Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Sirius Huang and Version 4 by Ting Yu.
Immune checkpoint inhibitors (ICIs), antibodies that target the checkpoints in immune cells, work to activate inhibited T-cells and other cells of the innate and adaptive arms, resulting in the robust activation of the immune system and productive antitumor immune responses. However, ICIs-related cardiotoxicity has been recognized as a rare but fatal consequence. Although there has been extensive research based on different types of ICIs, these studies have not indicated whether cardiotoxicity is specific to a type of cancer.
- immune checkpoint inhibitors
- cardiotoxicity
- cardio-oncology
- cancer-type-specific
1. Introduction
Cardiovascular disease (CVD) and cancer are global health issues with high morbidity and mortality [1], and numerous studies suggest that there is an overlap in epidemiology, risk factors, and pathophysiologic processes (Figure 1) .
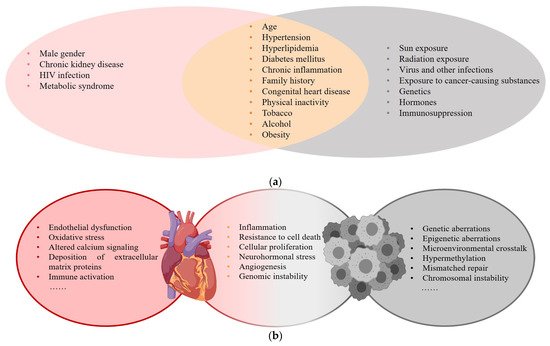
Figure 1. (a) Risk factors for CVD and cancer; (b) Common pathophysiologic processes of CVD and cancer.
With the widespread application of anticancer drugs, the survival of patients has significantly improved, but the related cardiotoxicity affects long-term therapeutic outcomes, and this has attracted considerable attention. Immune checkpoint inhibitors (ICIs), antibodies that target the checkpoints in immune cells, work to activate inhibited T-cells and other cells of the innate and adaptive arms, resulting in the robust activation of the immune system and productive antitumor immune responses. This new type of immunotherapy drug has significantly improved the survival of cancer patients [2][3][4]. However, their use is associated with adverse side effects involving different organs [5][6]. ICIs-related cardiotoxicity, which may develop even without a history of significant cardiac risk factors, includes myocarditis, pericarditis, heart failure, arrhythmias, and vasculitis [7]. In reported cases of adverse ICIs-related events, 6.2% were cardiac adverse events (CAEs), which can be the main determinants of quality of life and increased mortality [8][9][10].
2. Cardiotoxicity in Melanoma
In 16 studies, 24 of 6710 patients on ICIs [11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26] developed CAEs. This corresponded with an incidence of 0.20–4.93% in which grade 3–5 CAEs accounted for 41.7%. Commonly encountered cardiotoxicities included hypertension (50%), hypotension (16.7%), and myocarditis (8.3%). Treatment-related hypertension was linked to the application of lambrolizumab (58.3%) (PD-1). Nivolumab may have had a correlation with ICIs-related hypotension. Patients treated with a higher dose of ipilimumab, particularly 10 mg/kg × 4 doses/3 weeks, were more prone to fatal adverse events such as cardiac arrest (Table 1).
Table 1.
Cardiotoxicity in melanoma.
| Author, Year | Study Type | Phase | Sample Size | Drug | Dose and Frequency | Non-CAE | CAE | Manifestation | 3–5 Grade CAE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Study Type | Phase | Sample Size | Drug | Dose and Frequency | Non-CAE | CAE | Manifestation | 3–5 Grade CAE | ||||||||||
| Omid Hamid et al., 2017 [11] | Prospective study | II | 528 (178 vs. 179 vs. 171) | Pembrolizumab vs. Pembrolizumab vs. chemotherapy | 2 mg/kg/3 weeks vs. 10 mg/kg/3 weeks vs. standard dose | 528 | 0 | 0 | |||||||||||
| Kalyan R et al., 2019 [3727] | Retrospective study | NR | 252 (117 vs. 135) | Non-ICI vs. ICI (Nivolumab/Pembrolizumab) | 0 | ||||||||||||||
| Nivolumab (Niv) | Pembrolizumab (Pem) | Standard dose vs. increasing dose (Niv < 540 mg; 540~1440 mg; > 1440 mg Pem < 600 mg; 600~1707 mg; >1707 mg) | NR | 93 (42 vs. 51) | Arrhythmia 31 vs. 25; Cardiac-related chest pain 12 vs. 25; Valvular heart disease 4 vs. 2; Cardiomyopathy 13 vs. 20; Myopericardial disease 11; Pericardial disease 8; Myocarditis 1; Valvular-disease 2; Venous arterial thromboembolic events 8 | 40 (major CAE) | Caroline Robert et al., 2014 [12] | Prospective study | |||||||||||
| Scott N et al., 2015 [3828] | Prospective study (NSCLC) | III | 418 (210 vs. 208) | Nivolumab vs. Dacarbazine |
3 mg/kg/2 weeks vs. standard dose | 308 (153 vs. 155) | 5 | Hypotension 1 vs. 4 | 0 | ||||||||||
| I | 129 (33 vs. 37 vs. 59) | Nivolumab | 1 mg/kg vs. 3 mg/kg vs. 10 mg/kg intravenously/2 weeks in 8-week cycles for up to 96 weeks. | 91 (21 vs. 25 vs. 45) | 0 | 0 | 0 | Jeffrey S Weber et al., 2015 [13] | Prospective study | ||||||||||
| Tony S K Mok et al., 2019 | III | [3929 | 370 (268 vs. 102) | Nivolumab vs. ICC (Dacarbazine al) | 3 mg/kg/2 weeks vs. standard dose | ] | Prospective study (NSCLC) | III | 1251 (636 vs. 615) | Pembrolizumab vs. platinum-based chemotherapy | 200 mg/3 weeks for up to 35 cycles vs. platinum-based chemotherapy for four to six cycles.362 (181 vs. 81) | 1112 (515 vs. 597)0 | 1 (1 vs. 0)0 | Myocarditis 1 vs. 00 | |||||
| 1 | Paolo A Ascierto et al., 2017 [14] | Prospective study | |||||||||||||||||
| Achim Rittmeyer et al., 2017 [4030] | Prospective study (NSCLC) | III | III726 (364 vs. 362) | 1187 (609 vs. 578)Ipilimumab | Atezolizumab vs. Docetaxel10 mg/kg/4 doses/3 weeks vs. 3 mg/kg/4 doses/3 weeks | 1200 mg/3 weeks vs. 75 mg/m2514 (286 vs. 228) | 3 | Hypertension 1 vs. 0; Heart arrest 1 vs. 0; Pericarditis 1 vs. 0 | 3 | ||||||||||
| /3 weeks | 886 (390 vs. 496) | 0 | 0 | 0 | F Stephen Hodi et al., 2016 [15] | Prospective study | |||||||||||||
| S.J. Antonia et al., 2017 [4131] | II | 142 (95 vs. 47) | Nivolumab + Ipilimumab vs. Ipilimumab + placebo | 1 mg/kg + 3 mg/kg/4 doses/3 weeks vs. 3 mg/kg + placebo/4 doses/3 weeks | Prospective study (NSCLC) | III | 718 (475 vs. 234) | Durvalumab vs. Placebo140 (94 vs. 46) |
10 mg/kg/2 weeks for up to 12 months vs. placebo7 |
Hypotension 3 vs. 0; Ventricular arrhythmia 1 vs. 0; Ventricular tachycardia 1 vs. 0; Atrial fibrillation 1 vs. 0; Myocardial infarction 1 vs. 0 | 5 | ||||||||
| 421 (301 vs. 120) | 26 (21 vs. 5) | ACS 9 vs. 2; Arrhythmia 7 vs. 1; Heart failure 7 vs. 0; Cardiac arrest 2 vs. 1; Cardiogenic shock 1 vs. 0; Cardiomyopathy 1 vs. 0; Myocarditis 0 vs. 1; Pericardial effusion 2 vs. 0 | NR | Caroline Robert et al., 2015 [16] | |||||||||||||||
| Yuequan Shi et al., 2021 [ | Prospective study | III | 834 (278 vs. 277 vs. 256) | 4232] | Observational study (NSCLC/SCLC) | NR | 1905 (1162 vs. 743) (598 vs. 455 vs. 273 vs. 176 vs. 125 vs. 81 vs. 62 vs. 34 vs. 23)Pembrolizumab vs. Pembrolizumab vs. Ipilimumab |
10 mg/kg/2 weeks/doses vs. 10 mg/kg/3 weeks/ doses vs. 3 mg/kg/3 weeks/4 doses | 610 (221 vs. 202 vs. 187) | 4 | Hypertension | ICI (Pembrolizumab/Nivolumab/Camrelizumab/Treprizumab/Tisilizumab/Atezolizumab/Durvalumab/Ipilimumab) only vs. combination therapy |
at least one dose | 647 | 22 (22 vs. 0)3 vs. 1 vs. 0 | Elevated cTnI or myocarditis 222 | |||
| 9 | J. Weber, M. et al., 2017 [17] | Prospective study | III | 906 (453 vs. 453) | Nivolumab vs. Ipilimumab | 3 mg/kg/4 doses/2 weeks vs. 10 mg/kg/4 doses/3 weeks | 884 (438 vs. 446) | 0 | 0 | 0 | |||||||||
| Roy S Herbst et al., 2016 [4333] | Prospective study (NSCLC) | II/III | 991 (339 vs. 343 vs. 309) | Pembrolizumab vs. Docetaxel | Pem 2 mg/kg, Pem 10 mg/kg vs. Docetaxel 75 mg/m2/3 weeks | 690 (215 vs. 225 vs. 250) | 1 (0 vs. 1 vs. 1) | Myocardial infarction 0 vs. 1 vs. 0; Acute cardiac failure 0 vs. 0 vs. 1 | 1 | J.D. Wolchok et al., 2017 [18] | Prospective study | III | 937 (313 vs. 313 vs. 311) | Nivolumab + Ipilimumab vs. Nivolumab + p vs. | |||||
| Martin Reck et al., 2016 [44 | Ipilimumab + p | p(placebo) | 1 mg/kg+3 mg/kg | /3 weeks/4 doses vs. 3 mg/kg/2 weeks + placebo vs. 3 mg/kg/3 weeks/4 doses + placebo |
847 (300 vs. 279 vs. 268) | 0 | 0 | 0 | |||||||||||
| 34] | Prospective study (NSCLC) | III | 304 (154 vs. 150) | Pembrolizumab vs. platinum-based chemotherapy |
200 mg/3 weeks vs. standard dose | 52 (45 vs. 7) | 0 | 0 | 0 | Jedd D Wolchok et al., 2010 [19] | Prospective study | II | 217 (73 vs. 72 vs. 72) | Ipilimumab | 10 mg/kg vs. 3 mg/kg vs. 0.3 mg/kg/3 weeks/4 doses | 115 (50 vs. 46 vs. 19) | |||
| H. Borghaei et al., 2015 [4535] | Prospective study (NSCLC) | III | 0 | 0 | 0 | ||||||||||||||
| 555 (278 vs. 268) | Nivolumab vs. Docetaxel | 3 mg/kg/2 weeks vs. 75 mg/m | 2 | /3 weeks | 432 (196 vs. 236) | 3 (3 vs. 0) | Cardiac tamponade 1 vs. 0; Pericardial effusion 1 vs. 0 | Tachycardia 1 vs. 0 |
3 | Ines Pires da Silva et al., 2021 [20] | Retrospective study | NR (Not Reported) | 355 (193 vs. 162) | Ipilimumab + Nivolumab/Pembrolizumab/Atezolizumab vs. Ipilimumab | 3 mg/kg/3 weeks/4 doses + standard dose vs. 3 mg/kg/3 weeks/4 doses | 287 (163 vs. 124) | 1 (0 vs. 1) | Myocarditis 0 vs. 1 | 1 |
| Julie Brahmer et al., 2015 [4636] | Prospective study (NSCLC) | III | 272 (135:137) | Nivolumab vs. Docetaxel | 3 mg/kg/2 weeks vs. 75 mg/m2/3 weeks. | 187 (76 vs. 111) | 0 | 0 | 0 | Patrick Schöffski et al., 2022 [21] | Retrospective study | I/II | 255 (134 vs. 121) | LAG-3 inhibitor Ieramilimab vs. Ieramilimab + Spartalizumab |
Ieramilimab (escalating 1–15 mg/kg)/2 weeks or once/4 weeks vs. Ieramilimab + Spartalizumab q2w or q3w or q4w or Ieramilimab q2w + Spartalizumab q4w | 159 (75 vs. 84) | 0 | 0 | |
| D.P. Carbone et al., 2017 [4737] | 0 | ||||||||||||||||||
| Prospective study (NSCLC) | III | 530 (267 vs. 263) | Nivolumab vs. Chemotherapy(platinum-based) | 3 mg/kg/2 weeks vs. standard dose for six cycles. | 431 (188 vs. 243) | 2 (2 vs. 0) | Alexander M.M. et al., 2020 [22] | Prospective study | III | 1011 (509 vs. 502) | Pembrolizumab vs. placebo | 200 mg/3 weeks for 18 doses | 235 (190 vs. 45) | 1 (1 vs. 0) | Myocarditis 1 vs. 0 | NR | |||
| Myocardial infarction 1 vs. 0; Pericardial effusion malignant 1 vs. 0 | 2 | Omid Hamid et al., 2013 [23] | Prospective study | I | 135 (57 vs. 56 vs. 22) | Lambrolizumab | 10 mg/kg/2 weeks vs. 10 mg/kg/3 weeks vs. 2 mg/kg/3 weeks | 132 (55 vs. 55 vs. 22) | 7 (2 vs. 4 vs. 1) | Hypertension (2 vs. 4 vs. 1) | NR | ||||||||
| Margaret K. et al., 2018 [24] | Retrospective study | I | 94 (53 vs. 41) | Ipilimumab + Nivolumab Nivolumab (Niv) Ipilimumab (Ipi) |
Niv+Ipi(escalating doses)/3 weeks for four doses, followed by Niv 3 weeks for four doses, then Niv + Ipi/12 weeks for eight doses vs. Niv 1 mg/kg + Ipi 3 mg/kg/3 weeks for 4 doses, followed by Niv 3 mg/kg/2 weeks |
87 | 0 | 0 | 0 | ||||||||||
| Ulrich Keilholz et al., 2019 [25] | Prospective study | I | 51 | Avelumab | 10 mg/kg for one-hour intravenous infusion/2 weeks | 39 | 0 | 0 | 0 | ||||||||||
| Hussein A et al., 2022 [26] | Retrospective study | II-III | 714 (355 vs. 359) | Relatlimab + Nivolumab vs. Nivolumab | Relatlimab 160 mg + Nivolumab 480 mg vs. Nivolumab 480 mg | 504 (288 vs. 216) | 0 | 0 | 0 |
The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Grade 3: severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living. Grade 4: life-threatening consequences; urgent intervention indicated. Grade 5: Death related to adverse events.
3. Cardiotoxicity in Lung Cancer
A total of 11 studies [27][28][29][30][31][32][33][34][35][36][37] included 5404 patients on ICIs, and 101 developed CAEs for an incidence of 0.15–37.78% in which grade 3–5 CAEs accounted for 55.4%. Commonly encountered cardiotoxicities included arrhythmia (32.7%), cardiac-related chest pain (24.8%), elevated cTnI or myocarditis (23.8%), cardiomyopathy (20.8%), pericardial disease (11.9%), and acute coronary syndrome (10.9%). One study indicated that major adverse cardiovascular events (MACEs) were dose-independent of nivolumab and pembrolizumab in lung cancer patients [27]. Those treated with a higher dose of durvalumab, particularly 10 mg/kg × 4 doses/2 weeks, were more prone to fatal adverse events such as a cardiac arrest and cardiogenic shock [31]. One patient treated with pembrolizumab at 10 mg/kg for 3 weeks underwent a myocardial infarction, which led to death (Table 2) [33].
Table 2. Cardiotoxicity in lung cancer.
4. Renal Cell Carcinoma
In seven studies [4838][4939][5040][5141][5242][5343][5444] comprising 1971 patients with renal cell carcinomas on ICIs, 14 developed CAEs with an incidence of 0.20–2.19% in which grade 3–5 CAEs accounted for 35.7%. Commonly encountered cardiotoxicities included hypertension (85.7%) and myocarditis (7.1%). Treatment-related hypertension was linked to a nivolumab plus ipilimumab therapy (100%). Compared with melanomas and lung cancer, the ICI therapy caused mild cardiotoxicity in renal cell carcinomas. Fatal CAEs were not found.
5. Urothelial Carcinoma
In Seven studies [5545][5646][5747][5848][5949][6050][6151] 111 of 2550 patients with urothelial carcinomas on ICIs developed CAEs with an incidence of 0.22–10.60% in which grade 3–5 CAEs accounted for 52.3%. Commonly encountered cardiotoxicities included hypertension (28.8%), arrhythmia (14.4%) and hypotension (6.3%). The fluctuation of blood pressure was linked to treatment with atezolizumab. Hypertension was observed in 21 patients and hypotension was observed in 7 after application of atezolizumab. Patients treated with 200 mg pembrolizumab for 3 weeks (maximum 35 cycles) or at 1200 mg every three weeks were more prone to fatal adverse events such as a cardiac arrest.
6. Other Types of Cancer
The most commonly encountered ICIs-related type of cardiotoxicity in hematological malignancies was hypertension [6252][6353][6454][6555]. In other cancers, such as hepatocellular carcinomas and malignant pleural mesotheliomas, the relevant research did not present many cases [6656][6757][6858][6959][7060][7161]; these were almost all case reports of myocarditis [7262][7363][7464].
References
- Narayan, V.; Thompson, E.W.; Demissei, B.; Ho, J.E.; Januzzi, J.L., Jr.; Ky, B. Mechanistic Biomarkers Informative of Both Cancer and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2726–2737.
- Kaushik, I.; Ramachandran, S.; Zabel, C.; Gaikwad, S.; Srivastava, S.K. The evolutionary legacy of immune checkpoint inhibitors. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022.
- Lee, J.B.; Kim, H.R.; Ha, S.J. Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy. Immune Netw. 2022, 22, e2.
- Park, J.; Kwon, M.; Shin, E.C. Immune checkpoint inhibitors for cancer treatment. Arch. Pharmacal Res. 2016, 39, 1577–1587.
- Zhou, J.; Chau, Y.A.; Yoo, J.W.; Lee, S.; Ng, K.; Dee, E.C.; Liu, T.; Wai, A.K.C.; Zhang, Q.; Tse, G. Liver Immune-related Adverse Effects of Programmed Cell Death 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) Inhibitors: A Propensity Score Matched Study with Competing Risk Analyses. Clin. Oncol. (R Coll. Radiol.) 2022, 34, e316–e317.
- Zhou, J.; Lee, S.; Lakhani, I.; Yang, L.; Liu, T.; Zhang, Y.; Xia, Y.; Wong, W.T.; Bao, K.K.H.; Wong, I.C.K.; et al. Adverse Cardiovascular Complications following prescription of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors: A propensity-score matched Cohort Study with competing risk analysis. Cardiooncology 2022, 8, 5.
- Dolladille, C.; Akroun, J.; Morice, P.M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.F.; Legallois, D.; L’Orphelin, J.M.; et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: A safety meta-analysis. Eur. Heart J. 2021, 42, 4964–4977.
- Giza, D.E.; Iliescu, G.; Hassan, S.; Marmagkiolis, K.; Iliescu, C. Cancer as a Risk Factor for Cardiovascular Disease. Curr. Oncol. Rep. 2017, 19, 39.
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17.
- Master, S.R.; Robinson, A.; Mills, G.M.; Mansour, R.P. Cardiovascular complications of immune checkpoint inhibitor therapy. J. Clin. Oncol. 2019, 37, 2568.
- Hamid, O.; Puzanov, I.; Dummer, R.; Schachter, J.; Daud, A.; Schadendorf, D.; Blank, C.; Cranmer, L.D.; Robert, C.; Pavlick, A.C.; et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur. J. Cancer 2017, 86, 37–45.
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330.
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384.
- Ascierto, P.A.; Del Vecchio, M.; Robert, C.; Mackiewicz, A.; Chiarion-Sileni, V.; Arance, A.; Lebbé, C.; Bastholt, L.; Hamid, O.; Rutkowski, P.; et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017, 18, 611–622.
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568.
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532.
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835.
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356.
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T., Jr.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164.
- Pires da Silva, I.; Ahmed, T.; Reijers, I.L.M.; Weppler, A.M.; Betof Warner, A.; Patrinely, J.R.; Serra-Bellver, P.; Allayous, C.; Mangana, J.; Nguyen, K.; et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol. 2021, 22, 836–847.
- Schöffski, P.; Tan, D.S.W.; Martín, M.; Ochoa-de-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J. Immunother. Cancer 2022, 10, e003776.
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527.
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144.
- Callahan, M.K.; Kluger, H.; Postow, M.A.; Segal, N.H.; Lesokhin, A.; Atkins, M.B.; Kirkwood, J.M.; Krishnan, S.; Bhore, R.; Horak, C.; et al. Nivolumab Plus Ipilimumab in Patients With Advanced Melanoma: Updated Survival, Response, and Safety Data in a Phase I Dose-Escalation Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 391–398.
- Keilholz, U.; Mehnert, J.M.; Bauer, S.; Bourgeois, H.; Patel, M.R.; Gravenor, D.; Nemunaitis, J.J.; Taylor, M.H.; Wyrwicz, L.; Lee, K.W.; et al. Avelumab in patients with previously treated metastatic melanoma: Phase 1b results from the JAVELIN Solid Tumor trial. J. Immunother. Cancer 2019, 7, 12.
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34.
- Chitturi, K.R.; Xu, J.; Araujo-Gutierrez, R.; Bhimaraj, A.; Guha, A.; Hussain, I.; Kassi, M.; Bernicker, E.H.; Trachtenberg, B.H. Immune Checkpoint Inhibitor-Related Adverse Cardiovascular Events in Patients With Lung Cancer. JACC CardioOncol. 2019, 1, 182–192.
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2004–2012.
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830.
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265.
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929.
- Shi, Y.; Fang, J.; Zhou, C.; Liu, A.; Wang, Y.; Meng, Q.; Ding, C.; Ai, B.; Gu, Y.; Yao, Y.; et al. Immune checkpoint inhibitor-related adverse events in lung cancer: Real-world incidence and management practices of 1905 patients in China. Thorac. Cancer 2022, 13, 412–422.
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833.
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639.
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135.
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426.
- Abou Alaiwi, S.; Xie, W.; Nassar, A.H.; Dudani, S.; Martini, D.; Bakouny, Z.; Steinharter, J.A.; Nuzzo, P.V.; Flippot, R.; Martinez-Chanza, N.; et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J. Immunother. Cancer 2020, 8, e000144.
- Yekedüz, E.; Ertürk, İ.; Tural, D.; Karadurmuş, N.; Karakaya, S.; Hızal, M.; Arıkan, R.; Arslan, Ç.; Taban, H.; Küçükarda, A.; et al. Nivolumab in metastatic renal cell carcinoma: Results from the Turkish Oncology Group Kidney Cancer Consortium database. Future Oncol. 2021, 17, 4861–4869.
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290.
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Redman, B.G.; Kuzel, T.M.; Harrison, M.R.; Vaishampayan, U.N.; Drabkin, H.A.; George, S.; Logan, T.F.; et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1430–1437.
- McFarlane, J.J.; Kochenderfer, M.D.; Olsen, M.R.; Bauer, T.M.; Molina, A.; Hauke, R.J.; Reeves, J.A.; Babu, S.; Van Veldhuizen, P.; Somer, B.; et al. Safety and Efficacy of Nivolumab in Patients With Advanced Clear Cell Renal Cell Carcinoma: Results From the Phase IIIb/IV CheckMate 374 Study. Clin. Genitourin. Cancer 2020, 18, 469–476.e464.
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813.
- Vaishampayan, U.; Schöffski, P.; Ravaud, A.; Borel, C.; Peguero, J.; Chaves, J.; Morris, J.C.; Kotecki, N.; Smakal, M.; Zhou, D.; et al. Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: Phase Ib results from the JAVELIN Solid Tumor trial. J. Immunother. Cancer 2019, 7, 275.
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537.
- Ye, D.; Liu, J.; Zhou, A.; Zou, Q.; Li, H.; Fu, C.; Hu, H.; Huang, J.; Zhu, S.; Jin, J.; et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 2021, 112, 305–313.
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588.
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322.
- Van der Heijden, M.S.; Loriot, Y.; Durán, I.; Ravaud, A.; Retz, M.; Vogelzang, N.J.; Nelson, B.; Wang, J.; Shen, X.; Powles, T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur. Urol. 2021, 80, 7–11.
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920.
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945.
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218.
- Shi, Y.; Su, H.; Song, Y.; Jiang, W.; Sun, X.; Qian, W.; Zhang, W.; Gao, Y.; Jin, Z.; Zhou, J.; et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e12–e19.
- Shi, Y.; Wu, J.; Wang, Z.; Zhang, L.; Wang, Z.; Zhang, M.; Cen, H.; Peng, Z.; Li, Y.; Fan, L.; et al. Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: An open-label phase 2 study (Gxplore-002). J. Hematol. Oncol. 2021, 14, 12.
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer 2016, 4, 50.
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471.
- Wadhwa, D.; Fallah-Rad, N.; Grenier, D.; Krahn, M.; Fang, T.; Ahmadie, R.; Walker, J.R.; Lister, D.; Arora, R.C.; Barac, I.; et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: A retrospective study. Breast Cancer Res. Treat 2009, 117, 357–364.
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952.
- Quispel-Janssen, J.; van der Noort, V.; de Vries, J.F.; Zimmerman, M.; Lalezari, F.; Thunnissen, E.; Monkhorst, K.; Schouten, R.; Schunselaar, L.; Disselhorst, M.; et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1569–1576.
- Vos, J.L.; Elbers, J.B.W.; Krijgsman, O.; Traets, J.J.H.; Qiao, X.; van der Leun, A.M.; Lubeck, Y.; Seignette, I.M.; Smit, L.A.; Willems, S.M.; et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 7348.
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552.
- Monge, C.; Maeng, H.; Brofferio, A.; Apolo, A.B.; Sathya, B.; Arai, A.E.; Gulley, J.L.; Bilusic, M. Myocarditis in a patient treated with Nivolumab and PROSTVAC: A case report. J. Immunother. Cancer 2018, 6, 150.
- Mahmood, S.S.; Chen, C.L.; Shapnik, N.; Krishnan, U.; Singh, H.S.; Makker, V. Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: A case report. Gynecol. Oncol. Rep. 2018, 25, 74–77.
- Chen, Q.; Huang, D.-S.; Zhang, L.-W.; Li, Y.-Q.; Wang, H.-W.; Liu, H.-B. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin. Toxicol. 2018, 56, 667–671.
More
