molecular signaling
- exosomes
- human diseases
- therapeutics
1. Introduction
It is widely recognized that camel milk (CM) is a valuable nutritional source for people living in the hot and arid regions of the world. CM is a complex biological fluid that contains not only nutritional components, including macronutrients and micronutrients, but also non-nutritional components, such as hormones, growth factors, immune system products, and exosomes [1][2][3][1,2,3].
Evidence has shown that CM is used for therapeutic benefits against various diseases and conditions [4][5][6][7][8][9][4,5,6,7,8,9]. To this end, CM has been reported to provide therapeutic benefits against various pathophysiological conditions, including diabetes, hypertension, cancer, inflammatory and allergic responses as discussed in subsequent sections [1][7][10][11][12][13][1,7,10,11,12,13]. Amongst all these beneficial properties, its therapeutic potential against diabetes has been extensively explored. Reports have shown that CM consumption not only lowers the prevalence of diabetes, but also improves the detrimental effects of hyperglycemic condition, as well as reducing the insulin therapy required by type-1 diabetic patients [5][6][7][11][14][15][16][17][18][5,6,7,11,14,15,16,17,18]. The antidiabetic potentials of CM were mainly accredited to the presence of insulin and/or insulin-like peptides in CM. However, this belief has undergone a paradigm shift as a consequence of independent studies that have explicitly highlighted the fact that that these insulin/insulin-like peptides are completely hydrolyzed by gastrointestinal enzymes [19][20][21][19,20,21] This has prompted the researcher to study the CM protein hydrolysates and their bioactive peptides; indeed, lately, this area of research has garnered much attention all across the globe [5][22][23][24][5,22,23,24].
2. Brief Overview of Camel Milk (CM) Components
2.1. Nutritional Components (Macronutrients and Micronutrients)
Over the years, much attention has been diverted toward the consumption of CM as an alternative to milk from other sources, apparently due to its therapeutic benefits and lower allergenicity issues. Indeed, the physicochemical composition of CM differs considerably from that of milk from other domestic dairy animals. It has been argued that the physicochemical composition of CM is dependent on various factors, including geographical origin, breed, age, parity, season, ecology, feed and feeding approach, and also on the analytical measurement procedures used to measure the components [25][26][36,37]. Milk is a composite mixture of various proteins, carbohydrates, lipids, minerals, vitamins, etc., and CM is known to contain low amounts of fats, proteins, and oligosaccharides and high amounts of vitamins, minerals, and water [27][28][38,39]. Among macronutrients, according to meta-analysis and literature data, the fat composition of CM varies but is reportedly lower than that of milk from other domestic animals [26][37]. The fat component consists mainly of triglycerides and, importantly, very low levels of cholesterol [29][30][40,41]. Interestingly, the constituent of CM that is considered to have the greatest impact on its nutritional value and confer beneficial properties are proteins. Nevertheless, it is not entirely clear whether the beneficial effects of CM can be accredited to a particular component acting on one specific target or whether they are the result of the harmonious actions of multiple components in the system [7].
An overview of various active research endeavors related to CM proteins in food science is depicted in Figure 1. Basically, the main protein constituents of CM are casein proteins (CPs) and whey proteins (WPs). The proportion of CPs in CM is 50–80%, consisting of αS1-casein, αS2-casein, β-casein, and κ-casein, with an abundance of β-casein [31][42]. The abundance of β-casein in CM has been considered responsible for the distinctive and intriguing biological characteristics of CM, akin to those of human milk [32][25]. Furthermore, CPs of CM possess higher molecular weight than CPs of bovine milk (BM) [22][31][33][22,33,42]. In addition, the dimensions of casein micelles in CM are greater than those of casein micelles in BM [34][43]. WPs represent another important constituent, comprising approximately 30% of total CM proteins [35][36][44,45]. The major WPs in CM are α-lactalbumin (α-LA), camel serum albumin (CSA), lactoferrin (LF), and thermostable immunoglobulins (Igs) (IgG and IgM). In contrast to BM, wherein β-lactoglobulin (β-LG) represents the major WP component, the main WP component in CM is α-LA (50%), followed by CSA (35%) [22][32][37][22,25,46]. The levels of β-LG in CM are either very low or absent altogether. As β-LG is the main protein in BM responsible for eliciting allergic responses, the absence of β-LG in CM is likely responsible for its renowned anti-allergic characteristics [29][37][40,46]. Additionally, CM contains many proteins possessing immunomodulatory properties, including peptidoglycan recognition proteins (PGRPs), Igs, lactoperoxidase (LP), CSA, LF, insulin, and insulin-like proteins [26][27][38][37,38,47]. Among Igs, CM WPs are further reported to contain IgG1 and IgG2, IgG variants that are not present in BM. Apart from CPs and WPs, CM has some milk fat globule membrane proteins, such as milk fat globule-EGF factor 8, adipophilin, lactadherin, fatty acid synthase, and xanthine dehydrogenase. Furthermore, CM has been reported to possess high amounts of N-acetyl-β-glucosaminidase (NAGase), LP, and lysozymes (LZ) [24][39][24,48], which confer anti-bacterial, anti-fungal and anti-viral properties.
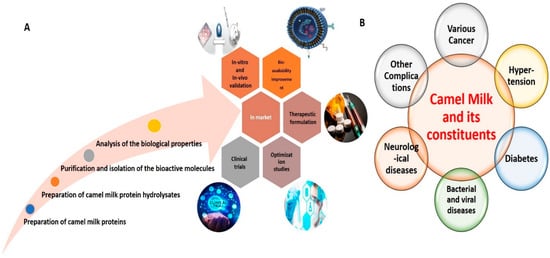

Figure 1. Representative image delineating various active research endeavors related to CM in food sciences (A). Representative image delineating the beneficial effects of CM and its constituents against various human diseases (B).
Among micronutrients, both water and fat-soluble vitamins, i.e., A, D, E, K, B complex, and C, are present in CM [28][40][41][39,49,50]. CM is also rich in minerals, which are present in the following order of abundance: K > Cl > Ca > P > Na > Mg, Cu, Fe, Zn, etc. [24][27][41][42][43][24,32,38,50,51]. It has been reported that the Fe content of CM is approximately 10-fold that in BM and that the K and Cu contents are also higher [27][41][38,50]. These elements play important roles in various biological processes, serving either as catalytic or structural components or having specific functions, all indispensable for cellular function [27][41][38,50], thereby imparting additional value to CM. In particular, the concentrations of heavy metals in CM are in the harmless range [27][41][38,50].
2.2. Pharmacological Properties of CM Bioactive Molecules against Various Pathological Conditions
2.2.1. Molecular Intricacies of Anti-Cancer Effects
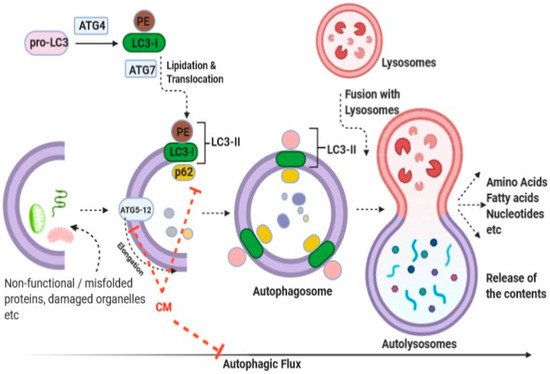
Cancer is a very complex disease caused by genetic and/or epigenetic changes that lead to uncontrolled cell growth. According to GLOBOCAN 2020 reports, cancer is the second leading cause of death worldwide, with an estimated 19.3 million cases diagnosed and 10.0 million deaths [44][91]. The report also predicts that the number of cases will nearly double by 2040 [44][91]. During tumorigenesis and tumor progression, cancer cells undergo several alterations, including gaining the ability to proliferate, independent of normal growth-promoting or inhibitory signals, invading and migrating to surrounding or distant tissues/sites, promoting angiogenesis, escaping apoptosis, and avoiding replicative senescence and immune responses. It has been posited that these characteristic features are acquired from alterations in cellular signaling pathways that, in normal cells, regulate controlled cell growth. Traditionally, it was believed that the consumption of CM helped reduce the incidence of cancers; however, molecular evidence for this health benefit is limited [45][46][47][70,71,74]. Various in vitro studies have revealed the inhibitory effects of CM and its constituents against various forms of cancer, apparently through induction of apoptotic pathways [2][12][47][48][49][50][51][52][2,12,69,72,73,74,75,76]. In parallel, animal experimental data have also demonstrated the inhibitory potential of CM and its constituents against various forms of cancer [53][54][55][77,78,79]. Interestingly, CM has been shown to interfere with the expression of cancer-related mediators at both gene and protein levels [48][69]. Furthermore, apart from investigations into the traditional processes, reports have linked autophagic responses with the ameliorative effects of CM against cancer [46][71]. Autophagy, a process through which the system maintains cellular homeostasis through the removal of dysfunctional organelles and/or dysfunctional/misfolded proteins, has also been studied in relation to particular complications [56][92]. To this end, Krishnankutty et al. showed that CM exerts anti-proliferative effects on human colorectal cancer cells by orchestration of autophagic responses [46][71]. An overview of the autophagy process, together with the plausible targets of CM, is illustrated in Figure 2. Interestingly, using the GFP-LC3 puncta assay, Krishnankutty et al. clearly showed an increment in LC3-II formation following treatment with CM. Moreover, a dose-dependent decrease in p62 (sequestosome 1) was observed following treatment with CM. A dose-dependent decrease in the expression of Atg5-12 proteins was observed in two cell lines studied following treatment with CM. Collectively, the group convincingly demonstrated that CM exerted cytotoxicity responses toward human colorectal cancer cells, seemingly through orchestration of autophagic responses, although the identity of the component of CM responsible was not ascertained [46][71]. Further studies in this direction would be highly instrumental in identifying the active “wonder” component(s) of CM that can selectively kill cancer cells.
Figure 2. Representative image delineating the anti-cancer effect of CM against human colorectal and breast cancer cells through induction of autophagic responses (abbreviations are: LC3: a microtubule- associated protein 1 light chain 3 (LC3-I precursor); PE: phosphatidylethanolamine; LC3-II: Lipidated LC3-I; ATG4 and ATG7, autophagy proteins; ATG5-12, a complex of autophagy proteins; p62: sequestosome 1). Modified from Izadi et al., Journal of Functional food [24].
Furthermore, as a matter of fact, widely used chemotherapeutic drugs have been shown to manifest many negative side effects. Nevertheless, CM has been shown to manifest many beneficial properties without any side effects. Interestingly, these studies suggest that components of CM can modulate signaling cascades and, thus, may represent an alternative to traditional chemotherapeutic drugs and/or act as adjuncts and complements in the management of various disorders, including cancer.
2.2.2. Molecular Intricacies of CM’s Anti-Hypertensive Potential
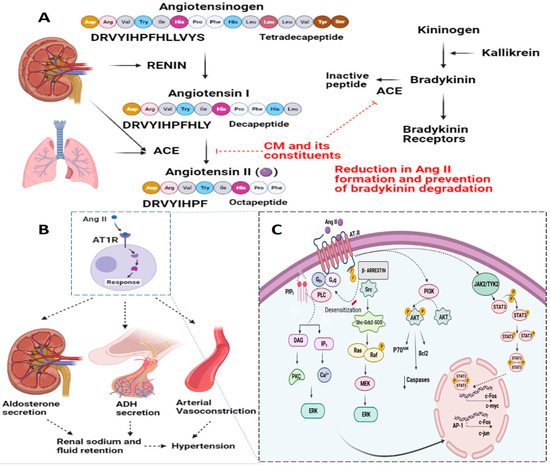
Hypertension, a high blood pressure (BP) condition, leads to severe health complications and increases the risk of heart disease, stroke, and eventually death. Elevated BP causes long-term detrimental effects on the heart and other organs. In recent years, due to the increasing incidence of hypertensive heart disease, hypertension has emerged as a leading cause of cardiovascular-related morbidity and mortality worldwide [57][58][59][57,58,93]. Several explanations for the mechanism governing hypertension have been proposed [60][61][62][94,95,96]. Accumulating studies have shown that CM and CM-derived proteins and peptides have anti-hypertensive effects [12][22][33][50][63][12,22,33,73,82]; generally attributed to the inhibition of angiotensin-converting enzyme (ACE) [33][64][65][33,81,97]. To this end, rwesearchers have recently performed comparative profiling of the bio-macromolecular fractions of CM and BM and ascertained their anti-hypertensive potentials following simulated gastrointestinal digestion [33], and theyour recent report identified novel anti-hypertensive bioactive peptides from CM protein hydrolysates (CMPHs) and delineated the underlying molecular mechanism of these peptides [10]. The renin–angiotensin system (RAS) is a principal regulatory hormonal system that plays important roles in hypertension [57][58][62][66][57,58,96,98]. The important effector RAS hormone angiotensin II (Ang II), a vasoconstrictive peptide, has been implicated in regulating the physiological effects of the regulation of BP and is associated with the pathophysiology of hypertension [62][66][67][96,98,99]. Ang II has also been associated with inflammatory responses, endothelial dysfunction, atherosclerosis, and congestive heart failure. Reports have indicated various effects of Ang II, depending on the cell/tissue type under consideration and the duration of exposure (acute versus chronic) [50][62][68][73,96,100]. Ang II is derived from Ang I through the activity of ACE [69][70][101,102]. ACE, or kininase II, also plays a key role in the kallikrein-kinin system by cleaving bradykinin to inactive peptides; which, in turn, also have effects on hypertensive responses (Figure 3). Ang II receptors are categorized into two types based on their structure: Ang II type 1 receptor (AT1R) and Ang II type 2 receptor (AT2R), each with a distinctive downstream signaling cascade [69][70][101,102]. Signaling through AT1R is envisaged to mediate vasoconstriction, aldosterone secretion, catecholamine release, and cardiac remodeling [71][103]. However, signaling through AT2R has opposite effects to those mediated by AT1R and has been shown to induce vasodilation [72][104] and attenuation of cardiac remodeling [73][74][105,106].
Figure 3. Representative image delineating the plausible anti-hypertensive effect of CM. At the molecular level, the renin-angiotensin system (RAS) is at the center of the regulation of hypertension. Angiotensin II (Ang II), an important effector RAS hormone, has been implicated in regulating the physiological effects of the regulation of blood pressure and is associated with the pathophysiology of hypertension. Basically, Ang II is derived from Ang I through the activity of Angiotensin Converting Enzyme (ACE). ACE, or kininase II, also plays a key role in the kallikrein-kinin system by cleaving bradykinin to inactive peptides; which, in turn, also affect hypertensive responses (A). Molecular signaling through AT1R is envisaged to mediate aldosterone secretion, ADH secretion, and the arterial vasoconstriction, which ultimately leads to hypertensive response (B). A detailed overview of the molecular signaling mediated by Ang II-AT1R is delineated (C). Interestingly, CM and its constituents have been reported to inhibit ACE.
Ang II has been shown to mediate downstream signaling through the action of G protein- and non-G protein-related signaling mediators [62][66][96,98]. However, Ang II also mediates its functions via various mitogen-activated protein kinases, receptor tyrosine kinases and non-receptor tyrosine kinases [66][98]. In addition, Ang II-AT1R-mediated NAD(P)H oxidase activation, which has been widely implicated in vascular inflammatory and fibrotic responses, has been studied in detail. These signaling pathways regulate normal cellular function and/or disease conditions. A detailed overview of the signal transduction pathways related to Ang II-mediated hypertensive responses is delineated in Figure 3. The plausible target for CM and its constituents has also been highlighted (Figure 3).
Interestingly, the anti-hypertensive potential of CM proteins, PHs and/or bioactive peptides is an important emerging area with promising prospects. It is important to understand how these CM bioactive molecules influence the cellular system at the molecular, cellular, and organelle levels. Further, it is anticipated that the underlying events do not function in isolation but rather influence each other either directly or indirectly, and ultimately affect the underlying responses. Thus, it is equally imperative to understand how these CM bioactive molecules influence these dynamically intertwined responses.、
