Biopolymers are materials obtained from renewable resources. Despite the exciting properties of biopolymers, such as biocompatibility and environmental sustainability, they do not present antimicrobial properties (except chitosan). However, this lack of antimicrobial properties can be solved by incorporating or encapsulating antimicrobial agents. Natural polymers possess low stability in aqueous media and limited mechanical strength, which could be improved through cross-linking strategies. Hydrogels are biocompatible materials that can be synthesized from natural polymers, forming a cross-linking material. Alginate, collagen, fibrin, chitosan, gelatin, and hyaluronic acid are some natural polymers used to synthesize hydrogels.
- natural polymers
- biocomposites
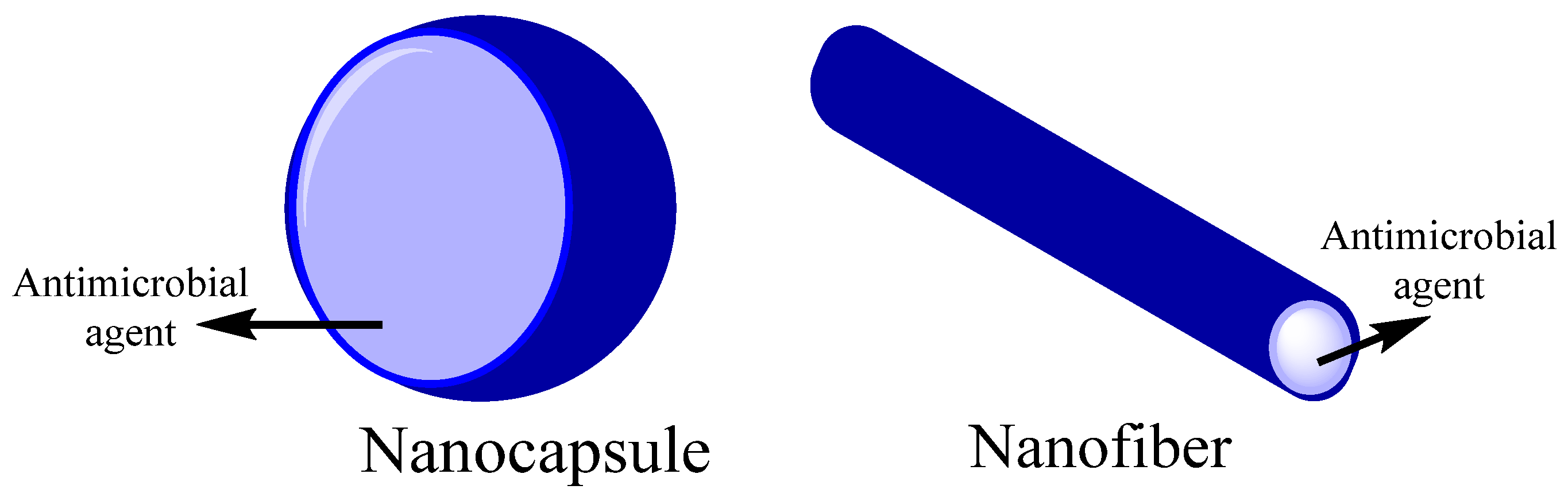
- antimicrobial agents
- antimicrobial activity
- biofilm
1. Cellulose-Based Composites
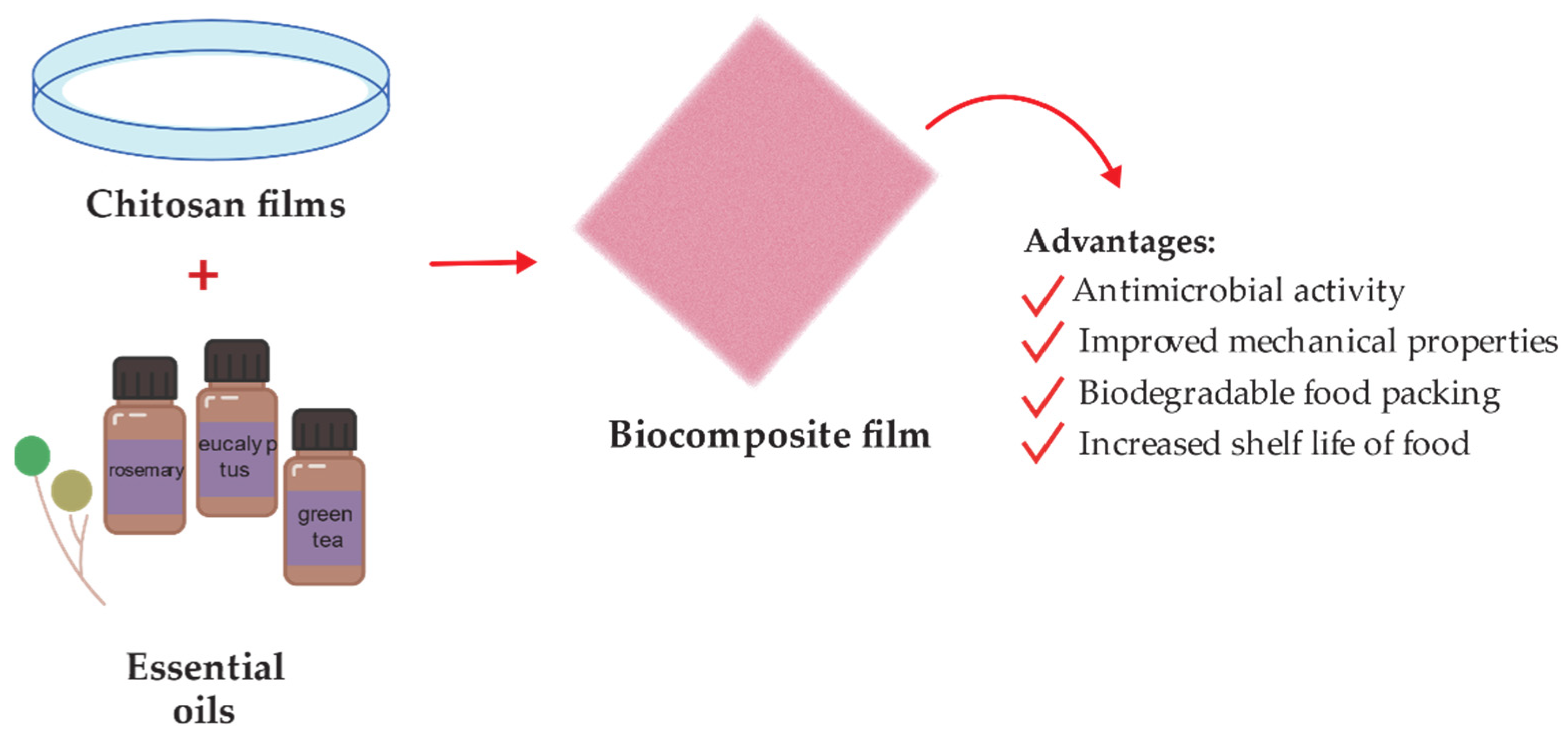
2. Chitosan-Based Composites
3. Starch-Based Composites
4. Collagen-Based Composites
5. Gelatin-Based Composites
Gelatin is a nontoxic natural biomacromolecule comprised of bioactive polypeptides derived from collagen in animal skin, bones, and connective tissues [177][74]. It is a protein obtained through controlled partial hydrolysis of collagen [101][75]. Gelatin has many glycine, proline, and 4-hydroxy proline residues [178][76]. Depending on the process employed, two types of gelatin can be obtained: type A gelatin produced by acid hydrolysis and type B obtained by an alkaline or lime process [101][75]. Gelatin has long attracted interest in food, packaging, pharmaceutical, and photographic industries, because of its physical and functional properties such as the reversible gel-to-sol transition of aqueous solution; viscosity behavior; protective colloid function, biodegradability, and solubility in hot water but insolubility in cold water. The gelatin-based film has a suitable matrix and compatibility that allows it to act as a medium for incorporating antimicrobial and antioxidant agents [179][77]. There is a considerable number of publications on the preparation of gelatin-based films with antimicrobial activity by incorporating naturally occurring and synthetic antimicrobials such as organic acids [180][78], proteins [181][79], enzymes [182][80], chelating agents [183][81], and EOs [184][82]. For instance, the effect of incorporating tannic acid (TA) and cellulose nanocrystals (CNC) on gelatin films was evaluated by Leite et al. [102][83]. The gelatin films containing nonoxidized TA and CNC (G-nTA-CNC) exhibited antimicrobial activity against S. aureus and E. coli due to the incorporation of TA. Moreover, G-nTA-CNC films showed an improvement in the gelatin’s antioxidant capacity antioxidant capacity, UV barrier, tensile strength, and water vapor barrier properties. Thus, the resulting approach is suitable for different applications, particularly food packaging. Developing wound dressing loaded with antimicrobial agents has also received much interest in reducing wound bacterial colonization [107][84]. Figure 53 shows a schematic illustration for the design of bioactive agent-loaded gelatin-based materials by electrospinning for the wound healing process. Recently, the design of a copper peroxide-loaded gelatin sponge with pH-controllable •OH delivery and effective antimicrobial activity for wound healing was reported. The experiments showed that the as-prepared wound dressing could release •OH, specifically in the bacterial-infected skin wound. In addition, in vitro experiments revealed that the wound dressing has good bactericidal properties against E. coli, S. aureus, and P. aeruginosa [103][85]. Pereda et al. reported the synthesis of biodegradable composite films based on gelatin and chitosan. Composite obtained showed a uniformity due to a compact structure indicating good compatibility between components, which could interact by strong hydrogen bonding. The researchers tested these films against E. coli and L. monocytogenes strains. However, only E. coli resulted be sensitive to the gelatin-chitosan composite [104][86]. Thongsrikhem and coworkers developed an antibacterial gelatin-bacterial cellulose nanocomposite (GCB) film using cinnamaldehyde as a crosslinker and an antibacterial additive. These films were evaluated using S. aureus and E. coli, resulting in a vigorous antibacterial activity against both bacteria strains [105][87]. In addition, Roy et al. synthesized Gelatin-based multifunctional composite films reinforcing various amounts of copper sulfide nanoparticles (CuSNPs). The gelatin/CuSNP composite film presented effective antibacterial activity against E. coli and some activity against L. monocytogenes, suggesting their use in food packaging [106][88].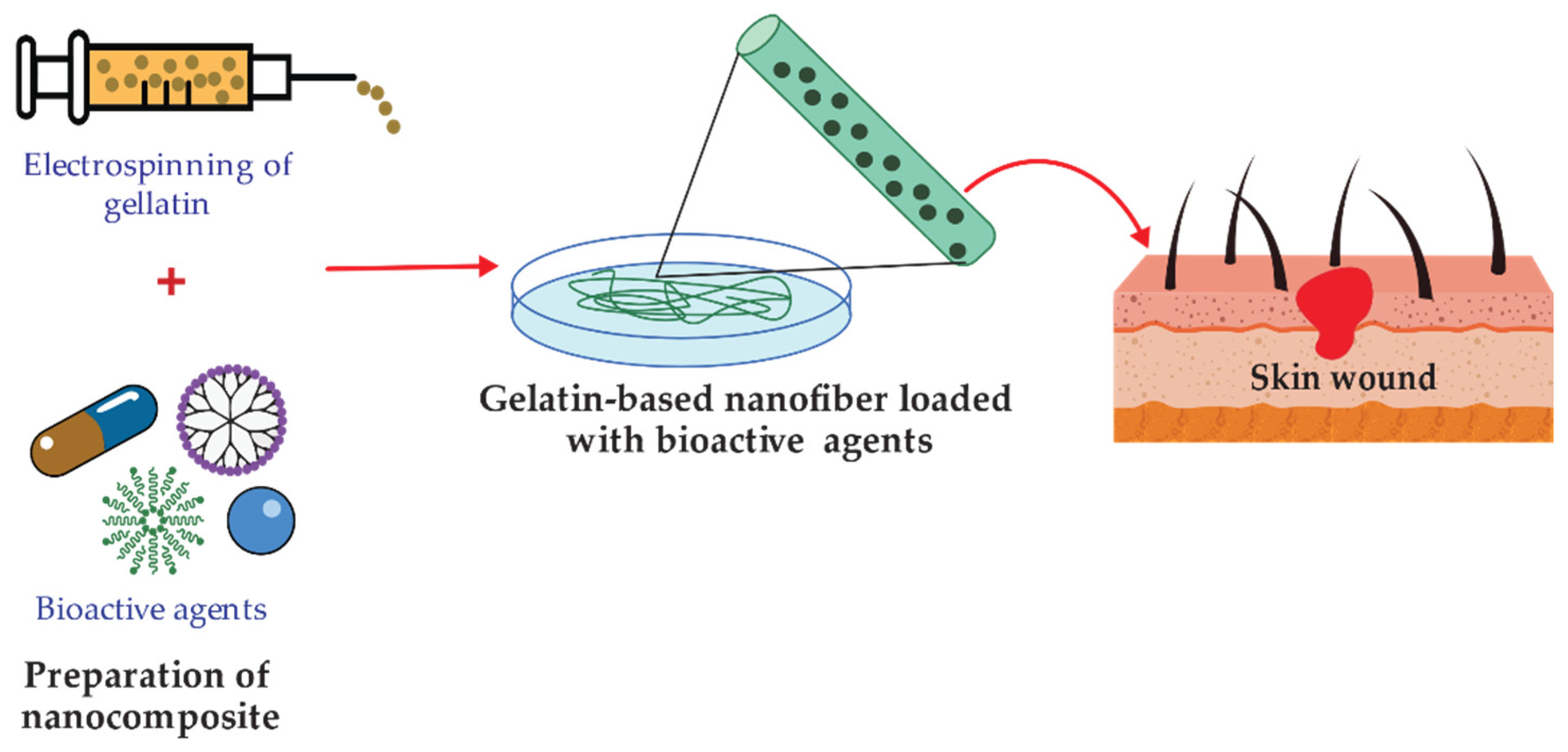
6. Hyaluronic Acid-Based Composites
Hyaluronic acid (HA) is a natural polymeric polysaccharide that contains N-acetyl glucosamine and glucuronic acid groups [185][89]. It is present in nature, mainly in mammalian connective tissues. HA is a highly reactive, biocompatible, biodegradable, no-inflammatory, and non-toxic polymer. However, HA has poor biomechanical properties in its native form, and various chemical modifications have been devised to provide mechanically and chemically robust materials [186][90]. HA can be cross-linked or conjugated with assorted biomacromolecules, and it is optimal to encapsulate different active agents [187][91]. Hyaluronic acid hydrogels are readily fabricated as microspheres, sponges, and fibers depending on the intended application [188][92]. However, unmodified HA has a poor residence time in vivo, which can be tailored via cross-linking reactions [189][93]. Among various polymers tested as antibacterial coatings, HA and some of its composites offer a well-established long-term safety profile and a proven ability to reduce bacterial adhesion and biofilm formation [190][94]. HA can interfere with bacterial adhesion to a cellular substrate concentration-dependent [191][95]. HA is bacteriostatic but not bactericidal and exhibits dose-dependent effects on different microorganisms in the planktonic phase [189][93]. HA and its derivate may offer a solution and long-term safety with a known ability to retard bacterial adhesion and biofilm formation [192][96]. However, some studies have shown that the bacteriostatic effect of soluble HA in vitro may be attributed to the saturation of the bacterial hyaluronidase by an excess of HA in the medium [193][97]. To impart antimicrobial properties, the polymeric matrix is commonly functionalized with antimicrobial agents such as quaternary ammonium compounds (QACs), improving antimicrobial efficiency through a contact killing mechanism [108][98]. The surface-functionalized scheme is present in Figure 64. HA carboxylic acid groups are modified by ester formation, while hydroxyl groups can be modified utilizing glutaraldehyde [194][99]. It is applied in ophthalmic treatments as a visual carrier material in a long-term antibiotic release.
7. Alginates-Based Composites
Alginate (ALG) is a natural polymer comprising β-D-mannuronic acid and α-L- guluronic acid extracted from brown seaweed [115][107]. This biomaterial exhibits several properties, including biocompatibility, gelation capability, low toxicity [196][108], mild gelation conditions, and simple modifications to prepare alginate derivatives with new properties [197][109], suggesting its use in biomedical and food industry applications. Alginate can absorb water and body fluids up to 20 times its weight, resulting in a hydrophilic gel [198][110]. The formed gel is weak, but it maintains a moist wound healing environment [198][110]. Linear Alginate polymer chains contain multiple carboxyl groups that can bind to divalent cations (Ca2+, Ba2+) to promote the formation of cross-linked structures [199][111]. Applications within biotechnology and medicine are mainly based on the temperature-independent sol-gel transition in multivalent cations (e.g., Ca2+), making alginates highly suitable as an immobilization matrix for living cells [200][112]. Several studies have investigated the effectiveness of incorporating antimicrobial agents such as EOs and NPs into alginate-based materials to induce an antimicrobial activity. Ahmed and Boateng reported the development of antimicrobial films for treating bacterial infections [116][113]. The calcium alginate films were loaded with ciprofloxacin and tested against E. coli, S. aureus, and P. aeruginosa. The results indicated a bacterial kill within 24 h and were highly biocompatible with human keratinocyte cells. In another study, biodegradable alginate films were prepared by adding zinc oxide nanoparticles (ZnONPs) and citronella essential oil (CEO) for cheese packaging. The ZnONPs act as a reinforcing agent and arrange the alginate polymer chains around them (Figure 75). Microbiological studies revealed a synergic effect between antibacterial activities of ZnO and CEO against two gram-negative (E. coli and Salmonella typhi) and two gram-positive (B. cereus and S. aureus) bacterial strains. Moreover, alginate/ZnO/CEO films showed better UV light barrier properties and lowered water vapor permeability (WVP) than pure alginate film [117][114]. Very similar research was reported by using spherical AgNPs and lemongrass essential oil (LGO) as antimicrobial agents, and the result indicated the feasibility of using alginate/Ag NPs/LGO films as antibacterial packaging to preserve the color, surface texture, and softness of cheese for 14 days [118][115].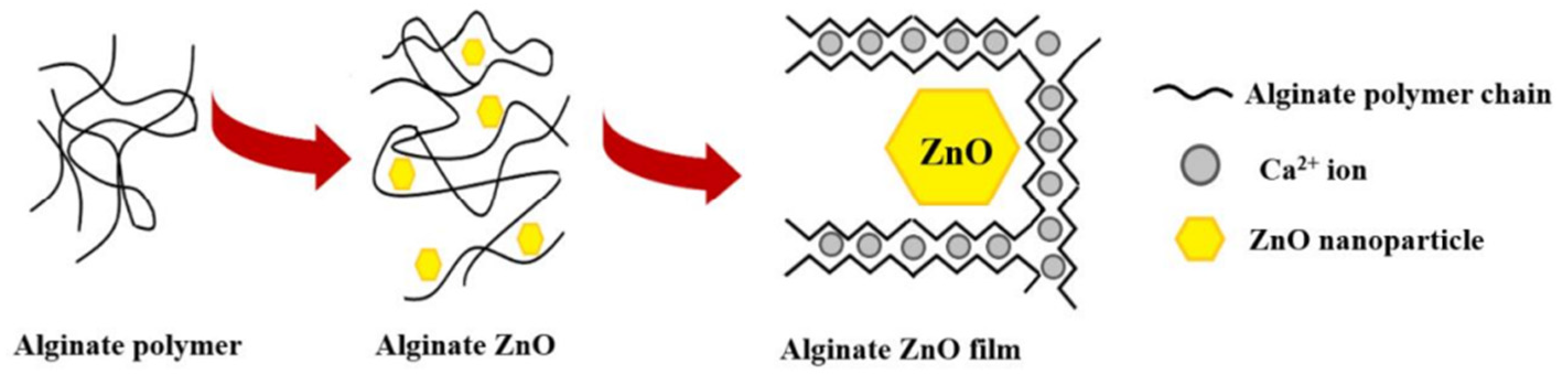
8. Fibrin-Based Composites
Fibrin, derived from critical proteins involved in blood clotting (fibrinogen and thrombin), is a self-assembling biopolymer [122][119]. Fibrin is a critical component of the blood clot that accelerates wound healing, prevents hemorrhage, and protects against bacterial infection [123][120]. Fibrin alone, or in combination with other biomaterials, was employed as a biological scaffold to promote stem or primary cells to regenerate [129][121]. In comparison to alginate-only gel-laden constructs, fibrin has an advantage in cytocompatibility due to cell adhesion moieties within the fibrin structure [201][122]. Several studies have reported the antimicrobial effect of leukocyte- and platelet-rich fibrin (L-PRF) against periodontal pathogens. Castro et al. assessed the antimicrobial properties of L-PRF against pathogens grown on agar plates and in planktonic cultures. A potent inhibition was found against Prevotella intermedia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and especially against Porphyromonas gingivalis [128][123]. The research conducted by Venante and co-workers [124] exhibited the effectiveness of fibrin biopolymer incorporating antimicrobial agents such as digluconate chlorhexidine and Punica granatum alcoholic extract to prevent the development of Candida albicans biofilm. In vitro results displayed the inhibition of the growth of C. albicans biofilm on poly(methyl methacrylate) (PMMA) substrates for up to 72 h, which suggests the excellent performance of the modified fibrin biopolymer as a drug delivery system, preventing the formation of denture biofilm. Fibrin sealant was used as a matrix for teicoplanin as an antimicrobial carrier applied externally to control infection sites [125]. Vancomycin impregnated fibrin sealant was developed to measure antibacterial activity and antibiotic release. This study uses fibrin sealant as a topical hemostat for post-operatory treatments in surgical fields [126]. AgNPs were studied in metal/fibrin nanocomposites, recognized as suitable materials for wound healing. AgNPs produce an antimicrobial effect related to the easy oxidation of silver. The action over the bacteria occurs due to the interaction between AgNPs/Ag+ and the cell membrane of the bacteria. The reaction was tested against E. coli and S. aureus [127]. A bioartificial human dermis substitute was developed for the treatment of infected wounds. It was based on a fibrin-agarose matrix with sodium colistimethate (SCM) and amikacin (AMK) as antimicrobial agents [202][128].References
- Brigham, C. Biopolymers. In Green Chemistry, 1st ed.; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 753–770. ISBN 9780128095492.
- Yahya, E.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Khalil, H. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648.
- Aravamudhan, A.; Ramos, D.; Nada, A.; Kumbar, S. Natural Polymers. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S., Laurencin, C.T., Deng, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 67–89. ISBN 9780123972903.
- Trejo-González, L.; Rodríguez-Hernández, A.; del Rocío López-Cuellar, M.; Martínez-Juárez, V.; Chavarría-Hernández, N. Antimicrobial pectin-gellan films: Effects on three foodborne pathogens in a meat medium, and selected physical-mechanical properties. CYTA J. Food 2018, 16, 469–476.
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.; Fernández-García, M. Bio-Based Polymers With Antimicrobial Properties Towards Sustainable Development. Materials 2019, 12, 641.
- Carvalho, J.; Silva, A.; Silvestre, A.; Freire, C.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744.
- Norrrahim, M.; Nurazzi, N.; Jenol, M.; Farid, M.; Janudin, N.; Ujang, F.; Yasim-Anuar, T.; Syed Najmuddin, S.; Ilyas, R. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551.
- Liu, Y.; Ahmed, S.; Sameen, D.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546.
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170.
- Vigo, T.L. Protection of Textiles from Biological Attack. In Handbook of Fibre Science and Technology; Lewin, M., Sello, S.B., Eds.; Marcel Dekker, INC.: New City, NY, USA, 1983; Volume 2, pp. 367–426. ISBN 9781351442732.
- Schindler, W.; Hauser, P. Chemical Finishing of Textiles, 1st ed.; Schindler, W., Hauser, P., Eds.; Woodhead Publishing: Cambridge, UK, 2004; pp. 165–174. ISBN 9781845690373.
- Simončič, B.; Tomšič, B. Recent Concepts of Antimicrobial Textile Finishes. In Textile Finishing: Recent Developments and Future Trends; Mital, K.L., Bahners, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1, pp. 3–68. ISBN 978-1-119-42685-1.
- Malis, D.; Jeršek, B.; Tomšič, B.; Štular, D.; Golja, B.; Kapun, G.; Simončič, B. Antibacterial Activity and Biodegradation of Cellulose Fiber Blends with Incorporated ZnO. Materials 2019, 12, 3399.
- Gopakumar, D.A.; Thomas, S.; Grohens, Y. Nanocelluloses as Innovative Polymers for Membrane Applications. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements, 1st ed.; Puglia, D., Fortunati, E., Kenny, J.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 253–275. ISBN 9780323417396.
- Kumar, R.; Kumar, G. Nanocellulose: Fascinating and sustainable nanomaterial for papermaking. In Nanotechnology in Paper and Wood Engineering, 1st ed.; Bhat, R., Kumar, A., Nguyen, T.A., Sharma, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 389–407. ISBN 9780323859639.
- Alvarado, D.; Argyropoulos, D.; Scholle, F.; Peddinti, B.; Ghiladi, R. A facile strategy for photoactive nanocellulose-based antimicrobial materials. Green Chem. 2019, 21, 3424–3435.
- Rashki, S.; Shakour, N.; Yousefi, Z.; Rezaei, M.; Homayoonfal, M.; Khabazian, E.; Atyabi, F.; Aslanbeigi, F.; Safaei Lapavandani, R.; Mazaheri, S.; et al. Cellulose-Based Nanofibril Composite Materials as a New Approach to Fight Bacterial Infections. Front. Bioeng. Biotechnol. 2021, 9, 732461.
- Littunen, K.; Snoei de Castro, J.; Samoylenko, A.; Xu, Q.; Quaggin, S.; Vainio, S.; Seppälä, J. Synthesis of cationized nanofibrillated cellulose and its antimicrobial properties. Eur. Polym. J. 2016, 75, 116–124.
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427.
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42.
- Orlando, I.; Basnett, P.; Nigmatullin, R.; Wang, W.; Knowles, J.; Roy, I. Chemical Modification of Bacterial Cellulose for the Development of an Antibacterial Wound Dressing. Front. Bioeng. Biotechnol. 2020, 8, 557885.
- Silva, Â.; Duarte, A.; Sousa, S.; Ramos, A.; Domingues, F. Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT 2016, 73, 481–489.
- Arakere, U.; Jagannath, S.; Krishnamurthy, S.; Chowdappa, S.; Konappa, N. Microbial bio-pesticide as sustainable solution for management of pests. In Biopesticides, 1st ed.; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Kumar, A., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 183–200. ISBN 9780128236147.
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839.
- Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182.
- Panda, P.; Yang, J.; Chang, Y.; Su, W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667.
- Samadi, F.; Mohammadi, Z.; Yousefi, M.; Majdejabbari, S. Synthesis of raloxifene–chitosan conjugate: A novel chitosan derivative as a potential targeting vehicle. Int. J. Biol. Macromol. 2016, 82, 599–606.
- Liakos, E.V.; Lazaridou, M.; Michailidou, G.; Koumentakou, I.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol 2021, 1, 130–154.
- Barbosa, M.; Pêgo, A.; Amaral, I. Chitosan. Compr. Biomater. 2011, 2, 221–237.
- Nurunnabi, M.; Revuri, V.; Huh, K.; Lee, Y. Polysaccharide based nano/microformulation: An effective and versatile oral drug delivery system. In Nanostructures for Oral Medicine, 1st ed.; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 409–433. ISBN 9780323477215.
- Jean, M.; Alameh, M.; De Jesus, D.; Thibault, M.; Lavertu, M.; Darras, V.; Nelea, M.; Buschmann, M.; Merzouki, A. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. Eur. J. Pharm. Sci. 2012, 45, 138–149.
- Das, B.; Patra, S. Antimicrobials. In Nanostructures for Antimicrobial Therapy, 1st ed.; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. ISBN 9780323461511.
- Kaczmarek, M.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243.
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209.
- Tsai, G.; Su, W. Antibacterial Activity of Shrimp Chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243.
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084.
- Bakshi, P.; Selvakumar, D.; Kadirvelu, K.; Kumar, N. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083.
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332.
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205.
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354.
- Varghese, S.; Siengchin, S.; Parameswaranpillai, J. Essential oils as antimicrobial agents in biopolymer-based food packaging—A comprehensive review. Food Biosci. 2020, 38, 100785.
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140.
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350.
- Alves, V.; Rico, B.; Cruz, R.; Vicente, A.; Khmelinskii, I.; Vieira, M. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT 2018, 89, 525–534.
- Joz Majidi, H.; Babaei, A.; Arab Bafrani, Z.; Shahrampour, D.; Zabihi, E.; Jafari, S. Investigating the best strategy to diminish the toxicity and enhance the antibacterial activity of graphene oxide by chitosan addition. Carbohydr. Polym. 2019, 225, 115220.
- Ao, H.; Yang, S.; Nie, B.; Fan, Q.; Zhang, Q.; Zong, J.; Guo, S.; Zheng, X.; Tang, T. Improved antibacterial properties of collagen I/hyaluronic acid/quaternized chitosan multilayer modified titanium coatings with both contact-killing and release-killing functions. J. Mater. Chem. B 2019, 7, 1951–1961.
- McKeen, L. The effect of heat aging on the properties of sustainable polymers. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers, 2nd ed.; William Andrew: Norwich, NY, USA, 2021; pp. 313–332. ISBN 978-0-323-85437-5.
- Winkler, S.; Kaplan, D. Biosynthesized Materials: Properties and Processing. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Jürgen, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E., Mahajan, S., Veyssière, P., Eds.; Pergamon: Oxford, UK, 2001; pp. 609–615. ISBN 9780080431529.
- Hutmacher, D. Polymers from Biotechnology. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Jürgen, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E., Mahajan, S., Veyssière, P., Eds.; Pergamon: Oxford, UK, 2001; pp. 7680–7683. ISBN 9780080431529.
- Van Soest, J.; Benes, K.; De Wit, D.; Vliegenthart, J. The influence of starch molecular mass on the properties of extruded thermoplastic starch. Polymer 1996, 37, 3543–3552.
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581.
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167.
- Mustapha, F.; Jai, J.; Nik Raikhan, N.; Sharif, Z.; Yusof, N. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus niger. Food Control 2019, 99, 106–113.
- Ojogbo, E.; Ward, V.; Mekonnen, T. Functionalized starch microparticles for contact-active antimicrobial polymer surfaces. Carbohydr. Polym. 2020, 229, 115422.
- Rezapour, N.; Rasekh, B.; Mofradnia, S.; Yazdian, F.; Rashedi, H.; Tavakoli, Z. Molecular dynamics studies of polysaccharide carrier based on starch in dental cavities. Int. J. Biol. Macromol. 2019, 121, 616–624.
- Saravanakumar, K.; Sriram, B.; Sathiyaseelan, A.; Mariadoss, A.; Hu, X.; Han, K.; Vishnupriya, V.; MubarakAli, D.; Wang, M. Synthesis, characterization, and cytotoxicity of starch-encapsulated biogenic silver nanoparticle and its improved anti-bacterial activity. Int. J. Biol. Macromol. 2021, 182, 1409–1418.
- Shapi’i, R.; Othman, S.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602.
- do Evangelho, J.; da Silva Dannenberg, G.; Biduski, B.; el Halal, S.; Kringel, D.; Gularte, M.; Fiorentini, A.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981.
- Li, X.; Zhang, Y.; Kong, W.; Zhou, J.; Hou, T.; Zhang, X.; Zhou, L.; Sun, M.; Liu, S.; Yang, B. Cross-Linking of Centrifugally Spun Starch/Polyvinyl Alcohol (ST/PVA) Composite Ultrafine Fibers and Antibacterial Activity Loaded with Ag Nanoparticles. ACS Omega 2022, 7, 7706–7714.
- Cheema, U.; Ananta, M.; Muder, V. Collagen: Applications of a Natural Polymer in Regenerative Medicine. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-51-4450-2.
- Balasubramanian, P.; Prabhakaran, M.; Sireesha, M.; Ramakrishna, S. Collagen in Human Tissues: Structure, Function, and Biomedical Implications from a Tissue Engineering Perspective. In Polymer Composites—Polyolefin Fractionation—Polymeric Peptidomimetics—Collagens, 1st ed.; Abe, A., Kausch, H.H., Möller, M., Pasch, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 173–206. ISBN 978-3-642-34330-8.
- Lin, K.; Zhang, D.; Macedo, M.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2018, 29, 1804943.
- Chung, H.; Steplewski, A.; Chung, K.; Uitto, J.; Fertala, A. Collagen Fibril Formation. Int. J. Biol. Chem. 2008, 283, 25879–25886.
- David, G. Collagen-based 3D structures—Versatile, efficient materials for biomedical applications. In Biopolymer-Based Formulations, 1st ed.; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W., Dubey, N.K., Majumder, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 881–906. ISBN 9780128168981.
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical applications. J. Food Sci. Technol. 2014, 52, 4703–4707.
- Patino, M.; Neiders, M.; Andreana, S.; Noble, B.; Cohen, R. Collagen as an Implantable Material in Medicine and Dentistry. J. Oral Implantol. 2002, 28, 220–225.
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017, 63, 360–366.
- Ge, L.; Xu, Y.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of Antibacterial Collagen-Based Composite Wound Dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166.
- Neacsu, I.; Leau, S.; Marin, S.; Holban, A.; Vasile, B.; Nicoara, A.; Ene, V.; Bleotu, C.; Albu Kaya, M.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153.
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769.
- Alvarez, G.; Hélary, C.; Mebert, A.; Wang, X.; Coradin, T.; Desimone, M. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660.
- Vladkova, T.; Ivanova, I.; Staneva, A.; Albu-Kaya, M.; Shalaby, A.; Moskova-Doumanova, V.; Kostadinova, A. Preparation and Biological Activity of New Collagen Composites, Part III. Collagen/(Ag/RGO) and Collagen/(Ag/RGO/SiO2) Composites. J. Arch. Mil. Med. 2017, 5, e57454.
- Gavaric, N.; Mozina, S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021.
- Narayanaswamy, R.; Kanagesan, S.; Pandurangan, A.; Padmanabhan, P. Basics to different imaging techniques, different nanobiomaterials for image enhancement. In Nanobiomaterials in Medical Imaging, 1st ed.; Grumezescu, A.M., Ed.; William Andrew: Norwich, NY, USA, 2016; Volume 8, pp. 101–129. ISBN 9780323417389.
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.; Khadheer Pasha, S.; Bhagat, P.; Chidambaram, K. Biopolymer Composites With High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics, 1st ed.; Sadasivuni, K., Ponnamma, D., Kim, J., Cabibihan, J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. ISBN 9780081009741.
- Pereda, M.; Ponce, A.; Marcovich, N.; Ruseckaite, R.; Martucci, J. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381.
- Said, N.; Howell, N.; Sarbon, N. A Review on Potential Use of Gelatin-based Film as Active and Smart Biodegradable Films for Food Packaging Application. Food Rev. Int. 2021, 1–23.
- Clarke, D.; Molinaro, S.; Tyuftin, A.; Bolton, D.; Fanning, S.; Kerry, J. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control 2016, 64, 202–211.
- Jridi, M.; Hajji, S.; Ayed, H.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379.
- Bower, C.; Avena-Bustillos, R.; Olsen, C.; McHugh, T.; Bechtel, P. Characterization of Fish-Skin Gelatin Gels and Films Containing the Antimicrobial Enzyme Lysozyme. J. Food Sci 2006, 71, M141–M145.
- Abarca, R.; Medina, J.; Alvarado, N.; Ortiz, P.; Carrillo López, B. Biodegradable gelatin-based films with nisin and EDTA that inhibit Escherichia coli. PLoS ONE 2022, 17, e0264851.
- Martucci, J.; Gende, L.; Neira, L.; Ruseckaite, R. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015, 71, 205–213.
- Leite, L.; Pham, C.; Bilatto, S.; Azeredo, H.; Cranston, E.; Moreira, F.; Mattoso, L.; Bras, J. Effect of Tannic Acid and Cellulose Nanocrystals on Antioxidant and Antimicrobial Properties of Gelatin Films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549.
- Khosravimelal, S.; Chizari, M.; Farhadihosseinabadi, B.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. J. Mater. Sci. Mater. Med. 2021, 32, 114.
- Cui, H.; Liu, M.; Yu, W.; Cao, Y.; Zhou, H.; Yin, J.; Liu, H.; Que, S.; Wang, J.; Huang, C.; et al. Copper Peroxide-Loaded Gelatin Sponges for Wound Dressings with Antimicrobial and Accelerating Healing Properties. ACS Appl. Mater. Interfaces 2021, 13, 26800–26807.
- Zeng, W.; Li, Y.; Wang, Y.; Cao, Y. Tissue Engineering of Blood Vessels. In Encyclopedia of Tissue Engineering and Regenerative Medicine, 1st ed.; Reis, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 413–424. ISBN 9780128137000.
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial activity in gelatin-bacterial cellulose composite film by thermally crosslinking with cinnamaldehyde towards food packaging application. Food Packag. Shelf Life 2022, 31, 100766.
- Roy, S.; Rhim, J.-W. Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications. Appl. Sci. 2021, 11, 6307.
- Guvendiren, M.; Purcell, B.; Burdick, J. Photopolymerizable Systems. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 413–438. ISBN 9780080878621.
- Burd, A. Hyaluronan and Scarring. In Chemistry and Biology of Hyaluronan, 1st ed.; Garg, H.G., Hales, C.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 367–394. ISBN 9780080472225.
- Bayer, I. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649.
- Burdick, J.; Stevens, M. Biomedical hydrogels. Biomaterials, Artificial Organs and Tissue Engineering, 1st ed.; Hench, L., Jones, J., Eds.; Woodhead Publishing: Cambridge, UK, 2005; pp. 107–115. ISBN 9781845690861.
- Zamboni, F.; Okoroafor, C.; Ryan, M.; Pembroke, J.; Strozyk, M.; Culebras, M.; Collins, M. On the bacteriostatic activity of hyaluronic acid composite films. Carbohydr. Polym. 2021, 260, 117803.
- Romanò, C.; Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72.
- Drago, L.; Cappelletti, L.; De Vecchi, E.; Pignataro, L.; Torretta, S.; Mattina, R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS 2014, 122, 1013–1019.
- Zamboni, F.; Wong, C.; Collins, M. Hyaluronic acid association with bacterial, fungal and viral infections: Can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioact. Mater. 2022, 19, 458–473.
- Carlson, G.; Dragoo, J.; Samimi, B.; Bruckner, D.; Bernard, G.; Hedrick, M.; Benhaim, P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem. Biophys. Res. Commun. 2004, 321, 472–478.
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.; Ashrafizadeh, M.; Zare, E.; Agarwal, T.; Padil, V.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 12.
- Schanté, C.; Zuber, G.; Herlin, C.; Vandamme, T. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489.
- Zhang, Z.; Suner, S.; Blake, D.; Ayyala, R.; Sahiner, N. Antimicrobial activity and biocompatibility of slow-release hyaluronic acid-antibiotic conjugated particles. Int. J. Pharm. 2020, 576, 119024.
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Azua, I.; Benito, V.; Bilbao, A.; Vilas-Vilela, J. Development of multiactive antibacterial multilayers of hyaluronic acid and chitosan onto poly (ethylene terephthalate). Eur. Polym. J. 2019, 112, 31–37.
- Walvekar, P.; Gannimani, R.; Salih, M.; Makhathini, S.; Mocktar, C.; Govender, T. Self-assembled oleylamine grafted hyaluronic acid polymersomes for delivery of vancomycin against methicillin resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces. 2019, 182, 110388.
- Kłodzińska, S.; Wan, F.; Jumaa, H.; Sternberg, C.; Rades, T.; Nielsen, H. Utilizing nanoparticles for improving anti-biofilm effects of azithromycin: A head-to-head comparison of modified hyaluronic acid nanogels and coated poly (lactic-co-glycolic acid) nanoparticles. J. Colloid Interface Sci. 2019, 555, 595–606.
- Lequeux, I.; Ducasse, E.; Jouenne, T.; Thebault, P. Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. Eur. Polym. J. 2014, 51, 182–190.
- Harris, L.; Richards, R. Staphylococcus aureus adhesion to different treated titanium surfaces. J. Mater. Sci. Mater. Med. 2004, 15, 311–314.
- Felgueiras, H.; Wang, L.; Ren, K.; Querido, M.; Jin, Q.; Barbosa, M.; Ji, J.; Martins, M. Octadecyl Chains Immobilized onto Hyaluronic Acid Coatings by Thiol–ene “Click Chemistry” Increase the Surface Antimicrobial Properties and Prevent Platelet Adhesion and Activation to Polyurethane. ACS Appl. Mater. Interfaces 2017, 9, 7979–7989.
- Preman, N.; Jain, S.; Sanjeeva, S.; Johnson, R. Alginate derived nanoassemblies in drug delivery and tissue engineering. In Polysaccharide Nanoparticles, 1st ed.; Venkatesan, J., Kim, S., Anil, S., Rekha, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–280. ISBN 9780128223567.
- Ahmad Raus, R.; Wan Nawawi, W.; Nasaruddin, R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306.
- Lee, K.; Mooney, D. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Aramwit, P. Introduction to biomaterials for wound healing. In Wound Healing Biomaterials, 1st ed.; Agren, M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; Volume 2, pp. 3–38. ISBN 9780081006061.
- Hamai, R.; Anada, T.; Suzuki, O. Novel scaffold composites containing octacalcium phosphate and their role in bone repair. In Octacalcium Phosphate Biomaterials, 1st ed.; Suzuki, O., Insley, G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 121–145. ISBN 9780081025123.
- Skjåk-Bræk, G.; Draget, K. Alginates. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 213–220.
- Ahmed, A.; Boateng, J. Calcium alginate-based antimicrobial film dressings for potential healing of infected foot ulcers. Ther. Deliv. 2018, 9, 185–204.
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.; Andronescu, E.; Holban, A. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020.
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Ene, V.; Vasile, B.; Andronescu, E.; Holban, A. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377.
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Lillelund Aachmann, F.; Serrano-Aroca, Á. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of Carbon Nanofibers (0.1% w/w). Appl. Sci. 2021, 11, 2311.
- Agren, M. Zinc in Wound Repair. Arch. Dermatol. 1999, 135, 1273–1274.
- Asadpoor, M.; Ithakisiou, G.; van Putten, J.; Pieters, R.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605.
- Catelas, I. Fibrin. In Comprehensive Biomaterials, 1st ed.; Ducheyne, P., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2011; Volume 2, pp. 303–328. ISBN 9780080552941.
- Ahmed, T.; Dare, E.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 199–215.
- Climov, M.; Leavitt, T.; Molnar, J.; Orgill, D. Natural Biomaterials for Skin Tissue Engineering. In Skin Tissue Engineering and Regenerative Medicine, 1st ed.; Albanna, M., Holmes, J.H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 145–161. ISBN 9780128017975.
- Carrow, J.; Kerativitayanan, P.; Jaiswal, M.; Lokhande, G.; Gaharwar, A. Polymers for Bioprinting. In Essentials of 3D Biofabrication and Translation, 1st ed.; Atala, A., Yoo, J.J., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 229–248. ISBN 9780128010150.
- Castro, A.; Herrero, E.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188.
- Venante, H.; Chappuis-Chocano, A.; Marcillo-Toala, O.; da Silva, R.; da Costa, R.; Pordeus, M.; Barraviera, B.; Ferreira Junior, R.; Lara, V.; Neppelenbroek, K.; et al. Fibrin Biopolymer Incorporated with Antimicrobial Agents: A Proposal for Coating Denture Bases. Materials 2021, 14, 1618.
- Tan, R.; Lee, H.; Ma, H.; Lee, H.; Han, S. Antibacterial Effect of Antibiotic-Saturated Fibrin Sealant; In Vitro Study. J. Wound Manag. Res. 2018, 14, 12–17.
- Shin, D.; Sohn, M.; Cho, C.; Koo, H.; Yoon, S. Evaluation of Cumulative and Conditional Antibiotic Release from Vancomycin-Embedded Fibrin Sealant and Its Antibacterial Activity: An In Vitro Study. J. Korean Neurosurg. Soc. 2020, 63, 45–55.
- Zahran, M.; Marei, A. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596.
- Chato-Astrain, J.; Chato-Astrain, I.; Sánchez-Porras, D.; García-García, Ó.; Bermejo-Casares, F.; Vairo, C.; Villar-Vidal, M.; Gainza, G.; Villullas, S.; Oruezabal, R.; et al. Generation of a novel human dermal substitute functionalized with antibiotic-loaded nanostructured lipid carriers (NLCs) with antimicrobial properties for tissue engineering. J. Nanobiotechnol. 2020, 18, 174.
