1. Diversity of Intercellular Interactions in the Tumor Microenvironment
The appropriate action of the immune system is based on distinguishing the correct cells from the foreign cells and destroying the latter with the use of both mechanisms of its activity—specific and non-specific. However, it should be remembered that in a strongly immunosuppressive neoplastic microenvironment, the immune system cells undergo reprogramming and, most often, they no longer fulfill their original function
[1][12]. A summary of the most important populations of immune system cells involved in the anti-cancer response and the change in their functioning in the neoplastic microenvironment is presented in
Table 1. Complex intercellular interactions within the tumor microenvironment are presented in
Figure 1.
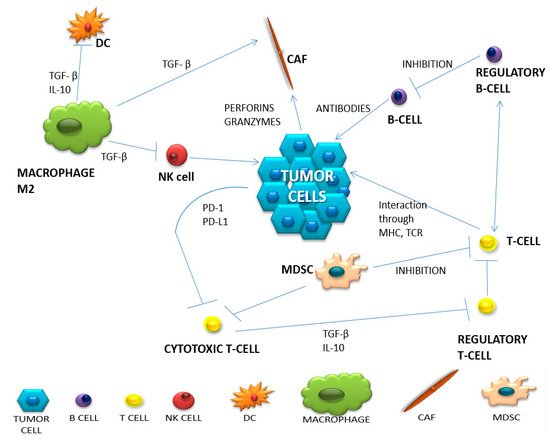
Figure 1. The network of intercellular interactions in the tumor microenvironment (based on Borros Arneth, 2019).
Table 1. Different cell populations in the tumor microenvironment
[2][3][4][5][7,13,14,15].
The population of T lymphocytes is the main component of the specific immune response and within this group we can distinguish several subpopulations of T cells that are extremely important for the proper development of the immune response. The most important subpopulation of T lymphocytes during an anti-tumor response is one composed of cytotoxic T lymphocytes, which are designed to directly destroy the neoplastic cell after its specific recognition. Moreover, they produce a huge amount of proinflammatory cytokines (e.g., IL-2, INF-
γ). Th1 lymphocytes arise when inactivated T lymphocytes are stimulated by IL-12 and express the transcription factor T-box, which induces the secretion of IFN-
γ by Th1 lymphocytes. The main function of IFN-
γ is an antitumor activity, stimulating cytotoxic T lymphocytes and NK cells. On the other hand, it has an inhibitory effect on Treg lymphocytes and recruits macrophages for their anti-tumor activity. In turn, by acting on dendritic cells, it stimulates MHC expression and antigen presentation
[6][16]. Moreover, Th2 and Th17 helper lymphocytes secrete numerous cytokines (including IL-4, IL-5, IL-13, IL-17, IL-21 and IL-22) that contribute to increased tissue inflammation and thus stimulate tumor growth. Regulatory T cells, defined as cells positive for CD4 and CD25, and with the intracellular expression of FoxP3 protein, are one of the most important populations of lymphocytes responsible for extinguishing the activity of other immune system cells. An extremely important marker for this function is the CTLA-4 molecule that is induced on lymphocytes by TGF-beta released by tumor cells. Regulatory T lymphocytes inhibit the activity of other lymphocytes, mainly T cytotoxic ones, but also the ability of phagocytic cells. The mechanism of tryptophan consumption, which is another phenomenon of suppressing the immune response, should also be mentioned. Regulatory T lymphocytes use tryptophan, an amino acid essential for the functioning of cytotoxic T lymphocytes, to inhibit long-term inflammation. At the same time, they produce toxic products of metabolism such as hydrogen peroxide and nitric oxide (H
2O
2 and NO), disrupting the functioning of other immune cells. Another mechanism of inhibiting the function of immune cells is the production of adenosine by neoplastic cells during the process of ATP dephosphorylation due to prolonged inflammation. Outside the cell, in its presence, the expression of CD39 and CD73 increases and, having strong inhibitory properties, it leads to the disappearance of cytotoxic T lymphocytes, while stabilizing the activity of regulatory T lymphocytes
[7][17]. In the context of different functions performed by selected populations of T lymphocytes, information, not only about the infiltration of the tumor site by the T cells, but also about the quality of this infiltration, seems to be extremely important. The cellular composition of the lymphocytic infiltration in the neoplastic tissue may be of good prognostic value
[8][18]. The presence of CD8-positive cytotoxic T lymphocytes in the tumor environment is a positive prognostic factor in patients before starting treatment, because it plays one of the most important roles in the immune response
[5][9][15,19].
Antigen recognition and antibody production are the main tasks of B lymphocytes. Even though B lymphocytes are known primarily for their stimulating role on the immune response, Ammirante et al. proved, in a mouse model of prostate cancer, that under the influence of the CXCL13 chemokine, they acquire a pro-tumor function
[8][10][11][18,20,21]. In turn, under the influence of the produced cytokine IL-10 and the formation of complexes with IgG antibodies, they can promote the infiltration of immunosuppressive cells by destroying the ECM and stimulating angiogenesis. The presence of these cells in the tumor environment is associated with a good prognosis in patients with hepatocellular, biliary and breast cancer. In turn, in lung cancer, melanoma and pancreatic cancer, the presence of B lymphocytes worsens the prognosis of patients
[2][12][7,22].
NK cells are attracted to the tumor by inflammation and chemokines in dendritic cells. Through perforins and granzymes, pro-inflammatory cytokines and chemokines (IFN-γ, IL-6, GM-CSF, CCL-5 and TNF), they are able to destroy foreign cells. However, their function in the tumor microenvironment is inhibited by impaired maturation, which means negligible expression of DX5, CD11b and CD27. For NK cells, the TIGIT receptor is an important checkpoint, which causes the blockade of the inhibitory pathway to restore their potent anti-tumor function
[13][23].
Due to their cytotoxic and phagocytic nature, macrophages have long been considered as immunoactivating cells. However, the tumor microenvironment is such a complex structure that even macrophages undergo plastic modifications. Depending on the received signals, they modify phenotypes assuming pro-tumor functions (M2) or anti-tumor macrophages (M1). TAMs (tumor-associated macrophages) show defective activation of NF-κB in the presence of TNF-α and bacterial LPS (lipopolysaccharide) and maintain the inflammatory phenotype in the tumor. Some macrophages, under the influence of GM-CSF, IFN-γ and TNF-α, are transformed into the M1 macrophage population. Activated M1 macrophages stimulate cytotoxic T lymphocytes and NK cells, making them capable of killing cancer cells. However, the tumor microenvironment is usually infiltrated by M2 cells arising from the influence of IL-4, IL-10 and IL-13
[1][14][12,24].
Dendritic cells are known as the main antigen-presenting cells that stimulate T cell activity and their presence correlates with a good prognosis. There are several groups of a tumor infiltrating dendritic cells, which, depending on their function, may be either immunostimulatory or immunosuppressive. Among them, we can distinguish: plasmacytoid dendritic cells (pDCs), classical dendritic cells (cDCs 1 and 2) and monocyte-derived dendritic cells (moDCs). The main role of pDCs is to develop the body’s tolerance to cancer and its progression. They are divided into two types, depending on the presence of the CD2 antigen on the surface. They secrete IFN-α and –β; however, only cells with high CD2 expression, by secreting IL-12, promote proliferation of CD4-positive T cells. Another important group of dendritic cells are classical DCs, which can also be divided into two subgroups: cDC1 and cDC2. The first group is responsible for the secretion of inflammatory cytokines: IL-6, IL-8, IL-12 and TNF-α. In turn, cDC2 constitutes a large percentage of the entire population of dendritic cells, demonstrating high efficiency in antigen presentation and increasing the CD4-positive T cell population. Therefore, their presence in the tumor microenvironment suggests the generation of an anti-tumor response. The last subpopulation of dendritic cells are monocytes transformed into inflammatory dendritic cells. They show similar expression of surface antigens, as in the case of cDC2s. The presence of this population in lung cancer has been proven
[15][25]. In inflammation, dendritic cells can start producing TNF and nitric oxide (NO), which is essential for CD8-positive T cells to perform an anti-cancer function. However, neoplastic cells secreting CXCL1, CXCL5, CCL2 and VEGF inhibit dendritic cell maturation, with the side effect of changing the function of a dendritic cell into a pro-neoplastic cell
[7][16][17,26].
Neutrophils, in a properly functioning organism, perform a defensive function, mainly through the phagocytosis of dead cells and as antigen-presenting cells. In the cancer microenvironment, tumor-associated neutrophils (TANs) secret various cytokines and metabolic products (myeloperoxidase—MPO), therefore they influence the recruitment of monocytes and macrophages, providing them with pro- or anti-tumor functions. Under the influence of TGF-β, neutrophils can transform into having a pro-neoplastic function (N2 subtype), and they can stimulate angiogenesis, while IFN-γ promotes the anti-tumor N1 subtype. This subtype has an antitumor character which is manifested by the possibility of phagocytosis and by bringing foreign cells into apoptosis. The presence of neutrophils in the neoplastic microenvironment lowers the prognostic value of the disease
[2][14][17][7,24,27].
Adipocytes are the main component of adipose tissue, where they act as an energy reservoir. In the tumor microenvironment, they secrete a multitude of growth factors, hormones and cytokines. They fulfill their mainly pro-neoplastic function by secreting adipokines, which include leptin and the hepatocyte growth factor, causing inflammation and increasing the likelihood of metastasis. On the other hand, excessive stimulation of adipocytes contributes to the growth of collagen and stiffening of the structure of the microenvironment
[4][14].
Cancer-associated fibroblasts (CAFs) are the main source of collagen for cells, they affect the surrounding tumor, immune and endothelial cells. Their presence contributes to resistance to therapies and more frequent relapses of many neoplastic diseases, and is associated with a poor prognosis. They secrete many cytokines and growth factors such as: TGF-
β, IL-6, exosomes, CXCL2, CCL7, HGF, IGF and CTGF. It has been proven that CAFs promote tumor development by causing inflammation, stimulation of the angiogenesis process, secretion of growth factors and modification of ECM
[18][19][20][28,29,30].
In breast cancer, adipocytes are the main ingredients that build the tumor microenvironment. Cancer-associated adipocytes (CAA) are characterized by a smaller size, they secrete more chemokine ligand 2 and 5 (CCL2, CCL5), IL-1β, IL-6, leptin, VEGF and TNF-α, but lower the expression of adiponectin. These are largely responsible for storing energy in the form of triacylglycerols to be released as free fatty acids when needed. CAAs are also able to influence the functions of immune cells through the release of pro-inflammatory cytokines IL-6, IL-8 and TNF-α. Thus, they attract monocytes and macrophages, creating chronic inflammation. As a result of lipolysis, free fatty acids are released, which disturbs lipid homeostasis and also influences the maturation of cells of the immune system
[21][22][23][31,32,33].
The cells strongly influencing the suppression of immune cells in the tumor microenvironment are myeloid-derived suppressor cells (MDSC). They come from the bone marrow, inhabiting the peripheral lymph nodes or the tumor microenvironment. Depending on the target location, they could perform different functions. In TME, they support neoplastic growth, metastases and participate in angiogenesis. There are two types of MDSC: M-MDSC (monocytic-MDSC) and PMN-MDSC (polymorphonuclear-MDSC). In TME, M-MDSC transforms into TAM (tumor-associated macrophages). This shows the complexity in the formation and functioning of the neoplastic microenvironment. Additionally, the complex network of cellular interactions may make it difficult to respond to treatment
[24][34]. An example of this is a recent study in mice that were administered a STAT3 inhibitor. The observations made showed a reduction in the amount of MDSC in the spleen, while it remained unchanged in the tumor
[25][35].
2. Three Categories of Tumor Microenvironments Based on Their Immunophenotype
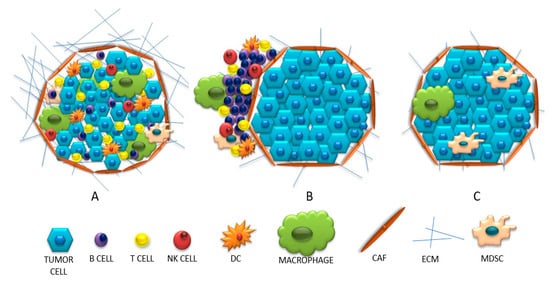
Based on the presence and the strength of the immune system invasion into the tumor site, neoplastic tumors may have different immunophenotypes. The following immunoprofiles, based on the activation and infiltration of the immune system, could distinguish: (1) “hot” tumors, which are strongly infiltrated by T lymphocytes and with many inflammatory signals; (2) “cold” tumors, which are scant of any immune cell infiltration nor inflammatory signs; (3) tumors with immune exclusion, where immune cells are at the periphery or within the stromal tissue.
The infiltrated tumors, so called “hot”, inflammatory tumors, are defined by strong leukocyte infiltration, including a huge diversity of cells: B lymphocytes, CD4-positive and CD8-positive T cells, Treg lymphocytes, macrophages, fibroblasts and MDSC. Additionally, these tumors are characterized by the presence of intra-tumor chemokines (e.g., CXCL9, CXCL10, CCL5)
[26][10]. IFN-γ is also released, which, although it has an anti-tumor effect, stimulates the immunosuppressive response by induction of IDO (indole 2,3-dioxygenase) and PD-L1 expression. It has also been proven that the presence of these molecules indicates the presence of cytotoxic T lymphocytes
[26][27][28][10,36,37]. In this type of tumor, there are many interactions between negative immune checkpoints on effector T cells and their ligands on APC and tumor cells. The main signal stimulating T cells to activate is the association of CD28 on their surface with CD80 and CD86 on tumor cells or APC. However, when CD28 binds to CTLA-4 on a cancer cell (which has a greater affinity for CD28 than CD80 or CD86), T cells become depleted, anergic and killed. The “hot” tumors are associated with denser PD-1-positive T lymphocyte infiltration, with a pre-existing primed immune response and are more likely to respond to the anti-PD-1 or anti-PD-L1 blockade used as monotherapy.
“Cold” (non-inflammatory) tumors can be divided into two types: the ones with invasion of immune cells and the so-called “immune desert”. The first group is characterized by an influx of cells in the vicinity of the tumor without penetrating its center. It is suspected that APCs may play a role here; in the absence of stimulation, they inhibit the influx of T lymphocytes into the center of the tumor or release an insufficient amount of chemokines recruiting T lymphocytes
[8][28][18,37]. The second concept for this behavior of lymphocytes is the inability to pass the barrier surrounding the tumor parenchyma (caused, for example, by a compact network of collagen fibers)
[8][18]. Therefore, in this type of tumor, little or no intercellular interactions are observed due to the ‘barrier character ‘of fibroblast cells. Whereas the second group describes a condition in which there is no infiltration or activation of T lymphocytes, with the presence of Treg lymphocytes, macrophages and MDSC. These cells inhibit the maturation of dendritic cells and make it difficult for T cells to be infiltrated. When the amount of adhesion molecules (CD34, E-selectin, vascular cell adhesion molecule—VCAM and intercellular adhesion molecule—ICAM) is reduced under the influence of VEGF and FGF, VEGF stimulates blood vessel formation and reduces cell adhesion. In turn, the stimulated ligand for the Fas receptor (FasL) inhibits the influx of T lymphocytes
[28][37]. The presence of IDO, TGF-β and PD-L1 molecules in the microenvironment is not conducive to the survival and activation of T lymphocytes, but may additionally stimulate MDSC cells. These, using arginine, reduce the survival of T lymphocytes. This condition is called endothelial cell anergy, which results in the inability to adhere T lymphocytes in the tumor blood vessel and its infiltration
[8][27][28][29][18,36,37,38]. A comparison of the discussed immunophenotypes is presented in
Figure 2.
Figure 2. (A) “Hot” (inflamed), (B)”cold”(excluded), (C) “cold “(ignored) tumors.