You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Harleen Kaur Chela.
Esophageal cancer is rapidly increasing across the world. It is the sixth most common cause of death from cancer and is the eighth most common cancer worldwide. Esophageal cancer is a devastating malignancy which can be detected at an early stage but is more often diagnosed as an advanced process. It affects both men and women and inflicts the young and the elderly. There are multiple underlying factors involved in the pathogenesis of this cancer including inflammation.
- cancer
- pathogenesis
- inflammation
- esophagus
1. Smoking
Smoking is a risk factor for both ESCC and adenocarcinoma of the esophagus [2,5][1][2]. Smoking predisposes to the development of Barrett’s and also increases the risk for adenocarcinoma [2][1]. Smoking duration and amount is also correlated to the overall risk [11][3]. The magnitude of risk is also higher for squamous cell carcinoma as compared to adenocarcinoma [11][3]. The risk for male smokers is noted to be increased as compared to female smokers [12][4]. Smoking generates a pro-inflammatory response with production of several inflammatory cytokines and molecules. It promotes an inflammatory state by inducing the generation of cytokines such as interleukin (IL)-1, IL-6 and IL-8 and tumor necrosis factor-α (TNF-α) [13][5]. It reduces the levels of IL-10, which is an anti-inflammatory medicator [13][5]. The NF-kB (Nuclear factor kappa B) pathway is one of the main factors underlying the stimulation of inflammatory cells secondary to smoking [14][6]. Tobacco smoke is also a source of free radicals such as ROS and reactive nitrogen species (RNS) that lead to oxidative stress [15][7]. These include hydrogen peroxide, hydroxyl radicals, superoxide, and nitric oxide [15][7]. These reactive oxygen species and reactive nitrogen species lead to the activation of the NF-kB pathway and the creation of a pro-inflammatory state [16][8]. These reactive nitrogen and oxygen species cause oxidative stress and damage to lipids, proteins and nucleic acids, which not only lead to inflammation but contribute to carcinogenesis [17][9]. The peroxidation of lipids can cause the formation of products that cause damage to DNA and proteins [18][10].
2. Alcohol
Alcohol is metabolized by the enzyme alcohol dehydrogenase, with ethanol being converted to acetaldehyde [9][11]. Acetaldehyde exerts a carcinogenic effect by forming DNA adducts and alters the genes to lead to a mutation [9][11]. The consumption of alcohol is clearly linked to the development of ESCC but not as strongly to adenocarcinoma [11][3]. A prospective cohort study conducted in the Netherlands did not find a significant link between adenocarcinoma and alcohol use [19][12]. However with regard to ESCC, the risk can be up to three to five times greater in those who have a history of alcohol consumption [19,20][12][13]. In a large meta-analysis of case control and cohort studies conducted by Islami et al., it was seen that moderate and high consumption of alcohol was linked to a higher risk of ESCC in Asian and non-Asian countries [21][14]. Light consumption of alcohol was only noted to be connected to a higher risk for ESCC in Asian countries [21][14]. However, a dose-dependent association was seen between alcohol intake and risk of ESCC in Asian and non-Asian countries [21][14]. The type of alcoholic beverages and association with esophageal malignancy is not as clearly established [11][3].
Alcohol can lead to inflammation in the gastrointestinal tract along with dysbiosis, bacterial overgrowth and intestinal hyperpermeability [22][15]. Alcohol is metabolized predominantly in the hepatocytes, with conversion of alcohol to acetaldehyde via the enzyme alcohol dehydrogenase [22][15]. The acetaldehyde, which is harmful, is then metabolized to acetate via the enzyme acetaldehyde dehydrogenase [22][15]. The oxidative metabolism of alcohol also occurs in the intestines and bacteria generates acetaldehyde in the gut [23][16]. In cases of chronic alcohol consumption, there is a nonoxidative metabolism of alcohol in the intestines that involves phospholipids and free fatty acids and can have harmful effects [24][17]. The microsomal ethanol–oxidizing system is another form of metabolism in which alcohol is subjected to and leads to the generation of free radicals with resultant injury to cells [22][15]. By increasing the permeability of the wall of the intestines, alcohol permits bacteria to translocate, and due to the release of endotoxins cause local and systemic inflammation with the release of cytokines [22][15]. Alcohol also promotes an inflammatory state by decreasing immunity and lowering the activity of the Paneth cells [22][15].
3. Diet
Diet can play a pivotal role in the predisposition of esophageal malignancy. A diet high in meat and fast foods akin to the Western diet increases the risk for Barrett’s esophagus and subsequent adenocarcinoma [25][18]. Whereas a diet that is abundant in fruits, vegetables, nonfried fish is linked to a decreased risk for cancer of the esophagus, as all of these contain antioxidants [25][18]. A diet that is high in omega-3-fatty-acids, total fiber, polyunsaturated fats, and fiber is linked to a decreased risk for Barrett’s esophagus [26][19]. The intake of vitamin D, beta-carotene, and vitamin C also has protective effects against Barrett’s esophagus [27][20]. Consumption of meat can lead to the development of esophageal cancer through the production of mutagenic heterocyclic amines and polycyclic aromatic hydrocarbons when it is prepared at high temperatures [28][21]. Meat is a source of iron and a source of nitrates and nitrites when processed, and this can lead to the generation of N-nitroso compounds, which have carcinogenic properties [28][21]. Though ESCC shares some similar underlying risks, there are other dietary factors that are more pertinent to the development of ESCC.
In developing countries across the world such as in Northern China and Northeastern Iran, the dietary factors leading to ESCC have been well studied. The Linxian area in Northern China has a high incidence and mortality from ESCC [29][22]. A diet lacking in fruits and vegetables along with a deficiency of micronutrients such as carotenoids, riboflavin and vitamins A, C and E is associated with a risk for ESCC [29][22]. Diet-derived carcinogens such as nitrosamines and polycyclic aromatic hydrocarbons also predispose to ESCC [29][22]. Preformed nitrosamines and endogenously produced nitrosamines both play a role in carcinogenesis [29][22]. Nitrosamines are generated from nitrites (formed by the oxidation of nitrogenous elements in the water) and secondary amines (can be found in substances such as moldy corn) [29][22]. A nitrosation reaction of moldy corn can produce carcinogens such as methylbenzylnitrosamine, diethylnitrosamine and N-1-methylacetonyl-N-3-methylbutylnitrosamine [30][23]. Polycyclic aromatic hydrocarbons are formed due to the burning of wood and coal and have harmful effects as well [30][23]. The deficiency of micronutrients such as selenium and zinc may also be linked to esophageal cancer [31][24].
The ingestion of pickled vegetables has also been shown in some studies to be a risk factor for ESCC [30][23]. The proliferation of yeasts and fungi in the pickled vegetables can lead to formation of harmful substances such as mycotoxins, Roussin red methyl ester and N-nitrosamines [30,32,33][23][25][26]. Thermal damage to the lining of the esophagus can occur from the ingestion of hot food and beverages and increase the risk for ESCC [34][27]. The chewing of betel quid, which is common in areas like India and Taiwan, can cause irritation of the esophageal mucosa and lead to ESCC, and risk increases in conjunction with the use of tobacco [35][28]. The Mediterranean diet, which is increasing in popularity, had been found to have some beneficial effects with regard to esophageal cancer. A Mediterranean diet is plant based and is rich in whole grains, vegetables, and fruits, and the predominant fat is olive oil [36][29]. A study conducted in the Netherlands revealed that consuming a Mediterranean diet was related to a decreased risk for both ESCC and EAC [36][29]. This diet has high levels of anti-oxidants and can decrease oxidative damage of the DNA and reduce inflammation [37][30]. The presence of dietary fiber as well in this diet can be protective against the mutagenic properties of N-nitroso compounds by being a nitrite scavenger [37][30]. As the Mediterranean diet is low in meat, the harmful effects that occur due to meat as described above are much less [28][21].
Maté is made from a herb called Ilex paraguayensis, and when hot maté is consumed, especially in large amounts, it is linked to the development of esophageal cancer [31,38][24][31]. The recurrent thermal injury which occurs due to ingestion of hot maté and the polycyclic aromatic hydrocarbons that it contains lead to carcinogenesis [38][31]. Carbonated soft drinks have been speculated to have an association with EAC due to increased reflux secondary to gaseous distention of the stomach and their acidic nature [31][24]. However, other studies have not shown any correlation and hence the findings are inconclusive [31][24].
4. Gastro-Esophageal Reflux Disease (GERD)
The presence of uncontrolled and untreated reflux is an important factor in the pathogenesis of Barrett’s esophagus and the development of esophageal cancer [12][4]. Longstanding GERD induces acid related damage to the esophageal lining leading to chronic inflammation in the esophagus (Figure 21) and the eventual metaplastic change known as Barrett’s esophagus (Figure 32). The intestinal metaplasia with columnar cells and goblet cells is believed to be more resilient towards the deleterious effects of chronic acid exposure [39][32]. Continuous exposure to inflammation causes the production of substances such as cytokines, chemokines, and reactive oxygen species that can induce harmful effects [9][11]. They can ultimately lead to augmentation of cell growth, stimulate invasion and promote the growth of blood vessels [9][11]. Inflammatory cells may also produce mediators that can inhibit immune functions, and this also promotes carcinogenesis [40][33]. This progresses over time to dysplastic and neoplastic changes, as Barrett’s esophagus is a well-known risk factor for adenocarcinoma of the esophagus. GERD is not known to be a risk factor for ESCC.
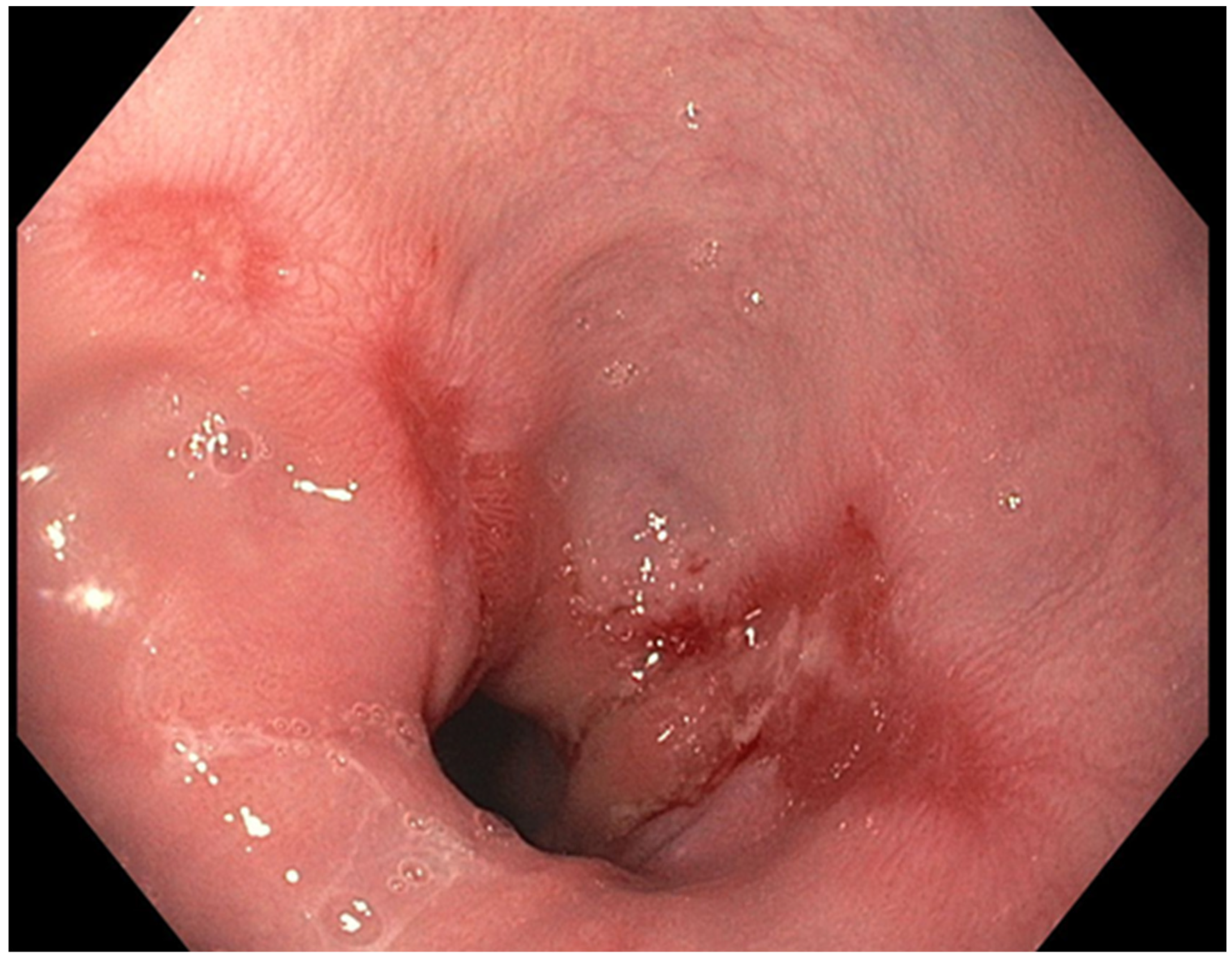
Figure 21.
Los Angeles Grade B esophagitis.
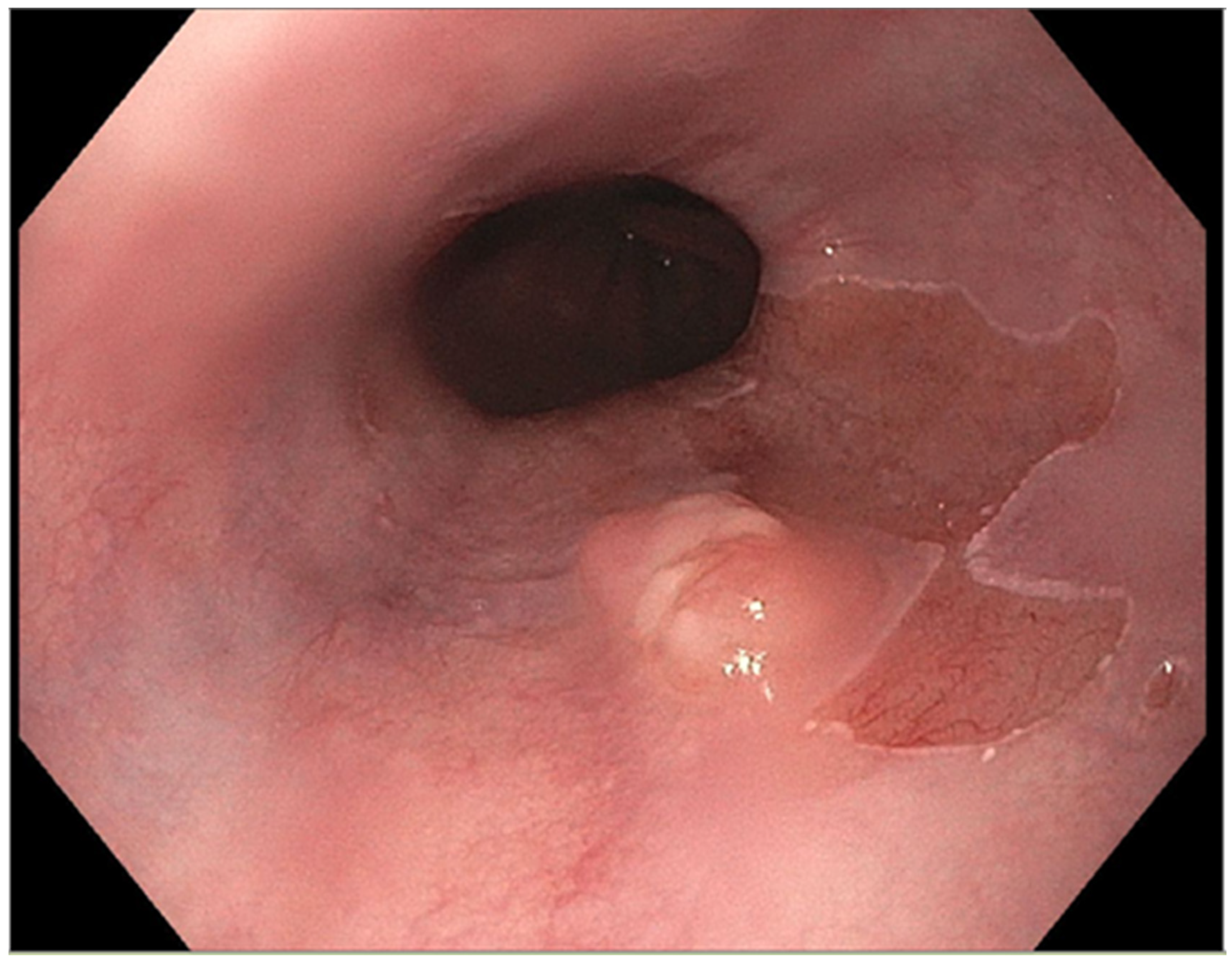
Figure 32.
Barrett’s esophagus with nodule.
5. Obesity
Obesity has been shown to have an association with adenocarcinoma with a higher BMI and abdominal obesity having a linkage to EAC [41][34]. A BMI greater than 25 kg/m2 raises the risk for adenocarcinoma in males and females, and the higher the BMI the greater the risk [41][34]. Obesity raises the intra-abdominal pressure and this worsens reflux and thereby predisposes the development of Barrett’s esophagus and the subsequent risk for EAC [42][35]. Inversely, an increased BMI is associated with a decreased risk for ESCC [12][4]. Obesity is also associated with a chronic state of low inflammation that involves adipocytes [43][36]. It leads to elevated levels of C-reactive protein and adipocyte induced activation of pathways of inflammation [43][36]. Cytokines that promote inflammation are produced by the adipocytes as well as immunological cells (such as T lymphocytes and macrophages) [44][37]. T lymphocytes produce interferon-gamma (INF-γ), IL-1, and IL-17 and the macrophages produce IL-12 and TNF-α [45][38]. Other cytokines that may play a causative role in carcinogenesis in the state of obesity include insulin-like growth factor-1 (IGF-1), transforming growth factor-beta (TGF-β), and vascular endothelium growth factor [46][39]. These factors are linked to cell proliferation and the promotion of angiogenesis and hence the possible causal link to carcinogenesis [46][39].
6. Infections
There are some infectious agents that have been speculated to be implicated in the pathogenesis of esophageal cancer. Bacteria such as Helicobacter pylori and viral agents such as the Human papillomavirus types 16 and 18 may contribute to the development of esophageal cancer. Helicobacter pylori is a possible factor in ESCC [47][40], but results are inconclusive, as other studies show no correlation. For EAC, H. pylori shows a protective effect according to some studies and was found to have a decreased risk for EAC [48,49][41][42]. H. pylori lessens the generation of acid in the stomach and leads to decreased reflux of gastric acid, thereby mitigating the effect for Barrett’s and EAC [50][43]. H. pylori may also reduce the generation of ghrelin, which enhances appetite and is a hormone formed in the stomach [51][44]. By decreasing appetite, a lower level of ghrelin may decrease obesity and hence lower the risk for EAC [52][45]. Due to use of antibiotics and improvements in sanitation, there is a decreased colonization of H. pylori in western countries, and this could be contributing to the increase in EAC [31,53][24][46]. The human papilloma virus (HPV) has oncogenic variants such as HPV 16 and 18, and though the findings are controversial, there are some studies that implicate these variants in the pathogenesis of esophageal cancer [54][47]. Epstein-Barr virus (EBV) infects the B lymphocytes and has known oncogenic potential, but with regards to esophageal cancer, the available data is conflicting, with some studies showing an association but others showing none [55][48].
7. Anatomical Factors
Anatomical changes such as hiatal hernia, achalasia, and gastric atrophy can all predispose to esophageal cancer. A hiatal hernia, especially one that is large, can lead to a higher risk for EAC, as it increases the reflux of gastric acid and can cause the development of Barrett’s esophagus [56,57][49][50]. This is not considered to be a risk factor for ESCC. Gastric atrophy leads to increased risk for ESCC as noted by some studies that show that decreased levels of serum pepsinogens are linked to a higher risk for ESCC [58][51]. When there is gastric atrophy there is decreased production of gastric acid, and bacteria can grow in the stomach and produce carcinogenic compounds such as acetaldehyde and nitrosamines [31,59][24][52]. Achalasia is a disorder of esophageal dysmotility that is characterized by lack of peristalsis in the distal esophagus along with the absence of lower esophageal sphincter relaxation [31][24]. Due to the stagnation of food and subsequent fermentation, it leads to inflammation and is linked to a risk for esophageal malignancy [60][53]. A large study conducted in Sweden showed that achalasia increased the risk for both esophageal adenocarcinoma and squamous cell carcinoma [61][54].
8. Pre-Malignant Esophageal Disorders
Certain conditions of the esophagus are considered premalignant due to their predisposition to esophageal cancer. Tylosis, also called hyperkeratosis palmaris et plantaris, is an autosomal dominant disorder that is linked to a very high risk for developing esophageal cancer [62][55]. Genetic mutations involving RHBDF2 located on 17q25.1 are believed to occur [62][55]. Esophageal involvement in the form of small, white colored polypoid areas are seen in the esophagus, along with oral leukokeratosis and cutaneous manifestations [62][55]. The ingestion of caustic substances such as lye have been shown to cause not only the formation of strictures but also the development of esophageal cancer [63][56]. Plummer-Vinson syndrome is another pre-cancerous condition involving the esophagus that is characterized by the triad of iron deficiency anemia, dysphagia and esophageal web [64][57]. It is seen more frequently in women of middle age and has a significant association with the development of squamous cell carcinoma [64][57].
9. Genetic Factors
Changes at the genome level are known to play a part in the pathogenesis of esophageal cancer. Genes that regulate the cell cycle are mutated (such as CDKN2A, NFE2L2, RB1, CHEK1, CHEK2) or are over-expressed (such as CCND1, CDK4/CDK6, MDM2) in cases of ESCC [65][58]. Mutations in genes that are involved in cell differentiation (NOTCH1, NOTCH3) also occur in ESCC [65][58]. Over expression of epidermal growth factor receptor is seen in cases of ESCC and is linked to worse prognosis [66][59]. Mutations in receptor tyrosine kinase and RAS signaling pathways also occurs in ESCC [65][58]. Epigenetic changes such as histone modification, DNA methylation, and loss of genome imprinting are also implicated in the development of ESCC [67][60]. In EAC the expression of B-cell translocation gene 3 (encodes protein that modulates progression of cell cycle) [68][61] and the level of which has been linked to lymph node metastasis as well as tumor staging [68,69][61][62]. Increased expression of vascular endothelial growth factor (VEGF)-C has been seen in adenocarcinoma along with high levels of cyclin E (encoded by cyclin E1 gene), which plays a role in tumor progression [12,70][4][63].
References
- Domper Arnal, M.J.; Ferrández Arenas, Á.; Lanas Arbeloa, Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943.
- Pickens, A.; Orringer, M.B. Geographical distribution and racial disparity in esophageal cancer. Ann. Thorac. Surg. 2003, 76, S1367–S1369.
- Dong, J.; Thrift, A.P. Alcohol, smoking and risk of oesophago-gastric cancer. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 509–517.
- Huang, F.L.; Yu, S.J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215.
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265.
- Gonçalves, R.B.; Coletta, R.D.; Silvério, K.G.; Benevides, L.; Casati, M.Z.; Silva, J.S.; Nociti, F.H. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflamm. Res. 2011, 60, 409–424.
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Identification of possible cigarette smoke constituents responsible for muscle catabolism. J. Muscle Res. Cell Motil. 2012, 33, 199–208.
- Rom, O.; Avezov, K.; Aizenbud, D.; Reznick, A.Z. Cigarette smoking and inflammation revisited. Respir. Physiol. Neurobiol. 2013, 187, 5–10.
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Mutat. Res. 2021, 787, 108365.
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101.
- Abdel-Latif, M.M.; Duggan, S.; Reynolds, J.V.; Kelleher, D. Inflammation and esophageal carcinogenesis. Curr. Opin. Pharmacol. 2009, 9, 396–404.
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: A prospective cohort study. Gut 2009, 59, 39–48.
- Pandeya, N.; Williams, G.; Green, A.C.; Webb, P.M.; Whiteman, D.C.; Study, A.C. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009, 136, 1215–1224.
- Islami, F.; Fedirko, V.; Tramacere, I.; Bagnardi, V.; Jenab, M.; Scotti, L.; Rota, M.; Corrao, G.; Garavello, W.; Schüz, J.; et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: A systematic review and meta-analysis. Int. J. Cancer 2010, 129, 2473–2484.
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. Curr. Rev. 2017, 38, 163–171.
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685.
- Elamin, E.; Masclee, A.; Dekker, J.; Jonkers, D.M. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr. Rev. 2013, 71, 483–499.
- Kubo, A.; Levin, T.R.; Block, G.; Rumore, G.J.; Quesenberry, C.P.; Buffler, P.; Corley, D.A. Dietary Patterns and the Risk of Barrett’s Esophagus. Am. J. Epidemiol. 2008, 167, 839–846.
- Kubo, A.; Block, G.; Quesenberry, C.P.; Buffler, P.; Corley, D.A. Effects of Dietary Fiber, Fats, and Meat Intakes on the Risk of Barrett’s Esophagus. Nutr. Cancer 2009, 61, 607–616.
- Kubo, A.; Levin, T.R.; Block, G.; Rumore, G.J.; Quesenberry, J.C.P.; Buffler, P.; Corley, D.A. Dietary Antioxidants, Fruits, and Vegetables and the Risk of Barrett’s Esophagus. Am. J. Gastroenterol. 2008, 103, 1614–1623.
- Cross, A.J.; Freedman, N.D.; Ren, J.; Ward, M.H.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R.; Abnet, C. Meat Consumption and Risk of Esophageal and Gastric Cancer in a Large Prospective Study. Am. J. Gastroenterol. 2011, 106, 432–442.
- Yang, C.S.; Chen, X.; Tu, S. Etiology and Prevention of Esophageal Cancer. Gastrointest. Tumors 2016, 3, 3–16.
- Yang, C.S. Research on esophageal cancer in China: A review. Cancer Res. 1980, 40, 2633–2644.
- Kamangar, F.; Chow, W.-H.; Abnet, C.C.; Dawsey, S.M. Environmental Causes of Esophageal Cancer. Gastroenterol. Clin. N. Am. 2009, 38, 27–57, vii.
- Cheng, S.; Sala, M.; Li, M.; Courtois, I.; Chouroulinkov, I.; Alexandrov, V.A.; Anisimov, V.N.; Belous, N.M.; Vasilyeva, I.A.; Mazon, V.B. Promoting effect of Roussin’s red identified in pickled vegetables from Linxian China. Carcinogenesis 1981, 2, 313–319.
- Zhang, W.X.; Xu, M.S.; Wang, G.H.; Wang, M.Y. Quantitative analysis of Roussin red methyl ester in pickled vegetables. Cancer Res. 1983, 43, 339–341.
- Islami, F.; Boffetta, P.; Ren, J.-S.; Pedoeim, L.; Khatib, D.; Kamangar, F. High-temperature beverages and foods and esophageal cancer risk-A systematic review. Int. J. Cancer 2009, 125, 491–524.
- Wen, C.P.; Tsai, M.K.; Chung, W.S.I.; Hsu, H.L.; Chang, Y.C.; Chan, H.T.; Chiang, P.H.; Cheng, T.-Y.D.; Tsai, S.P. Cancer risks from betel quid chewing beyond oral cancer: A multiple-site carcinogen when acting with smoking. Cancer Causes Control 2010, 21, 1427–1435.
- Schulpen, M.; Peeters, P.H.; Brandt, P.A.V.D. Mediterranean diet adherence and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Gastric Cancer 2019, 22, 663–674.
- Verberne, L.; Bach-Faig, A.; Buckland, G.; Serra-Majem, L. Association Between the Mediterranean Diet and Cancer Risk: A Review of Observational Studies. Nutr. Cancer 2010, 62, 860–870.
- Kamangar, F.; Schantz, M.M.; Abnet, C.C.; Fagundes, R.B.; Dawsey, S.M. High Levels of Carcinogenic Polycyclic Aromatic Hydrocarbons in Mate Drinks. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1262–1268.
- Fitzgerald, R.C.; Omary, M.B.; Triadafilopoulos, G. Dynamic effects of acid on Barrett’s esophagus. An ex vivo proliferation and differentiation model. J. Clin. Investig. 1996, 98, 2120–2128.
- Oka, M.; Attwood, S.E.; Kaul, B.; Smyrk, T.C.; DeMeester, T.R. Immunosuppression in patients with Barrett’s esophagus. Surgery 1992, 112, 11–17.
- Corley, D.A.; Kubo, A.; Zhao, W. Abdominal Obesity and the Risk of Esophageal and Gastric Cardia Carcinomas. Cancer Epidemiol. Biomark. Prev. 2008, 17, 352–358.
- Corley, D.A.; Kubo, A.; Levin, T.R.; Block, G.; Habel, L.; Zhao, W.; Leighton, P.; Quesenberry, C.; Rumore, G.J.; Buffler, P.A. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology 2007, 133, 34–41.
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445.
- Harvey, A.E.; Lashinger, L.M.; Hursting, S.D. The growing challenge of obesity and cancer: An inflammatory issue. Ann. N. Y. Acad. Sci. 2011, 1229, 45–52.
- Conroy, M.J.; Dunne, M.R.; Donohoe, C.L.; Reynolds, J.V. Obesity-associated cancer: An immunological perspective. Proc. Nutr. Soc. 2015, 75, 125–138.
- Mazzarella, L. Why does obesity promote cancer? Epidemiology, biology, and open questions. Ecancermedicalscience 2015, 9, 554.
- El-Omar, E.M.; Rabkin, C.S.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Schoenberg, J.B.; Stanford, J.L.; Mayne, S.T.; Goedert, J.; Blot, W.J.; et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 2003, 124, 1193–1201.
- Islami, F.; Kamangar, F. Helicobacter pylori and esophageal cancer risk: A meta-analysis. Cancer Prev. Res. 2008, 1, 329–338.
- Zhuo, X.; Zhang, Y.; Wang, Y.; Zhuo, W.; Zhu, Y.; Zhang, X. Helicobacter pylori Infection and Oesophageal Cancer Risk: Association Studies via Evidence-based Meta-analyses. Clin. Oncol. 2008, 20, 757–762.
- Chow, W.H.; Blaser, M.J.; Blot, W.J.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Perez, G.P.; Schoenberg, J.B.; Stanford, J.L.; Rotterdam, H.; et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998, 58, 588–590.
- Wren, A.; Bloom, S. Gut Hormones and Appetite Control. Gastroenterology 2007, 132, 2116–2130.
- Whiteman, D.C.; Sadeghi, S.; Pandeya, N.; Smithers, B.M.; Gotley, D.C.; Bain, C.J.; Webb, P.M.; Green, A.C.; Australian Cancer Study. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2007, 57, 173–180.
- Blaser, M.J. Who are we? EMBO Rep. 2006, 7, 956–960.
- Yao, P.-F.; Li, G.-C.; Li, J.; Xia, H.-S.; Yang, X.-L.; Huang, H.-Y.; Fu, Y.-G.; Wang, R.-Q.; Wang, X.-Y.; Sha, J.-W. Evidence of human papilloma virus infection and its epidemiology in esophageal squamous cell carcinoma. World J. Gastroenterol. 2006, 12, 1352–1355.
- Xu, W.; Liu, Z.; Bao, Q.; Qian, Z. Viruses, Other Pathogenic Microorganisms and Esophageal Cancer. Gastrointest. Tumors 2015, 2, 2–13.
- Chow, W.H.; Finkle, W.D.; McLaughlin, J.K.; Frankl, H.; Ziel, H.K.; Fraumeni, J.F. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA 1995, 274, 474–477.
- Farrow, D.C.; Vaughan, T.L.; Sweeney, C.; Gammon, M.D.; Chow, W.-H.; Risch, H.A.; Stanford, J.L.; Hansten, P.D.; Mayne, S.T.; Schoenberg, J.B.; et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control 2000, 11, 231–238.
- Ye, W.; Held, M.; Lagergren, J.; Engstrand, L.; Blot, W.J.; McLaughlin, J.K.; Nyrén, O. Helicobacter pylori Infection and Gastric Atrophy: Risk of Adenocarcinoma and Squamous-Cell Carcinoma of the Esophagus and Adenocarcinoma of the Gastric Cardia. J. Natl. Cancer Inst. 2004, 96, 388–396.
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740.
- Leeuwenburgh, I.; Haringsma, J.; Van Dekken, H.; Scholten, P.; Siersema, P.D.; Kuipers, E.J. Long-term risk of oesophagitis, Barrett’s oesophagus and oesophageal cancer in achalasia patients. Scand. J. Gastroenterol. 2006, 41, 7–10.
- Zendehdel, K.; Nyrén, O.; Edberg, A.; Ye, W. Risk of Esophageal Adenocarcinoma in Achalasia Patients, a Retrospective Cohort Study in Sweden. Am. J. Gastroenterol. 2011, 106, 57–61.
- Ellis, A.; Risk, J.M.; Maruthappu, T.; Kelsell, D.P. Tylosis with oesophageal cancer: Diagnosis, management and molecular mechanisms. Orphanet J. Rare Dis. 2015, 10, 126.
- Moore, W.R. Caustic Ingestions. Pathophysiology, diagnosis, and treatment. Clin. Pediatr. 1986, 25, 192–196.
- Verma, S.; Mukherjee, S. Plummer Vinson Syndrome; StatPearls: Treasure Island, FL, USA, 2021.
- Song, Y.; Li, L.; Ou, Y.; Gao, Z.; Li, E.; Li, X.; Zhang, W.; Wang, J.; Xu, L.; Zhou, Y.; et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 2014, 509, 91–95.
- Zhang, W.; Zhu, H.; Liu, X.; Wang, Q.; Zhang, X.; He, J.; Sun, K.; Liu, X.; Zhou, Z.; Xu, N.; et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann. Thorac. Surg. 2014, 98, 513–519.
- Ahrens, T.; Werner, M.; Lassmann, S. Epigenetics in esophageal cancers. Cell Tissue Res. 2014, 356, 643–655.
- Winkler, G.S. The mammalian anti-proliferative BTG/Tob protein family. J. Cell. Physiol. 2010, 222, 66–72.
- Du, Y.; Liu, P.; Zang, W.; Wang, Y.; Chen, X.; Li, M.; Zhao, G. BTG3 upregulation induces cell apoptosis and suppresses invasion in esophageal adenocarcinoma. Mol. Cell. Biochem. 2015, 404, 31–38.
- Zhou, Z.; Bandla, S.; Ye, J.; Xia, Y.; Que, J.; Luketich, J.D.; Pennathur, A.; Peters, J.H.; Tan, D.; Godfrey, T.E. Cyclin E involved in early stage carcinogenesis of esophageal adenocarcinoma by SNP DNA microarray and immunohistochemical studies. BMC Gastroenterol. 2014, 14, 78.
More
