The COVID-19 pandemic has triggered a significant range of dermatologic sequela. Etiologies of lesions continue to be investigated. Proposed mechanisms include inflammatory response to spike protein, vitamin D deficiency, ACE2 receptor activation, androgen levels, and increased psychosocial stress. This encyclopedia article reflects a novel literature review (Pendlebury et al.) of dermatological manifestations associated with the Coronavirus Disease 2019 (COVID-19) pandemic. This literature review is the first published broad-spectrum examination that analyzes a range of dermatological manifestations related to the COVID-19 pandemic: infection, vaccinations, personal protective equipment (PPE), and psychosocial factors.
- COVID-19
- COVID-19 pandemic
- SARS-CoV-2 infection
- cutaneous manifestations
- COVID arm
- pandemic psychosocial stress
- personal protective equipment
- COVID vaccinations
- psychodermatology
- teledermatology
1. Introduction
2. COVID-19 Specific Dermatological Manifestations
2.1. Exanthematous (Morbilliform) Rash
2.2. Pernio (Chilblain)-like Acral Lesions
2.3. Urticaria
2.4. Livedo Reticularis
2.5. Livedo Racemosa/Retiform Purpura
2.6. Vesicular (Varicella-like) Eruptions
2.7. Papulosquamous Rashes and Pityriasis Rosea
2.8. Multisystem Inflammatory Syndrome in Children
-
Fever
-
Inflammatory markers
-
Failure or involvement of two organ systems
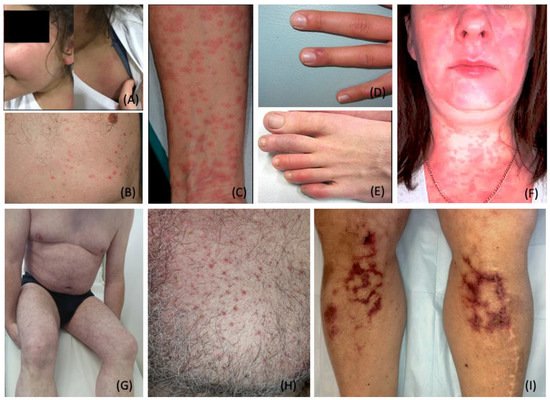
3. Dermatological Conditions Associated with Personal Protective Equipment and Hygiene Products
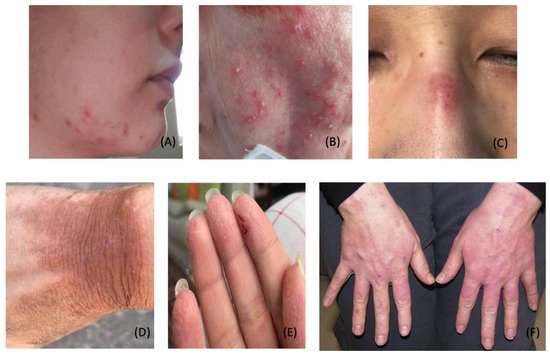
4. COVID-19 Vaccine-Induced Dermatological Manifestations
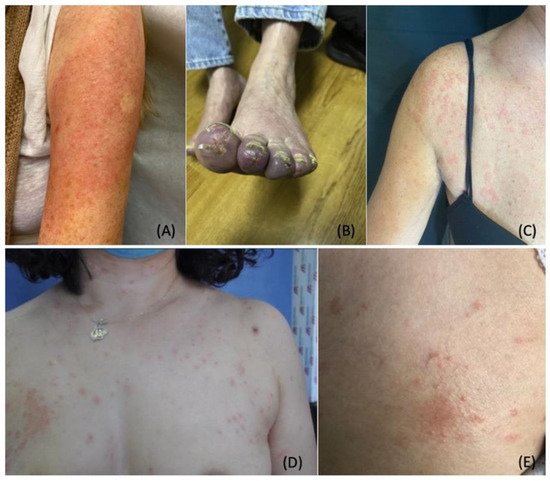
5. Stress-Induced Dermatological Conditions
5.1. Telogen Effluvium
Telogen effluvium (TE) is a common, self-limiting hair loss condition usually seen in women with a history of a recent stressor. Stressors associated with TE include systemic disease, infections, stressful events, drugs, nutritional deficiencies, postpartum hormonal changes, and major surgeries [44]. TE usually presents three months following the onset of the stressor. It is classified as acute (hair loss lasting up to six months) or chronic (hair loss exceeding six months) [170][193]. Coined as “COVID scalp,” increased cases of TE have been associated with the COVID-19 pandemic [171][194]. The cause of TE during the COVID-19 pandemic is multifactorial, and it is thought to be stress-induced or a direct manifestation of the inflammatory process during the infection phase.6. Pathomechanisms: Cutaneous Manifestations in SARS-CoV-2
International reports have identified a range of potential cutaneous manifestations related to COVID-19. The frequency and timing of these lesions to COVID-19 infection are difficult to ascertain. Multiple established associations in the scientific literature offer valuable insights into plausible mechanisms behind cutaneous manifestations of SARS-CoV-2. One proposed pathomechanism explains the presence of ACE2 in keratinocytes [19]. Another promising association describes the significance of androgen levels in the increased expression of the TMPRSS2 gene [13][14][13,14].
A strong association between increased androgens and increased TMPRSS2 gene expression has been identified in the literature [13][14][13,14]. ACE2 receptors and TMPRSS2 are expressed in multiple tissues throughout the body including the skin. In conjunction, the presence of ACE2 receptors in keratinocytes may play a significant role in the manifestation of dermatological conditions related to COVID-19. Furthermore, the association between androgen levels and TMPRSS2 can further contribute to the development of skin lesions [13][14][19][13,14,19]. As such, high levels of androgens combined with ACE2 distribution in the skin can provide invaluable clinical insight into the pathomechanism of skin manifestations.
Androgens have been shown to uniquely activate the TMPRSS2 gene. TMPRSS2 plays an essential role in the activation of spike protein and facilitates viral entry through ACE2 receptors. This correlation helps explain the greater susceptibility to COVID-19 in males, as males typically maintain higher levels of androgens. Moreover, it has been shown that males with prostate cancer receiving androgen deprivation therapy (ADT) have a lower risk of COVID-19 compared to prostate cancer patients who did not receive ADT [14]. As such, ADT may play a role in reducing the severity of symptoms in patients with COVID-19 [13][14][13,14]. However, more research is needed to confirm these findings. Additionally, it is well established that children have a lower rate of COVID-19 infection compared to adult males and females. The less symptomatic disease in children may be explained by overall low expressions of androgen receptors and androgen levels [7][13][14][15][16][17][7,13,14,15,16,17].7. The Role of Vitamin D in SARS-CoV-2
Additional susceptibility to COVID-19 has been strongly correlated with low vitamin D levels. Studies continue to identify and analyze the increased severity of COVID-19 infection with concomitant low vitamin D levels [172][173][174][224,225,226]. Vitamin D can be administered in various forms including (but not limited to) cholecalciferol, calcifediol, and calcitriol. Vitamin D supplementation has been proposed to reduce the risk of COVID-19 infection through multiple protective mechanisms. Such mechanisms include modulation of the host immune system, upregulation of ACE2 concentration, vitamin D receptor activation [174][226], reduction in endothelial damage, and reduction in proinflammatory cytokines [172][173][174][224,225,226]. Research studies have demonstrated that vitamin D receptors (VDRs) are expressed in high concentration levels in cuboidal alveolar type II cells (ACII) within the pulmonary system. Calcitriol, also known as 1,25 dihydroxyvitamin D (1,25(OH)2 D3), binds to VDRs in ACII cells. Calcitriol binding activates multiple intracellular signals which inhibit inflammatory cytokines and chemokines involved in Acute Respiratory Distress Syndrome (ARDS) [174][226]. Additional investigations have revealed that vitamin D signaling pathways prevent pulmonary vessel constriction, a manifestation associated with increased COVID-19 mortality [172][173][174][224,225,226]. VDR activation promotes vasodilatory effects through two mechanisms: inhibitions of angiotensin II (a potent vasoconstrictor) and upregulation of ACE2. Decreased expression of angiotensin II promotes pulmonary vasodilation [172][173][174][224,225,226]. Additionally, the induction of ACE2 expression in pulmonary tissues further dampens the effect of angiotensin II, thereby reducing respiratory distress symptoms. Prevention of pulmonary vasoconstriction greatly improves respiratory symptoms associated with SAR-CoV-2 infection. Therefore, ACE2 acts as an anti-inflammatory factor in the etiology of ARDS [172][173][174][224,225,226]. VDR activation has also been shown to inhibit Skp2 protein, which is utilized by SARS-CoV-2 to replicate inside cells. Thus, the binding of calcitriol and VDR activation reduce viral replication in pulmonary tissues and reduce the disease severity of COVID-19 [174][226]. Calcifediol, a vitamin D3 analog, rapidly increases serum levels of vitamin D 25-hydroxyvitamin D (25-OH-D), thereby promoting the protective properties associated with vitamin D [173][225]. A parallel randomized open label, double-masked clinical trial evaluated the effect of calcifediol on the severity of COVID-19 disease. The randomized clinical trial was conducted on 76 consecutive patients hospitalized with COVID-19 infection. All 76 patients clinically presented with acute respiratory infections, confirmed by radiographic patterns of viral pneumonia. Likewise, all 76 patients tested positive for SARS-CoV-2 through PCR tests. Lastly, all 76 patients were confirmed for appropriate hospital admission via the CURB65 Severity Scale [173][225]. All hospitalized patients received the best available therapy at the same standard of care. Of the 50 patients treated with calcifediol, one patient required intensive care unit (ICU) admission, and of the 26 untreated patients, 13 patients required ICU admission, with two deaths in the ICU. The remaining 11 untreated patients who did not receive calcifediol were discharged. Of all 50 patients treated with calcifediol, none died, and all patients were discharged with no complications [173][225]. A larger-scale observational cohort study with 930 patients also revealed significantly reduced ICU admissions and mortality rates associated with early vitamin D administration [175][227]. These clinical trials underscore the clinical value of vitamin D in a significant reduction in disease severity and disease mortality [173][175][225,227].8. The Role of Vitamin D in Cutaneous Manifestations Associated with COVID-19
With regards to infection-related cutaneous manifestations, most conditions have been attributed to the host’s inflammatory response to SARS-CoV-2. Likewise, the immune-boosting, anti-inflammatory properties of vitamin D alleviate and reduce the spread of cutaneous lesions [172][173][174][175][224,225,226,227]. In addition, vitamin D bioavailability and efficacy have been shown to increase with magnesium supplementation. Magnesium has been shown to facilitate vitamin D-related processes by activating vitamin D processing enzymes [176][228]. Vitamin D and magnesium supplementation are cost-effective measures that help prevent infection, reduce disease severity, and improve prognosis [176][228]. Likewise, patient education and adherence are essential factors for favorable clinical outcomes. Therefore, further investigations are warranted to identify the ideal maintenance dosage of vitamin D and magnesium to prevent infection. Additional research is recommended to elucidate an effective dosing regimen to reduce infection-related cutaneous lesions. Throughout the pandemic, clinical management for most COVID-19-associated and non-COVID-19 cutaneous manifestations have been similar in nature. For example, chilblain-like lesions in COVID-19 patients do not require treatment, but topical corticosteroids can relieve discomfort [62][64][177][62,64,229]. Other rashes, such as varicelliform-like/vesicular lesions, are self-limiting and therefore do not require treatment [48][102][48,102]. In contrast, maculopapular and urticarial eruptions can occur concomitantly or separately in moderate to severe cases of COVID-19. These lesions present with pruritus and pain which necessitates prompt treatment with therapeutic agents such as topical corticosteroids, oral antihistamines, oral corticosteroids, and vitamin C [48][178][179][180][181][48,230,231,232,233]. In addition to rash therapies, early treatment interventions have been shown to improve the overall prognosis for infected patients and reduce patient mortality [182][183][234,235].9. Global Implications of the COVID-19 Pandemic
The COVID-19 pandemic has caused an insurmountable disturbance on a global scale. The strict quarantine measures have caused significant psychosocial distress. Resultantly, many individuals have developed new or worsening pre-existing dermatological conditions, such as telogen effluvium, psoriasis, eczema, urticaria, and atopic dermatitis. Exacerbated lesions require supportive management, targeted treatment for underlying issues, and relevant psychosocial support. Additionally, interdisciplinary treatment involving mental health professionals should be implemented to help relieve stress, anxiety, and depression.
As the pandemic continues, strict measures and mandates have been implemented to promote mass vaccination. With increased immunizations, additional cases of anaphylaxis and other allergic reactions such as COVID toes, COVID arm, and urticaria have been documented in response to immunizations [37][38][154][155][156][159][37,38,175,176,177,180]. It has been proposed that lipid nanoparticles (LNPs) containing messenger RNA vaccines trigger allergic and anaphylactic reactions [184][240]. LNPs are composed of positively charged lipids at low pH to stabilize the messenger RNA [185][241]. Likewise, LNPs contain high amounts of polyethylene glycol (PEG), a highly hydrophilic molecule [186][187][239,242]. PEG helps to increase the hydrophilicity of LNPs and stabilize the mRNA. However, PEG within LNPs has been shown to trigger inflammatory responses through complement-mediated and direct mast cell activation [240]. Furthermore, when inoculated into the bloodstream, LNPs trigger nonclassical allergic reactions in certain patients. Such reactions involve preformed antibodies to PEG and other components of LNPs [184][240]. Moreover, LNPs destabilize during the freeze and thaw cycle of immunization preparation [184][240]. When injected, destabilized LNPs release the naked mRNA into the bloodstream. Naked mRNA is proinflammatory and has been shown to induce allergic and anaphylactic reactions [186][187][239,242]. Additionally, classical allergic reactions to PEG may be IgE-mediated [184][240]. As such, skin prick testing with PEG should be performed before receiving the vaccine to avoid anaphylactic reactions [147][168]. At this time, allergy testing has not been implemented to screen susceptible individuals and should be considered to maximize safety and minimize adverse events. Moreover, novel measures on vaccine risk reduction can improve outcomes and maximize safety.
10. Conclusions
The COVID-19 pandemic has posed considerable challenges across the entirety of medicine. As evidenced, the COVID-19 pandemic has triggered a significant range of dermatologic sequela. Various factors surrounding the pandemic have resulted in a multitude of other dermatological manifestations. Dermatologists play an integral role in the proper diagnosis and treatment of COVID-related lesions. Several years into the pandemic, there is still much to learn and understand. As more information is collected and assessed, theour comprehension of the pathogenesis and treatment of dermatologic manifestations will continue to evolve and guide the dermatological standard of care.
Early treatment regimens and timely prophylaxis have been shown to improve prognosis and reduce further infection-related sequelae. Moreover, novel measures on vaccine risk reduction can improve outcomes and maximize safety. Given the evolutionary nature of the pandemic, intentional observation and research are prudent in the diagnosis, management, and treatment of these cutaneous manifestations. Robust investigations are necessary to identify underlying dermatological pathomechanisms and improve lesion diagnosis. Data collection will help reveal pertinent risk factors and boost public health outcomes. Such studies will reduce disease burden and optimize quality of life as society continues to adapt and adjust to life after SARS-CoV-2.
