Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Ting Yu and Version 6 by Sirius Huang.
Immune checkpoint inhibitors (ICIs), antibodies that target the checkpoints in immune cells, work to activate inhibited T-cells and other cells of the innate and adaptive arms, resulting in the robust activation of the immune system and productive antitumor immune responses. However, ICIs-related cardiotoxicity has been recognized as a rare but fatal consequence. Although there has been extensive research based on different types of ICIs, these studies have not indicated whether cardiotoxicity is specific to a type of cancer.
- immune checkpoint inhibitors
- cardiotoxicity
- cardio-oncology
- cancer-type-specific
1. Introduction
Cardiovascular disease (CVD) and cancer are global health issues with high morbidity and mortality [1], and numerous studies suggest that there is an overlap in epidemiology, risk factors, and pathophysiologic processes (Figure 1) .
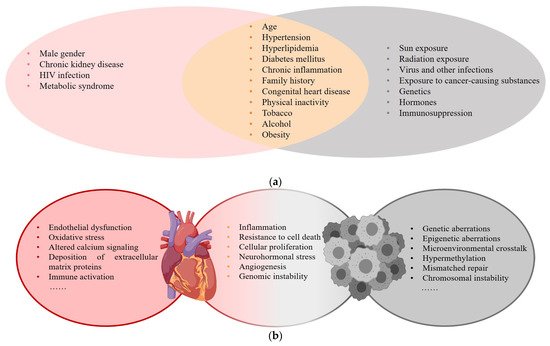
Figure 1. (a) Risk factors for CVD and cancer; (b) Common pathophysiologic processes of CVD and cancer.
With the widespread application of anticancer drugs, the survival of patients has significantly improved, but the related cardiotoxicity affects long-term therapeutic outcomes, and this has attracted considerable attention. Immune checkpoint inhibitors (ICIs), antibodies that target the checkpoints in immune cells, work to activate inhibited T-cells and other cells of the innate and adaptive arms, resulting in the robust activation of the immune system and productive antitumor immune responses. This new type of immunotherapy drug has significantly improved the survival of cancer patients [2][3][4]. However, their use is associated with adverse side effects involving different organs [5][6]. ICIs-related cardiotoxicity, which may develop even without a history of significant cardiac risk factors, includes myocarditis, pericarditis, heart failure, arrhythmias, and vasculitis [7]. In reported cases of adverse ICIs-related events, 6.2% were cardiac adverse events (CAEs), which can be the main determinants of quality of life and increased mortality [8][9][10].
2. Cardiotoxicity in Melanoma
In 16 studies, 24 of 6710 patients on ICIs [11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26] developed CAEs. This corresponded with an incidence of 0.20–4.93% in which grade 3–5 CAEs accounted for 41.7%. Commonly encountered cardiotoxicities included hypertension (50%), hypotension (16.7%), and myocarditis (8.3%). Treatment-related hypertension was linked to the application of lambrolizumab (58.3%) (PD-1). Nivolumab may have had a correlation with ICIs-related hypotension. Patients treated with a higher dose of ipilimumab, particularly 10 mg/kg × 4 doses/3 weeks, were more prone to fatal adverse events such as cardiac arrest (Table 1).
Table 1.
Cardiotoxicity in melanoma.
| Author, Year | Study Type | Author, Year | Study TypePhase | Sample Size | Drug | Phase | Sample SizeDose and Frequency | Non-CAE | Drug | Dose and Frequency | Non-CAECAE | Manifestation | 3–5 Grade CAE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAE | Manifestation | 3–5 Grade CAE | |||||||||||||||||
| Omid Hamid et al., 2017 [11] | Prospective study | II | 528 (178 vs. 179 vs. 171) | ||||||||||||||||
| Kalyan 卡利安R et al., 等人,2019 [27] | Retrospective study回顾性研究 | NR星期日 | 252 | Pembrolizumab vs. Pembrolizumab vs. chemotherapy | ((117 vs. 135)与. 135) | Non-ICI vs. ICI ((Nivolumab/Pembrolizumab)) Nivolumab ((Niv)2 mg/kg/3 weeks vs. 10 mg/kg/3 weeks vs. standard dose |
) Pembrolizumab ((Pem)) |
Standard dose vs. increasing dose (标准剂量与增加剂量(Niv < 540 mg; 540~1440 mg; > 1440 mg Pem < 600 mg; 600~1707 mg;528 | >1707 mg)0 | ) | NR0 | 星期日 | 93 0 | ||||||
| ( | ( | 42 | vs. 51) | 与. 51) | Arrhythmia | 心律失常 | 31 vs. 25; | Cardiac-related chest pain 心脏相关胸痛 12 vs. 25; Valvular heart disease 瓣膜性心脏病4 vs. 2; Cardiomyopathy 心肌病13 vs. 20; Myopericardial disease 11; Pericardial disease 8; Myocarditis 1; Valvular-disease 2; Venous arterial thromboembolic events 心包疾病11;心包疾病8;心肌炎1;瓣膜病2;静脉动脉血栓栓塞事件 8 | 40(主要 (major CAE)CAE) | Caroline Robert et al., 2014 [12] | |||||||||
| Scott 斯科特N et al., 等人,2015 | Prospective study | III | 418 (210 vs. 208) | [28] | Prospective study (NSCLC)前瞻性研究 | I我 | 129 ((Nivolumab vs. Dacarbazine |
3 mg/kg/2 weeks vs. standard dose | 308 (153 vs. 155) | 5 | Hypotension 1 vs. 4 | 33 vs. 37 vs. 59)对 37 对. 59)0 | |||||||
| Nivolumab | 尼沃鲁单抗 | 1 mg/kg vs. 3 mg/kg vs. 10 mg/kg | intravenously | ,静脉使用 | /2 weeks in 8-week cycles for up to 96 weeks.周,8 周周期,最长 96 周。 | 91 ((21 vs. 25 vs. 45)与. 25 与. 45) | 0 | 0 | 0 | Jeffrey S Weber et al., 2015 [13] | Prospective study | ||||||||
| Tony S K Mok et al.,, 2019 [29] | III | 370 (268 vs. 102) | Nivolumab vs. ICC (Dacarbazine al) | 3 mg/kg/2 weeks vs. standard dose | Prospective study (NSCLC) | 362 (181 vs. 81) | 前瞻性研究0 | 0 | 0 | ||||||||||
| III | 第三 | 1251 | ((636 vs. 615)与. 615) | Pembrolizumab vs. platinum-based chemotherapy彭布利珠单抗与铂类化疗 | 200 mg毫克/3 weeks for up to 35 cycles vs. platinum-based chemotherapy for four to six cycles.周,最多35个周期,而铂类化疗4至6个周期。 | 1112 ((515 vs. 597)与. 597) | 1 ((1 vs. 0)与 0) | Myocarditis心肌炎 1 vs. 0 | 1 | Paolo A Ascierto et al., 2017 [14] | Prospective study | III | 726 (364 vs. 362) | Ipilimumab | |||||
| Achim Rittmeyer et al.,, 2017 [30] | Prospective study (NSCLC)前瞻性研究 | III第三 | 1187 ((609 vs. 578)与. 578) | Atezolizumab | 10 mg/kg/4 doses/3 weeks vs. 3 mg/kg/4 doses/3 weeks | 阿替利珠单抗 vs. Docetaxel多西他赛 | 1200 mg毫克514 (286 vs. 228) | /33 | Hypertension 1 vs. 0; Heart arrest 1 vs. 0; Pericarditis 1 vs. 0 | weeks vs. 75 mg/m周对比75毫克/米23 | |||||||||
| /3 | weeks | 周 | 886 | ( | ( | 390 vs. 496)与. 496) | 0 | 0 | 0 | F Stephen Hodi et al., 2016 [15] | |||||||||
| S.J. Antonia et al., | Prospective study | , 2017 [31] | Prospective study (NSCLC)II | 142 (95 vs. 47) | Nivolumab + Ipilimumab vs. Ipilimumab + placebo | 1 mg/kg + 3 mg/kg/4 doses/3 weeks vs. 3 mg/kg + placebo/4 doses/3 weeks | 140 (94 vs. 46) | 7 | 前瞻性研究Hypotension 3 vs. 0; Ventricular arrhythmia 1 vs. 0; Ventricular tachycardia 1 vs. 0; Atrial fibrillation 1 vs. 0; Myocardial infarction 1 vs. 0 | 5 | |||||||||
| III | 第三 | 718 | ((475 vs. 234)与. 234) | Durvalumab杜瓦鲁单抗 vs. Placebo安慰剂 |
10 mg毫克/kg/2 weeks for up公斤/2 周,最长 to 12 months vs.个月,与 placebo安慰剂相比 |
421 ((301 vs. 120)与. 120) | 26 ((21 vs. 5)与. 5) | ACS 9 vs.对 2; Arrhythmia 7 vs. 1; Heart failure 心律失常 7 对 1;心力衰竭 7 vs. 0; Cardiac arrest 心脏骤停 2 vs. 1; Cardiogenic shock 1 vs. 0; Cardiomyopathy 心源性休克1对0;心肌病1 vs. 0; Myocarditis心肌炎 0 vs. 1; Pericardial effusion 心包积液 2 vs. 0 | NR星期日 | Caroline Robert et al., 2015 [16] | |||||||||
| Yuequan Shi et al.,石岳全等, 2021 | Prospective study | III | 834 (278 vs. 277 vs. 256) | Pembrolizumab vs. Pembrolizumab vs. Ipilimumab | 10 mg/kg/2 weeks/doses vs. 10 mg/kg/3 weeks/ doses vs. 3 mg/kg/3 weeks/4 doses | 610 (221 vs. 202 vs. 187) | [32]4 | Observational study (NSCLC/SCLC)观察性研究 | NR星期日 | 1905 ((1162 vs. 743)对 743) ((598 vs. 455 vs. 273 vs. 176 vs. 125 vs. 81 vs. 62 vs. 34 vs. 23)对 455 对 273 对 176 对 125 对 81 对 62 对 34 对 23) |
仅 ICI ((Hypertension 3 vs. 1 vs. 0 |
2 | |||||||
| Pembrolizumab/Nivolumab/Camrelizumab/Treprizumab/Tisilizumab/Atezolizumab/Durvalumab/Ipilimumab | ) only vs. combination therapy | )仅与联合治疗 | at least one dose | 至少一剂 | 647 | 22 | ((22 vs. 0)与. 0) | Elevated cTnI or myocarditis 升高或心肌炎 22 | 9 | J. Weber, M. et al., 2017 [17] | Prospective study | III | 906 (453 vs. 453) | Nivolumab vs. Ipilimumab | 3 mg/kg/4 doses/2 weeks vs. 10 mg/kg/4 doses/3 weeks | 884 (438 vs. 446) | 0 | ||
| Roy S Herbst et al. | 0 | ,, 2016 [33] | Prospective study (NSCLC)前瞻性研究 | II二/III三 | 991 ((339 vs与. 343 vs. 309)与. 309) | Pembrolizumab彭布罗利珠单抗 vs. Docetaxel多西他赛 | Pem 2 mg毫克/kg, 千克,Pem 10 mg/kg vs. Docetaxel 75 mg/m毫克/千克与多西紫杉醇75毫克/米2/3 weeks周 | 690 ((215 vs与. 225 vs. 250)与. 250) | 1 ((0 vs. 1 vs. 1)对 1 对 1)0 | ||||||||||
| Myocardial | 心肌梗死 | infarction 0 vs. 1 vs. 0; Acute cardiac failure | 0 对 1 对 0;急性心力衰竭 | 0 vs. 0 vs. 1 | 1 | J.D. Wolchok et al., 2017 [18] | Prospective study | III | 937 (313 vs. 313 vs. 311) | ||||||||||
| Martin Reck et al.,, 2016 [34] | Prospective study (NSCLC)前瞻性研究 | III第三 | 304 ((154 vs. 150)与. 150) | Nivolumab + Ipilimumab vs. Nivolumab + p vs. Ipilimumab + p p(placebo) |
Pembrolizumab vs. platinum-based彭布利珠单抗与铂1 mg/kg+3 mg/kg /3 weeks/4 doses vs. 3 mg/kg/2 weeks + placebo vs. 3 mg/kg/3 weeks/4 doses + placebo |
847 (300 vs. 279 vs. 268) | 0 |
0 | chemotherapy类化疗 | 200 mg毫克/3 weeks vs. standard dose0 | |||||||||
| 周与标准剂量相比 | 52 | ( | ( | 45 | vs. 7) | 与. 7) | 0 | 0 | 0 | Jedd D Wolchok et al., 2010 [19] | Prospective study | II | 217 (73 vs. 72 vs. 72) | Ipilimumab | |||||
| H. Borghaei et al. | 10 mg/kg vs. 3 mg/kg vs. 0.3 mg/kg/3 weeks/4 doses | ,, 2015 | 115 (50 vs. 46 vs. 19) | [35] | Prospective study (NSCLC)前瞻性研究 | III第三 | 555 ((278 vs. 268)与. 268) | Nivolumab尼沃鲁单抗 vs. Docetaxel多西他赛 | 3 mg毫克/kg/2 weeks vs. 75 mg/m千克/2 周对比 75 毫克/米2/3 weeks周 | 432 ((0 | 196 0 | 0 | |||||||
| vs. 236) | 与. 236) | 3 | ( | ( | 3 | vs. 0) | 与. 0) | Cardiac心脏压塞 tamponade 1 vs. 0; Pericardial effusion 心包积液 1 vs. 0 Tachycardia心动过速 1 vs. 0 |
3 | Ines Pires da Silva et al., 2021 [20] | Retrospective study | NR (Not Reported) | 355 (193 vs. 162) | Ipilimumab + Nivolumab/Pembrolizumab/Atezolizumab vs. Ipilimumab | 3 mg/kg/3 weeks/4 doses + standard dose vs. 3 mg/kg/3 weeks/4 doses | 287 (163 vs. 124) | 1 (0 vs. 1) | Myocarditis 0 vs. 1 | 1 |
| Julie Brahmer et al.,, 2015 [36] | Prospective study (NSCLC)前瞻性研究 | III第三 | 272 (135:137) | Nivolumab尼沃鲁单抗 vs. Docetaxel | 2/3 weeks.周。 | 187 ((76 vs. 111)与. 111) | 0 | 0 | 0 | Patrick Schöffski et al., 2022 [21] | Retrospective study | I/II | 255 (134 vs. 121) | LAG-3 inhibitor Ieramilimab vs. Ieramilimab + Spartalizumab |
Ieramilimab (escalating 1–15 mg/kg)/2 weeks or once/4 weeks vs. Ieramilimab + Spartalizumab q2w or q3w or q4w or Ieramilimab q2w + Spartalizumab q4w | 159 (75 vs. 84) | 0 | ||
| D.P. Carbone et al.,, 2017 [37] | Prospective study (NSCLC)前瞻性研究 | III第三 | 530 ((267 vs. 263)与. 263) | Nivolumab vs. Chemotherapy(platinum-based)尼沃鲁单抗与化疗(铂类) | 3毫克/ mg/kg/2 weeks vs. standard dose for six cycles.kg / 2周与标准剂量相比,六个周期。 | 431 | 0 | 0 | |||||||||||
| Alexander M.M. et al., 2020 [22] | Prospective study | III | 1011 (509 vs. 502) | Pembrolizumab vs. placebo | 200 mg/3 weeks for 18 doses | 235 (190 vs. 45) | 1 (1 vs. 0) | Myocarditis 1 vs. 0 | NR | ||||||||||
| Omid Hamid et al., 2013 [23] | Prospective study | I | 135 (57 vs. 56 vs. 22) | Lambrolizumab | 10 mg/kg/2 weeks vs. 10 mg/kg/3 weeks vs. 2 mg/kg/3 weeks | 132 (55 vs. 55 vs. 22) | 7 (2 vs. 4 vs. 1) | Hypertension (2 vs. 4 vs. 1) | NR | ||||||||||
| 多西他赛 | 3 | mg | 毫克 | / | kg/2 weeks vs. 75 mg/m千克/2 周对比 75 毫克/米 | Margaret K. et al., 2018 [24] | Retrospective study | I | 94 (53 vs. 41) | Ipilimumab + Nivolumab Nivolumab (Niv) Ipilimumab (Ipi) |
Niv+Ipi(escalating doses)/3 weeks for four doses, followed by Niv 3 weeks for four doses, then Niv + Ipi/12 weeks for eight doses vs. Niv 1 mg/kg + Ipi 3 mg/kg/3 weeks for 4 doses, followed by Niv 3 mg/kg/2 weeks |
87 | 0 | 0 | 0 | ||||
| Ulrich Keilholz et al., 2019 [25] | Prospective study | I | 51 | Avelumab | 10 mg/kg for one-hour intravenous infusion/2 weeks | 39 | 0 | 0 | 0 | ||||||||||
| Hussein A et al., 2022 [26] | Retrospective study | II-III | 714 (355 vs. 359) | Relatlimab + Nivolumab vs. Nivolumab | Relatlimab 160 mg + Nivolumab 480 mg vs. Nivolumab 480 mg | 504 (288 vs. 216) | 0 | 0 | 0 |
The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Grade 3: severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living. Grade 4: life-threatening consequences; urgent intervention indicated. Grade 5: Death related to adverse events.
3. Cardiotoxicity in Lung Cancer
A total of 11 studies [27][28][29][30][31][32][33][34][35][36][37] included 5404 patients on ICIs, and 101 developed CAEs for an incidence of 0.15–37.78% in which grade 3–5 CAEs accounted for 55.4%. Commonly encountered cardiotoxicities included arrhythmia (32.7%), cardiac-related chest pain (24.8%), elevated cTnI or myocarditis (23.8%), cardiomyopathy (20.8%), pericardial disease (11.9%), and acute coronary syndrome (10.9%). One study indicated that major adverse cardiovascular events (MACEs) were dose-independent of nivolumab and pembrolizumab in lung cancer patients [27]. Those treated with a higher dose of durvalumab, particularly 10 mg/kg × 4 doses/2 weeks, were more prone to fatal adverse events such as a cardiac arrest and cardiogenic shock [31]. One patient treated with pembrolizumab at 10 mg/kg for 3 weeks underwent a myocardial infarction, which led to death (Table 2) [33].
Table 2. Cardiotoxicity in lung cancer.
| ( | |||||||||
| ( | |||||||||
| 188 | |||||||||
| vs. 243) | |||||||||
| 与. 243) | |||||||||
| 2 | |||||||||
| ( | |||||||||
| ( | |||||||||
| 2 | |||||||||
| vs. 0) | 与. 0) | Myocardial | 心肌梗死 | infarction | 1 vs. 0; | Pericardial effusion malignant | 心包积液恶性 1 vs. 0 | 2 |
4. Renal Cell Carcinoma肾细胞癌的心脏毒性
In seven studies 在七项研究中[38][39][40][41][42][43][44] comprising 包括1971 patients with renal cell carcinomas on ICIs, 14 developed CAEs with an incidence of 0.20–2.19% in which grade 3–5 CAEs accounted for 35.7%. Commonly encountered cardiotoxicities included hypertension (85.7%) and myocarditis (7.1%). Treatment-related hypertension was linked to a nivolumab plus ipilimumab therapy (100%). Compared with melanomas and lung cancer, the ICI therapy caused mild cardiotoxicity in renal cell carcinomas. Fatal CAEs were not found.名ICIs肾细胞癌患者,14名开发CAE,发病率为0.20-2.19%,其中3-5级CAE占35.7%。常见的心脏毒性包括高血压(85.7%)和心肌炎(7.1%)。治疗相关的高血压与纳武鲁单抗加伊匹利单抗治疗(100%)有关。与黑色素瘤和肺癌相比,ICI治疗在肾细胞癌中引起轻度心脏毒性。未发现致命的 CAE。
5. Urothelial Carcinoma尿路上皮癌的心脏毒性
In Seven studies 在七项研究中[45][46][47][48][49][50][51] 111 of 在接受ICI治疗的2550 patients with urothelial carcinomas on ICIs developed CAEs with an incidence of 0.22–名尿路上皮癌患者中,有111例发展为CAE,发病率为0.22-10.60% in which grade 3–5 CAEs accounted for 52.3%. Commonly encountered cardiotoxicities included hypertension (28.8%), arrhythmia (14.4%) and hypotension (6.3%). The fluctuation of blood pressure was linked to treatment with atezolizumab. Hypertension was observed in 21 patients and hypotension was observed in 7 after application of ate,其中3-5级CAE占52.3%。常见的心脏毒性包括高血压(28.8%),心律失常(14.4%)和低血压(6.3%)。血压的波动与阿替珠单抗治疗有关。21例患者出现高血压,7例患者应用atezolizumab. Patients treated with 200 后出现低血压。用200mg pembrolizumab for 3 weeks (maximum 35 cycles) or at 1200 mg every three weeks were more prone to fatal adverse events such as a cardiac arrest.治疗3周(最多35个周期)或每3周以1200mg治疗的患者更容易发生致命的不良事件,如心脏骤停。
6. Other Types of Cancer其他类型癌症的心脏毒性
The most commonly encountered 在血液系统恶性肿瘤中,最常遇到的与ICIs-related type of cardiotoxicity in hematological malignancies was hypertension 相关的心脏毒性类型是高血压[52][53][54][55]. In other cancers, such as hepatocellular carcinomas and malignant pleural mesotheliomas, the relevant research did not present many cases 在其他癌症中,如肝细胞癌和恶性胸膜间皮瘤,相关研究并未提出很多病例。[56][57][58][59][60][61]; these were almost all case reports of myocarditis 这些几乎都是心肌炎的病例报告[62][63][64].
