The COVID-19 pandemic has triggered a significant range of dermatologic sequela. Etiologies of lesions continue to be investigated. Proposed mechanisms include inflammatory response to spike protein, vitamin D deficiency, ACE2 receptor activation, androgen levels, and increased psychosocial stress. This encyclopedia article reflects a novel literature review (Pendlebury et al.) of dermatological manifestations associated with the Coronavirus Disease 2019 (COVID-19) pandemic. This literature review is the first published broad-spectrum examination that analyzes a range of dermatological manifestations related to the COVID-19 pandemic: infection, vaccinations, personal protective equipment (PPE), and psychosocial factors.
- COVID-19
- COVID-19 pandemic
- SARS-CoV-2 infection
- cutaneous manifestations
- COVID arm
- pandemic psychosocial stress
- personal protective equipment
- COVID vaccinations
- psychodermatology
- teledermatology
1. Introduction
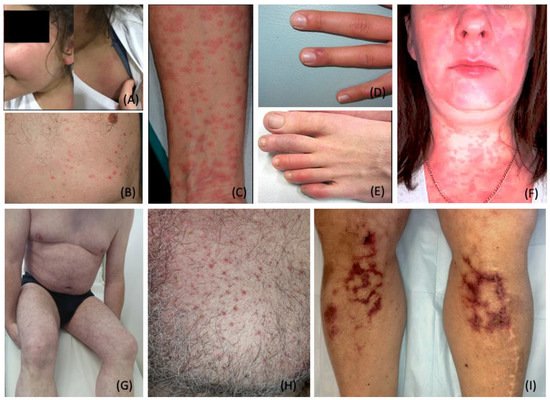
2. COVID-19 Specific Dermatological Manifestations
2.1. Exanthematous (Morbilliform) Rash
2.2. Pernio (Chilblain)-like Acral Lesions
2.3. Urticaria
2.4. Livedo Reticularis
2.5. Livedo Racemosa/Retiform Purpura
2.6. Vesicular (Varicella-like) Eruptions
2.7. Papulosquamous Rashes and Pityriasis Rosea
2.8. Multisystem Inflammatory Syndrome in Children
-
Fever
-
Inflammatory markers
-
Failure or involvement of two organ systems
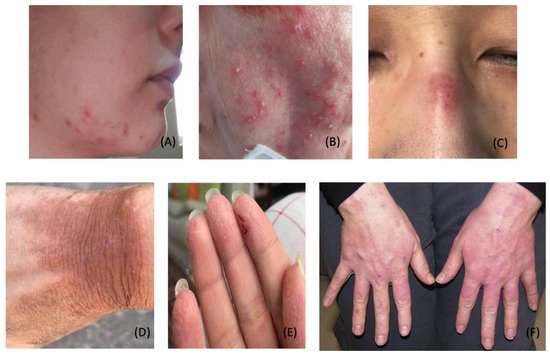
3. Dermatological Conditions Associated with Personal Protective Equipment and Hygiene Products
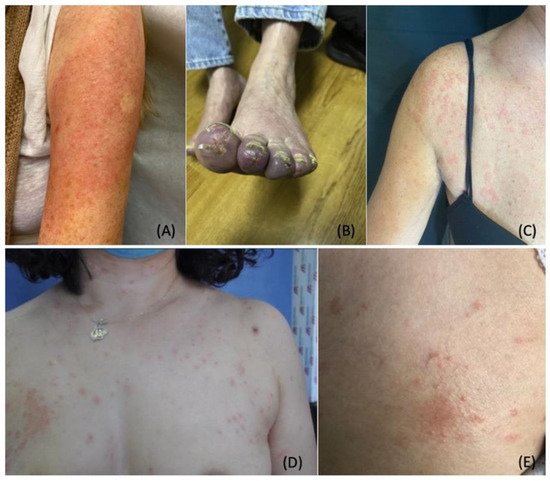
4. COVID-19 Vaccine-Induced Dermatological Manifestations
5. Stress-Induced Dermatological Conditions
5.1. Telogen Effluvium
Telogen effluvium (TE) is a common, self-limiting hair loss condition usually seen in women with a history of a recent stressor. Stressors associated with TE include systemic disease, infections, stressful events, drugs, nutritional deficiencies, postpartum hormonal changes, and major surgeries [44]. TE usually presents three months following the onset of the stressor. It is classified as acute (hair loss lasting up to six months) or chronic (hair loss exceeding six months) [193][170]. Coined as “COVID scalp,” increased cases of TE have been associated with the COVID-19 pandemic [194][171]. The cause of TE during the COVID-19 pandemic is multifactorial, and it is thought to be stress-induced or a direct manifestation of the inflammatory process during the infection phase.6. Pathomechanisms: Cutaneous Manifestations in SARS-CoV-2
International reports have identified a range of potential cutaneous manifestations related to COVID-19. The frequency and timing of these lesions to COVID-19 infection are difficult to ascertain. Multiple established associations in the scientific literature offer valuable insights into plausible mechanisms behind cutaneous manifestations of SARS-CoV-2. One proposed pathomechanism explains the presence of ACE2 in keratinocytes [19]. Another promising association describes the significance of androgen levels in the increased expression of the TMPRSS2 gene [13,14][13][14].
A strong association between increased androgens and increased TMPRSS2 gene expression has been identified in the literature [13,14][13][14]. ACE2 receptors and TMPRSS2 are expressed in multiple tissues throughout the body including the skin. In conjunction, the presence of ACE2 receptors in keratinocytes may play a significant role in the manifestation of dermatological conditions related to COVID-19. Furthermore, the association between androgen levels and TMPRSS2 can further contribute to the development of skin lesions [13,14,19][13][14][19]. As such, high levels of androgens combined with ACE2 distribution in the skin can provide invaluable clinical insight into the pathomechanism of skin manifestations.
Androgens have been shown to uniquely activate the TMPRSS2 gene. TMPRSS2 plays an essential role in the activation of spike protein and facilitates viral entry through ACE2 receptors. This correlation helps explain the greater susceptibility to COVID-19 in males, as males typically maintain higher levels of androgens. Moreover, it has been shown that males with prostate cancer receiving androgen deprivation therapy (ADT) have a lower risk of COVID-19 compared to prostate cancer patients who did not receive ADT [14]. As such, ADT may play a role in reducing the severity of symptoms in patients with COVID-19 [13,14][13][14]. However, more research is needed to confirm these findings. Additionally, it is well established that children have a lower rate of COVID-19 infection compared to adult males and females. The less symptomatic disease in children may be explained by overall low expressions of androgen receptors and androgen levels [7,13,14,15,16,17][7][13][14][15][16][17].7. The Role of Vitamin D in SARS-CoV-2
Additional susceptibility to COVID-19 has been strongly correlated with low vitamin D levels. Studies continue to identify and analyze the increased severity of COVID-19 infection with concomitant low vitamin D levels [224,225,226][172][173][174]. Vitamin D can be administered in various forms including (but not limited to) cholecalciferol, calcifediol, and calcitriol. Vitamin D supplementation has been proposed to reduce the risk of COVID-19 infection through multiple protective mechanisms. Such mechanisms include modulation of the host immune system, upregulation of ACE2 concentration, vitamin D receptor activation [226][174], reduction in endothelial damage, and reduction in proinflammatory cytokines [224,225,226][172][173][174]. Research studies have demonstrated that vitamin D receptors (VDRs) are expressed in high concentration levels in cuboidal alveolar type II cells (ACII) within the pulmonary system. Calcitriol, also known as 1,25 dihydroxyvitamin D (1,25(OH)2 D3), binds to VDRs in ACII cells. Calcitriol binding activates multiple intracellular signals which inhibit inflammatory cytokines and chemokines involved in Acute Respiratory Distress Syndrome (ARDS) [226][174]. Additional investigations have revealed that vitamin D signaling pathways prevent pulmonary vessel constriction, a manifestation associated with increased COVID-19 mortality [224,225,226][172][173][174]. VDR activation promotes vasodilatory effects through two mechanisms: inhibitions of angiotensin II (a potent vasoconstrictor) and upregulation of ACE2. Decreased expression of angiotensin II promotes pulmonary vasodilation [224,225,226][172][173][174]. Additionally, the induction of ACE2 expression in pulmonary tissues further dampens the effect of angiotensin II, thereby reducing respiratory distress symptoms. Prevention of pulmonary vasoconstriction greatly improves respiratory symptoms associated with SAR-CoV-2 infection. Therefore, ACE2 acts as an anti-inflammatory factor in the etiology of ARDS [224,225,226][172][173][174]. VDR activation has also been shown to inhibit Skp2 protein, which is utilized by SARS-CoV-2 to replicate inside cells. Thus, the binding of calcitriol and VDR activation reduce viral replication in pulmonary tissues and reduce the disease severity of COVID-19 [226][174]. Calcifediol, a vitamin D3 analog, rapidly increases serum levels of vitamin D 25-hydroxyvitamin D (25-OH-D), thereby promoting the protective properties associated with vitamin D [225][173]. A parallel randomized open label, double-masked clinical trial evaluated the effect of calcifediol on the severity of COVID-19 disease. The randomized clinical trial was conducted on 76 consecutive patients hospitalized with COVID-19 infection. All 76 patients clinically presented with acute respiratory infections, confirmed by radiographic patterns of viral pneumonia. Likewise, all 76 patients tested positive for SARS-CoV-2 through PCR tests. Lastly, all 76 patients were confirmed for appropriate hospital admission via the CURB65 Severity Scale [225][173]. All hospitalized patients received the best available therapy at the same standard of care. Of the 50 patients treated with calcifediol, one patient required intensive care unit (ICU) admission, and of the 26 untreated patients, 13 patients required ICU admission, with two deaths in the ICU. The remaining 11 untreated patients who did not receive calcifediol were discharged. Of all 50 patients treated with calcifediol, none died, and all patients were discharged with no complications [225][173]. A larger-scale observational cohort study with 930 patients also revealed significantly reduced ICU admissions and mortality rates associated with early vitamin D administration [227][175]. These clinical trials underscore the clinical value of vitamin D in a significant reduction in disease severity and disease mortality [225,227][173][175].8. The Role of Vitamin D in Cutaneous Manifestations Associated with COVID-19
With regards to infection-related cutaneous manifestations, most conditions have been attributed to the host’s inflammatory response to SARS-CoV-2. Likewise, the immune-boosting, anti-inflammatory properties of vitamin D alleviate and reduce the spread of cutaneous lesions [224,225,226,227][172][173][174][175]. In addition, vitamin D bioavailability and efficacy have been shown to increase with magnesium supplementation. Magnesium has been shown to facilitate vitamin D-related processes by activating vitamin D processing enzymes [228][176]. Vitamin D and magnesium supplementation are cost-effective measures that help prevent infection, reduce disease severity, and improve prognosis [228][176]. Likewise, patient education and adherence are essential factors for favorable clinical outcomes. Therefore, further investigations are warranted to identify the ideal maintenance dosage of vitamin D and magnesium to prevent infection. Additional research is recommended to elucidate an effective dosing regimen to reduce infection-related cutaneous lesions. Throughout the pandemic, clinical management for most COVID-19-associated and non-COVID-19 cutaneous manifestations have been similar in nature. For example, chilblain-like lesions in COVID-19 patients do not require treatment, but topical corticosteroids can relieve discomfort [62,64,229][62][64][177]. Other rashes, such as varicelliform-like/vesicular lesions, are self-limiting and therefore do not require treatment [48,102][48][102]. In contrast, maculopapular and urticarial eruptions can occur concomitantly or separately in moderate to severe cases of COVID-19. These lesions present with pruritus and pain which necessitates prompt treatment with therapeutic agents such as topical corticosteroids, oral antihistamines, oral corticosteroids, and vitamin C [48,230,231,232,233][48][178][179][180][181]. In addition to rash therapies, early treatment interventions have been shown to improve the overall prognosis for infected patients and reduce patient mortality [234,235][182][183].9. Global Implications of the COVID-19 Pandemic
The COVID-19 pandemic has caused an insurmountable disturbance on a global scale. The strict quarantine measures have caused significant psychosocial distress. Resultantly, many individuals have developed new or worsening pre-existing dermatological conditions, such as telogen effluvium, psoriasis, eczema, urticaria, and atopic dermatitis. Exacerbated lesions require supportive management, targeted treatment for underlying issues, and relevant psychosocial support. Additionally, interdisciplinary treatment involving mental health professionals should be implemented to help relieve stress, anxiety, and depression.
As the pandemic continues, strict measures and mandates have been implemented to promote mass vaccination. With increased immunizations, additional cases of anaphylaxis and other allergic reactions such as COVID toes, COVID arm, and urticaria have been documented in response to immunizations [37,38,175,176,177,180][37][38][154][155][156][159]. It has been proposed that lipid nanoparticles (LNPs) containing messenger RNA vaccines trigger allergic and anaphylactic reactions [240][184]. LNPs are composed of positively charged lipids at low pH to stabilize the messenger RNA [241][185]. Likewise, LNPs contain high amounts of polyethylene glycol (PEG), a highly hydrophilic molecule [239,242][186][187]. PEG helps to increase the hydrophilicity of LNPs and stabilize the mRNA. However, PEG within LNPs has been shown to trigger inflammatory responses through complement-mediated and direct mast cell activation [240]. Furthermore, when inoculated into the bloodstream, LNPs trigger nonclassical allergic reactions in certain patients. Such reactions involve preformed antibodies to PEG and other components of LNPs [240][184]. Moreover, LNPs destabilize during the freeze and thaw cycle of immunization preparation [240][184]. When injected, destabilized LNPs release the naked mRNA into the bloodstream. Naked mRNA is proinflammatory and has been shown to induce allergic and anaphylactic reactions [239,242][186][187]. Additionally, classical allergic reactions to PEG may be IgE-mediated [240][184]. As such, skin prick testing with PEG should be performed before receiving the vaccine to avoid anaphylactic reactions [168][147]. At this time, allergy testing has not been implemented to screen susceptible individuals and should be considered to maximize safety and minimize adverse events. Moreover, novel measures on vaccine risk reduction can improve outcomes and maximize safety.
10. Conclusions
The COVID-19 pandemic has posed considerable challenges across the entirety of medicine. As evidenced, the COVID-19 pandemic has triggered a significant range of dermatologic sequela. Various factors surrounding the pandemic have resulted in a multitude of other dermatological manifestations. Dermatologists play an integral role in the proper diagnosis and treatment of COVID-related lesions. Several years into the pandemic, there is still much to learn and understand. As more information is collected and assessed, ourthe comprehension of the pathogenesis and treatment of dermatologic manifestations will continue to evolve and guide the dermatological standard of care.
Early treatment regimens and timely prophylaxis have been shown to improve prognosis and reduce further infection-related sequelae. Moreover, novel measures on vaccine risk reduction can improve outcomes and maximize safety. Given the evolutionary nature of the pandemic, intentional observation and research are prudent in the diagnosis, management, and treatment of these cutaneous manifestations. Robust investigations are necessary to identify underlying dermatological pathomechanisms and improve lesion diagnosis. Data collection will help reveal pertinent risk factors and boost public health outcomes. Such studies will reduce disease burden and optimize quality of life as society continues to adapt and adjust to life after SARS-CoV-2.
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160.
- Worldmeter. Coronavirus Cases. Available online: https://www.worldometers.info/coronavirus/ (accessed on 1 June 2022).
- Li, J.; Jia, H.; Tian, M.; Wu, N.; Yang, X.; Qi, J.; Ren, W.; Li, F.; Bian, H. SARS-CoV-2 and Emerging Variants: Unmasking Structure, Function, Infection, and Immune Escape Mechanisms. Front. Cell Infect. Microbiol. 2022, 12, 869832.
- Liu, J.; Li, Y.; Liu, Q.; Yao, Q.; Wang, X.; Zhang, H.; Chen, R.; Ren, L.; Min, J.; Deng, F.; et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov. 2021, 7, 17.
- Conceicao, C.; Thakur, N.; Human, S.; Kelly, J.T.; Logan, L.; Bialy, D.; Bhat, S.; Stevenson-Leggett, P.; Zagrajek, A.K.; Hollinghurst, P.; et al. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020, 18, e3001016.
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192.
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140.
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018.
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 756–759, Erratum in Am. J. Respir. Crit. Care Med. 2021, 15, 782.
- Kabbani, N.; Olds, J.L. Does COVID19 Infect the Brain? If So, Smokers Might Be at a Higher Risk. Mol. Pharmacol. 2020, 97, 351–353.
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 2020, 12, 8.
- Kaya, G.; Kaya, A.; Saurat, J.H. Clinical and Histopathological Features and Potential Pathological Mechanisms of Skin Lesions in COVID-19: Review of the Literature. Dermatopathology 2020, 7, 2.
- Mjaess, G.; Karam, A.; Aoun, F.; Albisinni, S.; Roumeguère, T. COVID-19 and the male susceptibility: The role of ACE2, TMPRSS2 and the androgen receptor. Prog. Urol. 2020, 30, 484–487.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059, Erratum in JAMA 2020, 323, 2098.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069, Erratum in JAMA 2021, 325, 1113.
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032.
- Xue, X.; Mi, Z.; Wang, Z.; Pang, Z.; Liu, H.; Zhang, F. High Expression of ACE2 on Keratinocytes Reveals Skin as a Potential Target for SARS-CoV-2. J. Investig. Dermatol. 2021, 141, 206–209.e1.
- Wei, C.; Friedman, A.J. COVID-19 Pandemic: Are There Unique Cutaneous Manifestations in Patients Infected With SARS-CoV-2? J. Drugs Dermatol. 2020, 19, 554–555.
- Freeman, E.E.; McMahon, D.E.; Fitzgerald, M.E.; Fox, L.P.; Rosenbach, M.; Takeshita, J.; French, L.E.; Thiers, B.H.; Hruza, G.J. The American Academy of Dermatology COVID-19 registry: Crowdsourcing dermatology in the age of COVID-19. J. Am. Acad. Dermatol. 2020, 83, 509–510.
- Freeman, E.E.; Chamberlin, G.C.; McMahon, D.E.; Hruza, G.J.; Wall, D.; Meah, N.; Sinclair, R.; Balogh, E.A.; Feldman, S.R.; Lowes, M.A.; et al. Dermatology COVID-19 Registries: Updates and Future Directions. Dermatol. Clin. 2021, 39, 575–585.
- Freeman, E.E.; McMahon, D.E.; Lipoff, J.B.; Rosenbach, M.; Kovarik, C.; Desai, S.R.; Harp, J.; Takeshita, J.; French, L.E.; Lim, H.W.; et al. The spectrum of COVID-19-associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J. Am. Acad. Dermatol. 2020, 83, 1118–1129.
- Marzano, A.V.; Genovese, G.; Moltrasio, C.; Gaspari, V.; Vezzoli, P.; Maione, V.; Misciali, C.; Sena, P.; Patrizi, A.; Offidani, A.; et al. The clinical spectrum of COVID-19-associated cutaneous manifestations: An Italian multicenter study of 200 adult patients. J. Am. Acad. Dermatol. 2021, 84, 1356–1363.
- Lavery, M.J.; Bouvier, C.A.; Thompson, B. Cutaneous manifestations of COVID-19 in children (and adults): A virus that does not discriminate. Clin. Dermatol. 2021, 39, 323–328.
- Young, T.K.; Shaw, K.S.; Shah, J.K.; Noor, A.; Alperin, R.A.; Ratner, A.J.; Orlow, S.J.; Betensky, R.A.; Shust, G.F.; Kahn, P.J.; et al. Mucocutaneous Manifestations of Multisystem Inflammatory Syndrome in Children During the COVID-19 Pandemic. JAMA Dermatol. 2021, 157, 207–212.
- Naka, F.; Melnick, L.; Gorelik, M.; Morel, K.D. A dermatologic perspective on multisystem inflammatory syndrome in children. Clin. Dermatol. 2021, 39, 337–343.
- Damiani, G.; Gironi, L.C.; Grada, A.; Kridin, K.; Finelli, R.; Buja, A.; Bragazzi, N.L.; Pigatto, P.D.M.; Savoia, P. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): Multi-center, real-life, telemedical, and observational prospective study. Dermatol. Ther. 2021, 34, e14848.
- Rudd, E.; Walsh, S. Mask related acne (“maskne”) and other facial dermatoses. BMJ 2021, 373, n1304.
- Yu, J.; Chen, J.K.; Mowad, C.M.; Reeder, M.; Hylwa, S.; Chisolm, S.; Dunnick, C.A.; Goldminz, A.M.; Jacob, S.E.; Wu, P.A.; et al. Occupational dermatitis to facial personal protective equipment in health care workers: A systematic review. J. Am. Acad. Dermatol. 2021, 84, 486–494.
- Lin, P.; Zhu, S.; Huang, Y.; Li, L.; Tao, J.; Lei, T.; Song, J.; Liu, D.; Chen, L.; Shi, Y.; et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: A survey in Wuhan and its surrounding regions. Br. J. Dermatol. 2020, 183, 190–192.
- Lan, J.; Song, Z.; Miao, X.; Li, H.; Li, Y.; Dong, L.; Yang, J.; An, X.; Zhang, Y.; Yang, L.; et al. Skin damage among health care workers managing coronavirus disease-2019. J. Am. Acad. Dermatol. 2020, 82, 1215–1216.
- Zhou, N.Y.; Yang, L.; Dong, L.Y.; Li, Y.; An, X.J.; Yang, J.; Yang, L.; Huang, C.Z.; Tao, J. Prevention and Treatment of Skin Damage Caused by Personal Protective Equipment: Experience of the First-Line Clinicians Treating 2019-nCoV Infection. Int. J. Dermatol. Venereol. 2020; ahead of print.
- Vasireddy, D.; Atluri, P.; Malayala, S.V.; Vanaparthy, R.; Mohan, G. Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use. J. Clin. Med. Res. 2021, 13, 204–213, Erratum in J. Clin. Med. Res. 2021, 13, 412.
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Appendix-C (accessed on 10 February 2022).
- Meara, A.S.; Silkowski, M.; Quin, K.; Jarjour, W. A Case of Chilblains-like Lesions Post SARS-CoV-2 Vaccine? J. Rheumatol. 2021, 48, 1754.
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous reactions after SARS-CoV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Dermatol. 2022, 186, 142–152.
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55.
- Glowacz, F.; Schmits, E. Psychological distress during the COVID-19 lockdown: The young adults most at risk. Psychiatry Res. 2020, 293, 113486.
- Wang, Y.; Kala, M.P.; Jafar, T.H. Factors associated with psychological distress during the coronavirus disease 2019 (COVID-19) pandemic on the predominantly general population: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0244630.
- Kim, H.H.; Jung, J.H. Social Isolation and Psychological Distress during the COVID-19 Pandemic: A Cross-National Analysis. Gerontologist 2021, 61, 103–113.
- Rossi, A.; Magri, F.; Sernicola, A.; Michelini, S.; Caro, G.; Muscianese, M.; Di Fraia, M.; Chello, C.; Fortuna, M.C.; Grieco, T. Telogen Effluvium after SARS-CoV-2 Infection: A Series of Cases and Possible Pathogenetic Mechanisms. Skin Appendage Disord. 2021, 21, 1–5.
- Turkmen, D.; Altunisik, N.; Sener, S.; Colak, C. Evaluation of the effects of COVID-19 pandemic on hair diseases through a web-based questionnaire. Dermatol. Ther. 2020, 33, e13923.
- Rivetti, N.; Barruscotti, S. Management of telogen effluvium during the COVID-19 emergency: Psychological implications. Dermatol. Ther. 2020, 33, e13648.
- Mahil, S.K.; Yates, M.; Yiu, Z.; Langan, S.M.; Tsakok, T.; Dand, N.; Mason, K.J.; McAteer, H.; Meynell, F.; Coker, B.; et al. Describing the burden of the COVID-19 pandemic in people with psoriasis: Findings from a global cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e636–e640.
- Yazdany, J.; Manno, R.L. Delayed Hypersensitivity. In Current Medical Diagnosis & Treatment 2022; Papadakis, M.A., McPhee, S.J., Rabow, M.W., McQuaid, K.R., Eds.; McGraw Hill: New York, NY, USA, 2022; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=3081§ionid=258968670 (accessed on 20 May 2022).
- Giavedoni, P.; Podlipnik, S.; Pericàs, J.M.; Fuertes de Vega, I.; García-Herrera, A.; Alós, L.; Carrera, C.; Andreu-Febrer, C.; Sanz-Beltran, J.; Riquelme-Mc Loughlin, C.; et al. Skin Manifestations in COVID-19: Prevalence and Relationship with Disease Severity. J. Clin. Med. 2020, 9, 3261.
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020, 183, 71–77.
- Do, M.H.; Stewart, C.R.; Harp, J. Cutaneous Manifestations of COVID-19 in the Inpatient Setting. Dermatol. Clin. 2021, 39, 521–532.
- Rekhtman, S.; Tannenbaum, R.; Strunk, A.; Birabaharan, M.; Wright, S.; Grbic, N.; Joseph, A.; Lin, S.K.; Zhang, A.C.; Lee, E.C.; et al. Eruptions and related clinical course among 296 hospitalized adults with confirmed COVID-19. J. Am. Acad. Dermatol. 2021, 84, 946–952.
- Askin, O.; Altunkalem, R.N.; Altinisik, D.D.; Uzuncakmak, T.K.; Tursen, U.; Kutlubay, Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID-19. Dermatol. Ther. 2020, 33, e13896.
- Català, A.; Galván-Casas, C.; Carretero-Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa, A.; Navarro-Fernández, Í.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas-Velasco, M.; et al. Maculopapular eruptions associated to COVID-19: A subanalysis of the COVID-Piel study. Dermatol. Ther. 2020, 33, e14170.
- Ghimire, K.; Adhikari, N. Morbilliform rashes in a patient with COVID-19 infection: A case report. JNMA J. Nepal Med. Assoc. 2021, 59, 399–401.
- Kulkarni, R.B.; Lederman, Y.; Afiari, A.; Savage, J.A.; Jacob, J. Morbilliform Rash: An Uncommon Herald of SARS-CoV-2. Cureus 2020, 12, e9321.
- Fattori, A.; Cribier, B.; Chenard, M.P.; Mitcov, M.; Mayeur, S.; Weingertner, N. Cutaneous manifestations in patients with coronavirus disease 2019: Clinical and histological findings. Hum. Pathol. 2021, 107, 39–45.
- Ahouach, B.; Harent, S.; Ullmer, A.; Martres, P.; Bégon, E.; Blum, L.; Tess, O.; Bachmeyer, C. Cutaneous lesions in a patient with COVID-19: Are they related? Br. J. Dermatol. 2020, 183, e31.
- Najarian, D.J. Morbilliform exanthem associated with COVID-19. JAAD Case Rep. 2020, 6, 493–494.
- Hedrich, C.M.; Fiebig, B.; Hauck, F.H.; Sallmann, S.; Hahn, G.; Pfeiffer, C.; Heubner, G.; Lee-Kirsch, M.A.; Gahr, M. Chilblain lupus erythematosus—A review of literature. Clin. Rheumatol. 2008, 27, 949–954.
- Su, W.P.; Perniciaro, C.; Rogers, R.S., 3rd; White, J.W., Jr. Chilblain lupus erythematosus (lupus pernio): Clinical review of the Mayo Clinic experience and proposal of diagnostic criteria. Cutis 1994, 54, 395–399.
- Cappel, J.A.; Wetter, D.A. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin. Proc. 2014, 89, 207–215.
- de Masson, A.; Bouaziz, J.D.; Sulimovic, L.; Cassius, C.; Jachiet, M.; Ionescu, M.A.; Rybojad, M.; Bagot, M.; Duong, T.A.; SNDV (French National Union of Dermatologists-Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: A retrospective nationwide study from France. J. Am. Acad. Dermatol. 2020, 83, 667–670.
- Piccolo, V.; Neri, I.; Filippeschi, C.; Oranges, T.; Argenziano, G.; Battarra, V.C.; Berti, S.; Manunza, F.; Fortina, A.B.; Di Lernia, V.; et al. Chilblain-like lesions during COVID-19 epidemic: A preliminary study on 63 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e291–e293.
- Kolivras, A.; Dehavay, F.; Delplace, D.; Feoli, F.; Meiers, I.; Milone, L.; Olemans, C.; Sass, U.; Theunis, A.; Thompson, C.T.; et al. Coronavirus (COVID-19) infection-induced chilblains: A case report with histopathologic findings. JAAD Case Rep. 2020, 6, 489–492.
- El Hachem, M.; Diociaiuti, A.; Concato, C.; Carsetti, R.; Carnevale, C.; Ciofi Degli Atti, M.; Giovannelli, L.; Latella, E.; Porzio, O.; Rossi, S.; et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: Lights and shadows on the relationship with COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2620–2629.
- Kanitakis, J.; Lesort, C.; Danset, M.; Jullien, D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): Histologic, immunofluorescence, and immunohistochemical study of 17 cases. J. Am. Acad. Dermatol. 2020, 83, 870–875.
- Colmenero, I.; Santonja, C.; Alonso-Riaño, M.; Noguera-Morel, L.; Hernández-Martín, A.; Andina, D.; Wiesner, T.; Rodríguez-Peralto, J.L.; Requena, L.; Torrelo, A.; et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br. J. Dermatol. 2020, 183, 729–737.
- Santonja, C.; Heras, F.; Núñez, L.; Requena, L. COVID-19 chilblain-like lesion: Immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a polymerase chain reaction-negative patient. Br. J. Dermatol. 2020, 183, 778–780.
- Gambichler, T.; Reuther, J.; Stücker, M.; Stranzenbach, R.; Torres-Reyes, C.; Schlottmann, R.; Schmidt, W.E.; Hayajneh, R.; Sriram, A.; Becker, J.C.; et al. SARS-CoV-2 spike protein is present in both endothelial and eccrine cells of a chilblain-like skin lesion. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e187–e189.
- Ko, C.J.; Harigopal, M.; Damsky, W.; Gehlhausen, J.R.; Bosenberg, M.; Patrignelli, R.; McNiff, J.M. Perniosis during the COVID-19 pandemic: Negative anti-SARS-CoV-2 immunohistochemistry in six patients and comparison to perniosis before the emergence of SARS-CoV-2. J. Cutan. Pathol. 2020, 47, 997–1002.
- Baeck, M.; Hoton, D.; Marot, L.; Herman, A. Chilblains and COVID-19: Why SARS-CoV-2 endothelial infection is questioned. Br. J. Dermatol. 2020, 183, 1152–1153.
- Freeman, E.E.; McMahon, D.E.; Lipoff, J.B.; Rosenbach, M.; Desai, S.R.; Fassett, M.; French, L.E.; Lim, H.W.; Hruza, G.J.; Fox, L.P. Cold and COVID: Recurrent pernio during the COVID-19 pandemic. Br. J. Dermatol. 2021, 185, 214–216.
- Palamaras, I.; Kyriakis, K. Calcium antagonists in dermatology: A review of the evidence and research-based studies. Dermatol. Online J. 2005, 11, 8.
- Whitman, P.A.; Crane, J.S. Pernio; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Kayiran, M.A.; Akdeniz, N. Diagnosis and treatment of urticaria in primary care. North Clin. Istanb. 2019, 6, 93–99.
- Schaefer, P. Acute and Chronic Urticaria: Evaluation and Treatment. Am. Fam. Physician 2017, 95, 717–724.
- Sabroe, R.A.; Greaves, M.W. The pathogenesis of chronic idiopathic urticaria. Arch. Dermatol. 1997, 133, 1003–1008.
- Henry, D.; Ackerman, M.; Sancelme, E.; Finon, A.; Esteve, E. Urticarial eruption in COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e244–e245.
- Recalcati, S. Cutaneous manifestations in COVID-19: A first perspective. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e212–e213.
- Algaadi, S.A. Urticaria and COVID-19: A review. Dermatol. Ther. 2020, 33, e14290.
- De Giorgi, V.; Recalcati, S.; Jia, Z.; Chong, W.; Ding, R.; Deng, Y.; Scarfi, F.; Venturi, F.; Trane, L.; Gori, A.; et al. Cutaneous manifestations related to coronavirus disease 2019 (COVID-19): A prospective study from China and Italy. J. Am. Acad. Dermatol. 2020, 83, 674–675.
- Dastoli, S.; Bennardo, L.; Patruno, C.; Nisticò, S.P. Are erythema multiforme and urticaria related to a better outcome of COVID-19? Dermatol. Ther. 2020, 33, e13681.
- Jesenak, M.; Banovcin, P.; Diamant, Z. COVID-19, chronic inflammatory respiratory diseases and eosinophils-Observations from reported clinical case series. Allergy 2020, 75, 1819–1822.
- Rosenberg, H.F.; Foster, P.S. Eosinophils and COVID-19: Diagnosis, prognosis, and vaccination strategies. Semin. Immunopathol. 2021, 43, 383–392.
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in Asthma Patients Is Protective against Severe COVID-19 Illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3.
- Rodríguez-Jiménez, P.; Chicharro, P.; De Argila, D.; Muñoz-Hernández, P.; Llamas-Velasco, M. Urticaria-like lesions in COVID-19 patients are not really urticaria—A case with clinicopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e459–e460.
- Zipursky, J.S.; Croitoru, D. Urticaria and angioedema associated with SARS-CoV-2 infection. CMAJ 2021, 193, e1390.
- Hassan, K. Urticaria and angioedema as a prodromal cutaneous manifestation of SARS-CoV-2 (COVID-19) infection. BMJ Case Rep. 2020, 13, e236981.
- Najafzadeh, M.; Shahzad, F.; Ghaderi, N.; Ansari, K.; Jacob, B.; Wright, A. Urticaria (angioedema) and COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e568–e570.
- Proietti, I.; Mambrin, A.; Bernardini, N.; Tolino, E.; Balduzzi, V.; Maddalena, P.; Marchesiello, A.; Michelini, S.; Volpe, S.; Skroza, N.; et al. Urticaria in an infant with SARS-CoV-2 positivity. Dermatol. Ther. 2020, 33, e14043.
- Morey-Olivé, M.; Espiau, M.; Mercadal-Hally, M.; Lera-Carballo, E.; García-Patos, V. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An. Pediatr. 2020, 92, 374–375.
- Pagali, S.; Parikh, R.S. Severe urticarial rash as the initial symptom of COVID-19 infection. BMJ Case Rep. 2021, 14, e241793.
- Shanshal, M. Low- dose systemic steroids, an emerging therapeutic option for COVID-19 related urticaria. J. Dermatolog. Treat. 2022, 33, 1140–1141.
- Sajjan, V.V.; Lunge, S.; Swamy, M.B.; Pandit, A.M. Livedo reticularis: A review of the literature. Indian Dermatol. Online J. 2015, 6, 315–321.
- Verheyden, M.; Grosber, M.; Gutermuth, J.; Velkeniers, B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e684–e686.
- Khalil, S.; Hinds, B.R.; Manalo, I.F.; Vargas, I.M.; Mallela, S.; Jacobs, R. Livedo reticularis as a presenting sign of severe acute respiratory syndrome coronavirus 2 infection. JAAD Case Rep. 2020, 6, 871–874.
- Agnihothri, R.; Fox, L.P. Clinical Patterns and Morphology of COVID-19 Dermatology. Dermatol. Clin. 2021, 39, 487–503.
- Chand, S.; Rrapi, R.; Lo, J.A.; Song, S.; Gabel, C.K.; Desai, N.; Hoang, M.P.; Kroshinsky, D. Purpuric ulcers associated with COVID-19: A case series. JAAD Case Rep. 2021, 11, 13–19.
- Pincelli, M.S.; Echavarria, A.; Criado, P.R.; Marques, G.F.; Morita, T.; Valente, N.; de Carvalho, J.F. Livedo Racemosa: Clinical, Laboratory, and Histopathological Findings in 33 Patients. Int. J. Low Extrem. Wounds 2021, 20, 22–28.
- Jamshidi, P.; Hajikhani, B.; Mirsaeidi, M.; Vahidnezhad, H.; Dadashi, M.; Nasiri, M.J. Skin Manifestations in COVID-19 Patients: Are They Indicators for Disease Severity? A Systematic Review. Front. Med. 2021, 8, 634208.
- Wysong, A.; Venkatesan, P. An approach to the patient with retiform purpura. Dermatol. Ther. 2011, 24, 151–172.
- Daneshgaran, G.; Dubin, D.P.; Gould, D.J. Cutaneous Manifestations of COVID-19: An Evidence-Based Review. Am. J. Clin. Dermatol. 2020, 21, 627–639.
- Marzano, A.V.; Genovese, G.; Fabbrocini, G.; Pigatto, P.; Monfrecola, G.; Piraccini, B.M.; Veraldi, S.; Rubegni, P.; Cusini, M.; Caputo, V.; et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J. Am. Acad. Dermatol. 2020, 83, 280–285.
- Fernandez-Nieto, D.; Ortega-Quijano, D.; Jimenez-Cauhe, J.; Burgos-Blasco, P.; de Perosanz-Lobo, D.; Suarez-Valle, A.; Cortes-Cuevas, J.L.; Carretero, I.; Garcia-Del Real, C.; Fernandez-Guarino, M. Clinical and histological characterization of vesicular COVID-19 rashes: A prospective study in a tertiary care hospital. Clin. Exp. Dermatol. 2020, 45, 872–875.
- Trellu, L.T.; Kaya, G.; Alberto, C.; Calame, A.; McKee, T.; Calmy, A. Clinicopathologic Aspects of a Papulovesicular Eruption in a Patient With COVID-19. JAMA Dermatol. 2020, 156, 922–924.
- Mahé, A.; Birckel, E.; Merklen, C.; Lefèbvre, P.; Hannedouche, C.; Jost, M.; Droy-Dupré, L. Histology of skin lesions establishes that the vesicular rash associated with COVID-19 is not ‘varicella-like’. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e559–e561.
- Villalon-Gomez, J.M. Pityriasis Rosea: Diagnosis and Treatment. Am. Fam. Physician 2018, 97, 38–44.
- Kutlu, Ö.; Metin, A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol. Ther. 2020, 33, e14096.
- Merhy, R.; Sarkis, A.S.; Stephan, F. Pityriasis rosea as a leading manifestation of COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e246–e247.
- Dursun, R.; Temiz, S.A. The clinics of HHV-6 infection in COVID-19 pandemic: Pityriasis rosea and Kawasaki disease. Dermatol. Ther. 2020, 33, e13730.
- Veraldi, S.; Spigariolo, C.B. Pityriasis rosea and COVID-19. J. Med. Virol. 2021, 93, 4068.
- Martín Enguix, D.; Salazar Nievas, M.D.C.; Martín Romero, D.T. Pityriasis rosea Gibert type rash in an asymptomatic patient that tested positive for COVID-19. Med. Clin. 2020, 155, 273.
- Potekaev, N.N.; Zhukova, O.V.; Protsenko, D.N.; Demina, O.M.; Khlystova, E.A.; Bogin, V. Clinical characteristics of dermatologic manifestations of COVID-19 infection: Case series of 15 patients, review of literature, and proposed etiological classification. Int. J. Dermatol. 2020, 59, 1000–1009.
- Sanchez, A.; Sohier, P.; Benghanem, S.; L’Honneur, A.S.; Rozenberg, F.; Dupin, N.; Garel, B. Digitate Papulosquamous Eruption Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Dermatol. 2020, 156, 819–820.
- Drago, F.; Ciccarese, G.; Rebora, A.; Parodi, A. Human herpesvirus-6, -7, and Epstein-Barr virus reactivation in pityriasis rosea during COVID-19. J. Med. Virol. 2021, 93, 1850–1851.
- Welsh, E.; Cardenas-de la Garza, J.A.; Cuellar-Barboza, A.; Franco-Marquez, R.; Arvizu-Rivera, R.I. SARS-CoV-2 spike protein positivity in pityriasis rosea-like and urticaria-like rashes of COVID-19. Br. J. Dermatol. 2021, 184, 1194–1195.
- Perna, A.; Passiatore, M.; Massaro, A.; Terrinoni, A.; Bianchi, L.; Cilli, V.; D’Orio, M.; Proietti, L.; Taccardo, G.; De Vitis, R. Skin manifestations in COVID-19 patients, state of the art. A systematic review. Int. J. Dermatol. 2021, 60, 547–553.
- World Health Organization. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Available online: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 10 February 2022).
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665.
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608.
- Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (mis-C). Available online: https://www.cdc.gov/mis/misc/hcp/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fmis%2Fhcp%2Findex.html (accessed on 10 February 2022).
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among Children in China. Pediatrics 2020, 145, e20200702.
- Licciardi, F.; Pruccoli, G.; Denina, M.; Parodi, E.; Taglietto, M.; Rosati, S.; Montin, D. SARS-CoV-2-Induced Kawasaki-Like Hyperinflammatory Syndrome: A Novel COVID Phenotype in Children. Pediatrics 2020, 146, e20201711.
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778.
- Hennon, T.R.; Penque, M.D.; Abdul-Aziz, R.; Alibrahim, O.S.; McGreevy, M.B.; Prout, A.J.; Schaefer, B.A.; Ambrusko, S.J.; Pastore, J.V.; Turkovich, S.J.; et al. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; a Western New York approach. Prog. Pediatr. Cardiol. 2020, 57, 101232.
- Center for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 10 February 2022).
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346.
- Brumfiel, C.M.; DiLorenzo, A.M.; Petronic-Rosic, V.M. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin. Dermatol. 2021, 39, 329–333.
- Shakeel, S.; Ahmad Hassali, M.A. Post-COVID-19 Outbreak of Severe Kawasaki-like Multisystem Inflammatory Syndrome in Children. Malays. J. Med. Sci. 2021, 28, 109–116.
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020, 72, 1791–1805.
- Bursal Duramaz, B.; Yozgat, C.Y.; Yozgat, Y.; Turel, O. Appearance of skin rash in pediatric patients with COVID-19: Three case presentations. Dermatol. Ther. 2020, 33, e13594.
- Gianotti, R.; Recalcati, S.; Fantini, F.; Riva, C.; Milani, M.; Dainese, E.; Boggio, F. Histopathological Study of a Broad Spectrum of Skin Dermatoses in Patients Affected or Highly Suspected of Infection by COVID-19 in the Northern Part of Italy: Analysis of the Many Faces of the Viral-Induced Skin Diseases in Previous and New Reported Cases. Am. J. Dermatopathol. 2020, 42, 564–570.
- Bosch-Amate, X.; Giavedoni, P.; Podlipnik, S.; Andreu-Febrer, C.; Sanz-Beltran, J.; Garcia-Herrera, A.; Alós, L.; Mascaró, J.M. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID-19) coagulopathy. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e548–e549.
- Shanshal, M.; Ahmed, H.S.; Asfoor, H.; Salih, R.I.; Ali, S.A.; Aldabouni, Y.K. Impact of COVID-19 on medical practice: A nationwide survey of dermatologists and health care providers in Iraq. Clin. Dermatol. 2021, 39, 500–509.
- Chiriac, A.E.; Wollina, U.; Azoicai, D. Flare-up of Rosacea due to Face Mask in Healthcare Workers during COVID-19. Maedica 2020, 15, 416–417.
- Yin, Z.Q. Covid-19: Countermeasure for N95 mask-induced pressure sore. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e294–e295.
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J. Am. Acad. Dermatol. 2020, 83, 1730–1737.
- U.S. Food and Drug Administration. COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 10 February 2022).
- GOV.UK. Decision Conditions of Authorisation for COVID-19 Vaccine AstraZeneca (Regulation 174). Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca (accessed on 10 February 2022).
- Baraniuk, C. COVID-19: What do we know about Sputnik V and other Russian vaccines? BMJ 2021, 372, n743.
- Montano, D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front. Public Health 2022, 9, 756633.
- Centers for Disease Control and Prevention. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Moderna COVID-19 Vaccine—United States, December 21, 2020–January 10, 2021. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7004e1.htm (accessed on 10 February 2022).
- McLendon, K.; Sternard, B.T. Anaphylaxis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 15 May 2022.
- Sobczak, M.; Pawliczak, R. The risk of anaphylaxis behind authorized COVID-19 vaccines: A meta-analysis. Clin. Mol. Allergy 2022, 20, 1.
- Iguchi, T.; Umeda, H.; Kojima, M.; Kanno, Y.; Tanaka, Y.; Kinoshita, N.; Sato, D. Cumulative Adverse Event Reporting of Anaphylaxis After mRNA COVID-19 Vaccine (Pfizer-BioNTech) Injections in Japan: The First-Month Report. Drug Saf. 2021, 44, 1209–1214.
- Cabanillas, B.; Akdis, C.A.; Novak, N. Allergic reactions to the first COVID-19 vaccine: A potential role of polyethylene glycol? Allergy 2021, 76, 1617–1618.
- Garvey, L.H.; Nasser, S. Anaphylaxis to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit? Br. J. Anaesth. 2021, 126, e106–e108.
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437.
- Wenande, E.C.; Skov, P.S.; Mosbech, H.; Poulsen, L.K.; Garvey, L.H. Inhibition of polyethylene glycol-induced histamine release by monomeric ethylene and diethylene glycol: A case of probable polyethylene glycol allergy. J. Allergy Clin. Immunol. 2013, 131, 1425–1427.
- Bruusgaard-Mouritsen, M.A.; Johansen, J.D.; Garvey, L.H. Clinical manifestations and impact on daily life of allergy to polyethylene glycol (PEG) in ten patients. Clin. Exp. Allergy 2021, 51, 463–470.
- Sellaturay, P.; Nasser, S.; Ewan, P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J. Allergy Clin. Immunol. Pract. 2021, 9, 670–675.
- Brandt, N.; Garvey, L.H.; Bindslev-Jensen, U.; Kjaer, H.F.; Bindslev-Jensen, C.; Mortz, C.G. Three cases of anaphylaxis following injection of a depot corticosteroid with evidence of IgE sensitization to macrogols rather than the active steroid. Clin. Transl. Allergy 2017, 7, 2.
- Zhou, Z.H.; Stone, C.A., Jr.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.
- Kozma, G.T.; Mészáros, T.; Vashegyi, I.; Fülöp, T.; Örfi, E.; Dézsi, L.; Rosivall, L.; Bavli, Y.; Urbanics, R.; Mollnes, T.E. Pseudo-anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions. ACS Nano 2019, 13, 9315–9324.
- Tihy, M.; Menzinger, S.; André, R.; Laffitte, E.; Toutous-Trellu, L.; Kaya, G. Clinicopathological features of cutaneous reactions after mRNA-based COVID-19 vaccines. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2456–2461.
- Kempf, W.; Kettelhack, N.; Kind, F.; Courvoisier, S.; Galambos, J.; Pfaltz, K. ‘COVID arm’—Histological features of a delayed-type hypersensitivity reaction to Moderna mRNA-1273 SARS-CoV2 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e730–e732.
- Ramos, C.L.; Kelso, J.M. “COVID Arm”: Very delayed large injection site reactions to mRNA COVID-19 vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 2480–2481.
- Lindgren, A.L.; Austin, A.H.; Welsh, K.M. COVID Arm: Delayed Hypersensitivity Reactions to SARS-CoV-2 Vaccines Misdiagnosed as Cellulitis. J Prim Care Community Health. 2021, 12, 21501327211024431.
- Centers for Disease Control and Prevention. COVID-19 Vaccine Distribution Allocations by Jurisdiction—Pfizer. Available online: https://data.cdc.gov/Vaccinations/COVID-19-Vaccine-Distribution-Allocations-by-Juris/saz5–9hgg (accessed on 10 February 2022).
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95.
- Kelso, J.M.; Coda, A.B.; Keating, R.M.; Vaccari, D.M. “COVID Toes” After mRNA COVID-19 Vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 3196–3197.
- Lesort, C.; Kanitakis, J.; Donzier, L.; Jullien, D. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: A case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e630–e632.
- Tan, S.W.; Tam, Y.C.; Oh, C.C. Skin manifestations of COVID-19: A worldwide review. JAAD Int. 2021, 2, 119–133.
- Akdaş, E.; İlter, N.; Öğüt, B.; Erdem, Ö. Pityriasis rosea following CoronaVac COVID-19 vaccination: A case report. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e491–e493.
- Burlando, M.; Herzum, A.; Cozzani, E.; Parodi, A. Acute urticarial rash after COVID-19 vaccination containing Polysorbate 80. Clin. Exp. Vaccine Res. 2021, 10, 298–300.
- Hossain, M.M.; Tasnim, S.; Sultana, A.; Faizah, F.; Mazumder, H.; Zou, L.; McKyer, E.; Ahmed, H.U.; Ma, P. Epidemiology of mental health problems in COVID-19: A review. F1000Research 2020, 9, 636.
- Chaves, C.; Castellanos, T.; Abrams, M.; Vazquez, C. The impact of economic recessions on depression and individual and social well-being: The case of Spain (2006–2013). Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 977–986.
- Tapia Granados, J.A.; Christine, P.J.; Ionides, E.L.; Carnethon, M.R.; Diez Roux, A.V.; Kiefe, C.I.; Schreiner, P.J. Cardiovascular Risk Factors, Depression, and Alcohol Consumption During Joblessness and During Recessions Among Young Adults in CARDIA. Am. J. Epidemiol. 2018, 187, 2339–2345.
- Beaglehole, B.; Mulder, R.T.; Frampton, C.M.; Boden, J.M.; Newton-Howes, G.; Bell, C.J. Psychological distress and psychiatric disorder after natural disasters: Systematic review and meta-analysis. Br. J. Psychiatry. 2018, 213, 716–722.
- Reich, A.; Wójcik-Maciejewicz, A.; Slominski, A.T. Stress and the skin. G. Ital. Dermatol. Venereol. 2010, 145, 213–219.
- Malkud, S. Telogen Effluvium: A Review. J. Clin. Diagn. Res. 2015, 9, WE01–WE3.
- Sharquie, K.E.; Jabbar, R.I. COVID-19 infection is a major cause of acute telogen effluvium. Ir. J. Med. Sci. 2021, 1–5.
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361.
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751.
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719.
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol Treatment and COVID-19-Related Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, e4017–e4027.
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189.
- Gallizzi, R.; Sutera, D.; Spagnolo, A.; Bagnato, A.M.; Cannavò, S.P.; Grasso, L.; Guarneri, C.; Nunnari, G.; Mazza, F.; Pajno, G.B. Management of pernio-like cutaneous manifestations in children during the outbreak of COVID-19. Dermatol. Ther. 2020, 33, e14312.
- Mahé, A.; Birckel, E.; Krieger, S.; Merklen, C.; Bottlaender, L. A distinctive skin rash associated with coronavirus disease 2019? J. Eur. Acad. Dermatol. Venereol. 2020, 34, e246–e247.
- Iancu, G.M.; Solomon, A.; Birlutiu, V. Viral exanthema as manifestation of SARS-CoV-2 infection: A case report. Medicine 2020, 99, e21810.
- Abuelgasim, E.; Dona, A.C.M.; Sondh, R.S.; Harky, A. Management of urticaria in COVID-19 patients: A systematic review. Dermatol. Ther. 2021, 34, e14328.
- van Damme, C.; Berlingin, E.; Saussez, S.; Accaputo, O. Acute urticaria with pyrexia as the first manifestations of a COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e300–e301.
- McCullough, P.A.; Kelly, R.J.; Ruocco, G.; Lerma, E.; Tumlin, J.; Wheelan, K.R.; Katz, N.; Lepor, N.E.; Vijay, K.; Carter, H.; et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am. J. Med. 2021, 134, 16–22.
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev. Cardiovasc. Med. 2020, 21, 517–530.
- Risma, K.A.; Edwards, K.M.; Hummell, D.S.; Little, F.F.; Norton, A.E.; Stallings, A.; Wood, R.A.; Milner, J.D. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J. Allergy Clin. Immunol. 2021, 147, 2075–2082.e2.
- Preissner, K.T.; Fischer, S.; Deindl, E. Extracellular RNA as a Versatile DAMP and Alarm Signal That Influences Leukocyte Recruitment in Inflammation and Infection. Front. Cell Dev. Biol. 2020, 8, 619221.
- Manalo, I.F.; Smith, M.K.; Cheeley, J.; Jacobs, R. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J. Am. Acad. Dermatol. 2020, 83, 700.
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221.
