Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Jose Maria Maria Fernandez-Rodriguez.
Hydrotalcites are known in the bibliography as LDHs (Layered double hydroxides). Hydrotalcites, as such, are not good CO2 absorbents due to poor basic properties and presence of entities that hinder CO2 adsorption and are therefore subjected to thermal treatment (around 500 °C) to obtain nearly amorphous metastable mixed solid solutions known as calcined layered double hydroxides (CLDHs). There are several parameters that can make hydrotalcites suitable for use as CO2 sinks.
- CO2 sinks
- calcined hydrotalcite
- one-coat mortar
- CO2 curing
21. Calcined Hydrotalcite to Capture CO2
Calcined hydrotalcite or its use under high temperatures (around 400 °C so that the hydrotalcite becomes oxide and can be rebuilt in contact with CO2). LDH are poor CO2 adsorbents in their natural or unburned form, which is due to a poor basic property and the presence of entities that hinder CO2 adsorption. Hence, they are subjected to thermal treatment to obtain nearly amorphous metastable mixed solid solutions (CLDHs) [19][1]. Figure 1 shows two diagrams representing what happens during the calcination of hydrotalcite of Mg3AlCO3 as shown (A) by W.J. Long et al. [77][2] and (B) by Lauermannová et al. [73][3]. Both schemes attempt to represent the collapse of the structure due to the loss of interlayer anions and moisture.
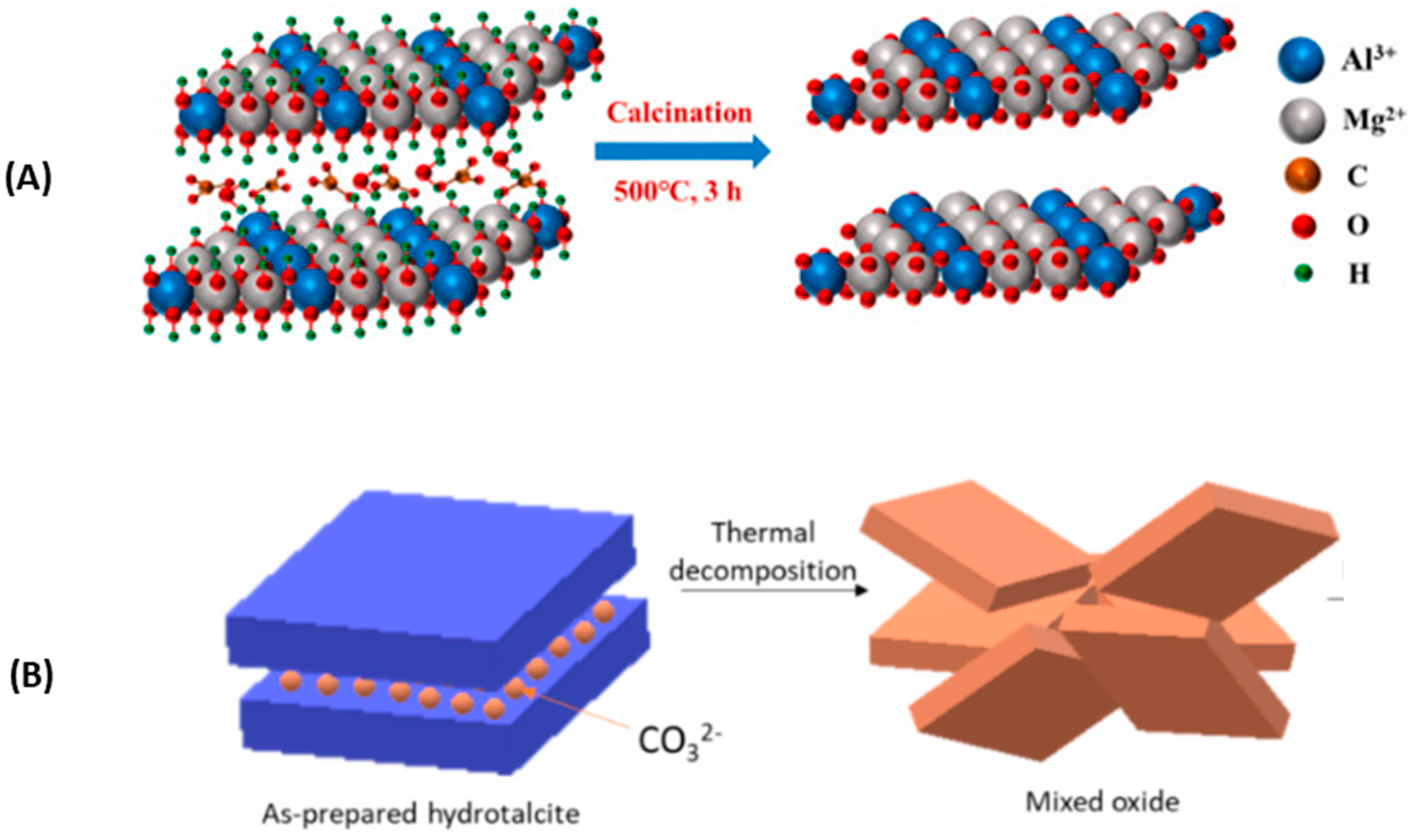
Figure 1. Structural changes of LDH after calcination according to adapted from W.J. Long et al. [77] (
B
) (open Access).
21.1. Thermal Behaviour of Hydrotalcite by TGA/DTA
LDH undergoes several stages until it reaches CLDHs. There is even research that attempts to explain this process in great detail and focuses on it alone [80,81][4][5]. It is very important to choose a suitable calcination temperature, avoiding it being too high (to avoid higher energy consumption), or being too low (not producing the collapse of the structure and hindering a higher CO2 capture). To distinguish the different stages, it is useful to rely on thermogravimetric analysis (TGA) and differential thermal analysis (DTA) carried out by Suescum-Morales et al. [33][6], shown in Figure 2. It should be noted that different variations in temperature ranges may be encountered, approximately as shown below.
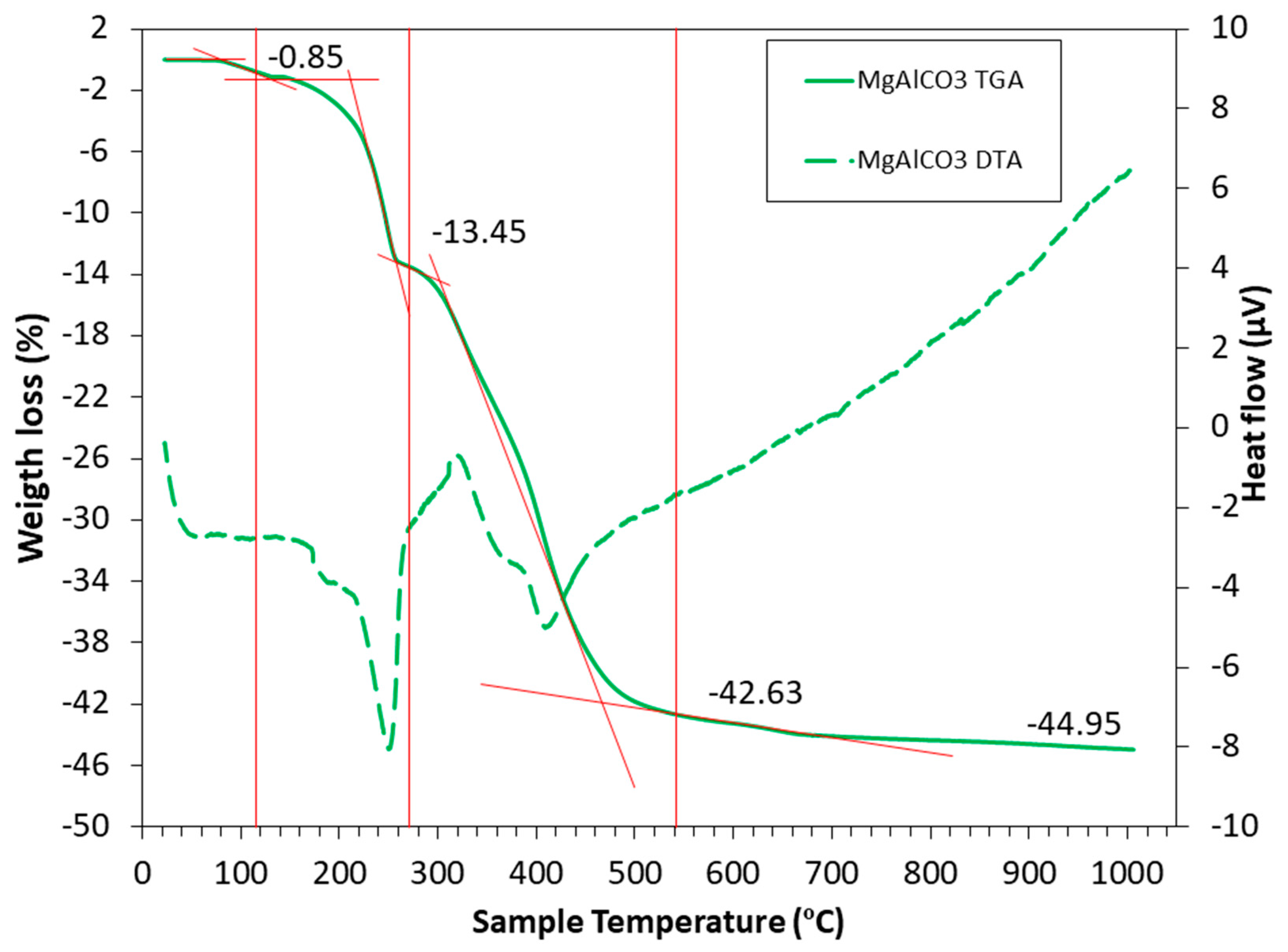
Figure 2.
TGA (solid lines) and DTA (dotted lines) for commercial hydrotalcite of MgAlCO
First of all, a loss of humidity is observed, up to a temperature of about 105 °C. The second stage occurs from 105 to 270 °C, where the water hydration of the hydrotalcite structure is lost (leading to a decrease in basal spacing) [82,83,84][7][8][9]. There are authors who indicate that between 200 and 300 °C, the OH− groups attached to Al3+ are lost [19][1]. The third stage, from 270 to 540 °C, is where the dehydroxilation of the hydrotalcite takes place and the loss of the carbonate anion of the interlayer in the form of CO2 occurs. The layer structure collapses (Figure 1), and the LDH converts to a mixed-oxide MgO-like phase [85][10]. In the last stage, from 540 to 1000 °C, very small weight losses are observed, attributed to the loss of residual OH− groups.
From the above, it can be seen that the calcination temperature affects the capacity to capture CO2 in CLDHs. Different structural characteristics are presented in the different stages of thermal decomposition. From this analysis, the ideal calcination temperature of the hydrotalcite under study can be determined, which is characteristic and unique depending on the type of hydrotalcite. Most research indicates that the calcination temperature of a Mg-Al LDH is around 400 °C [86,87,88][11][12][13]. However, the performance of a TGA/DTA for each specific case would allow observing the exact calcination temperature.
21.2. Thermal Behaviour of Hydrotalcite by XRD
The appropriate calcination temperature can also be determined by XRD temperature variation analysis. The thermal decomposition sequence of Mg-Al-CO3 hydrotalcite is well documented. Miyata, 1980 and Hibino et al., 1995 [82,89][7][14] studied the XRD variation at different temperatures, shown in Figure 3 (non-calcined, 150, 250, 350, 500, 850 and 1000 °C). The diffraction patterns of MgO can be identified between 400 °C and 850 °C, given the amorphous nature of Al2O3 at this temperature. For 900 °C the spinal phase (MgAl2O3) was formed. Given these experiences, the following chemical equations can be posed, with the different temperature ranges, to help us understand the process (Equations (31)–(53)):
Mg6Al2(OH)16CO3⋅4H2O →Mg6Al2(OH)16CO3+4H2O 100<T<250 ℃ (3)
Mg6Al2(OH)16CO3→6Mg(Al)O +8H2O +CO2400<T<850 ℃ (4)
6MgO +Al2O3→MgAl2O4+5MgO 900<T<1000 ℃ (5)
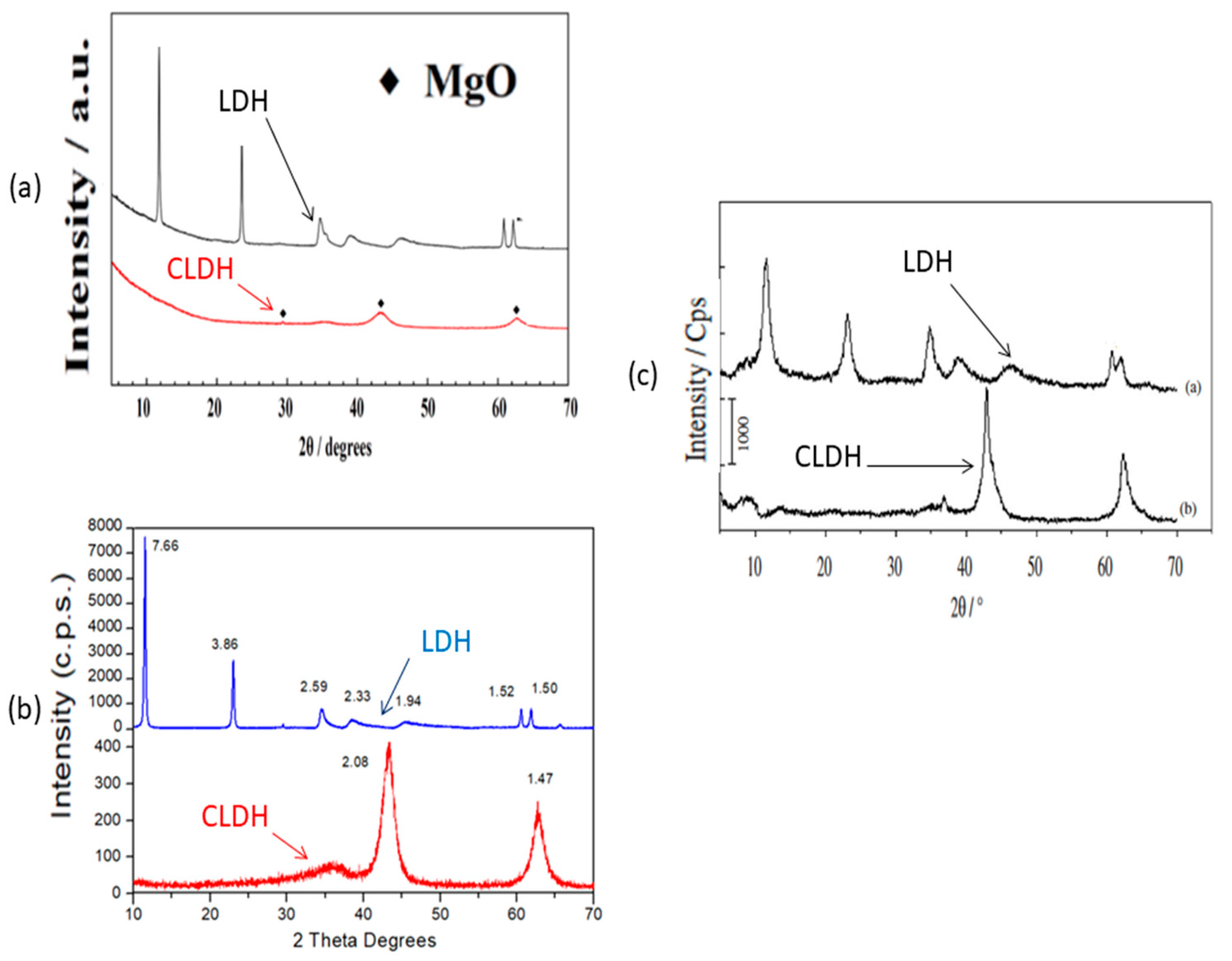
Figure 4 shows the similar XRD obtained by several authors by calcination at 500 °C for different periods: firstly, the XRD obtained by W.J. Long et al. [77][2] (Figure 4a) shows how at a temperature of 500 °C for 3 h the layered structure collapses and also shows the production of mixed oxides. Figure 4b shows a similar result, but in this case using a calcination time of 2 h, and the same temperature (500 °C) [33][6]. This leads to a large saving of energy in the calcination of LDH, with a consequent lowering of the carbon footprint. Already, Z. Yang et al. [67][15] calcined at 500 °C for 3 h, obtaining a similar result. Even Q.Tao et al. [90][16] heated at 500 °C for 4 h, obtaining similar results (Figure 4c). Similar results in XRD were obtained by S.I. Garcés Polo et al. [91][17] for CLDH. None of the previous authors [33,67,77,91][2][6][15][17] indicated the amount of sample used in the calcination, which may have led to these observed differences. Although different calcination temperatures have been used with similar results, there are no economic studies of the cost of calcination; studies of this type, with times, temperatures and quantities of LDH to be calcined, would be very useful for these materials in industrial applications. It would also be very important to carry out a real carbon footprint calculation (UNE EN 15804:2012), which determines the real CO2 sink capacity in each specific case (amount of hydrotalcite, type of furnace, etc.). Only two studies have been found that indicate the amount of hydrotalcite calcined (100 and 1 g respectively) [92,93][18][19]. Annotations of this type, i.e., what quantity is fed into the LDH kiln, are very important in order to maximise the efficiency of the calcination process.
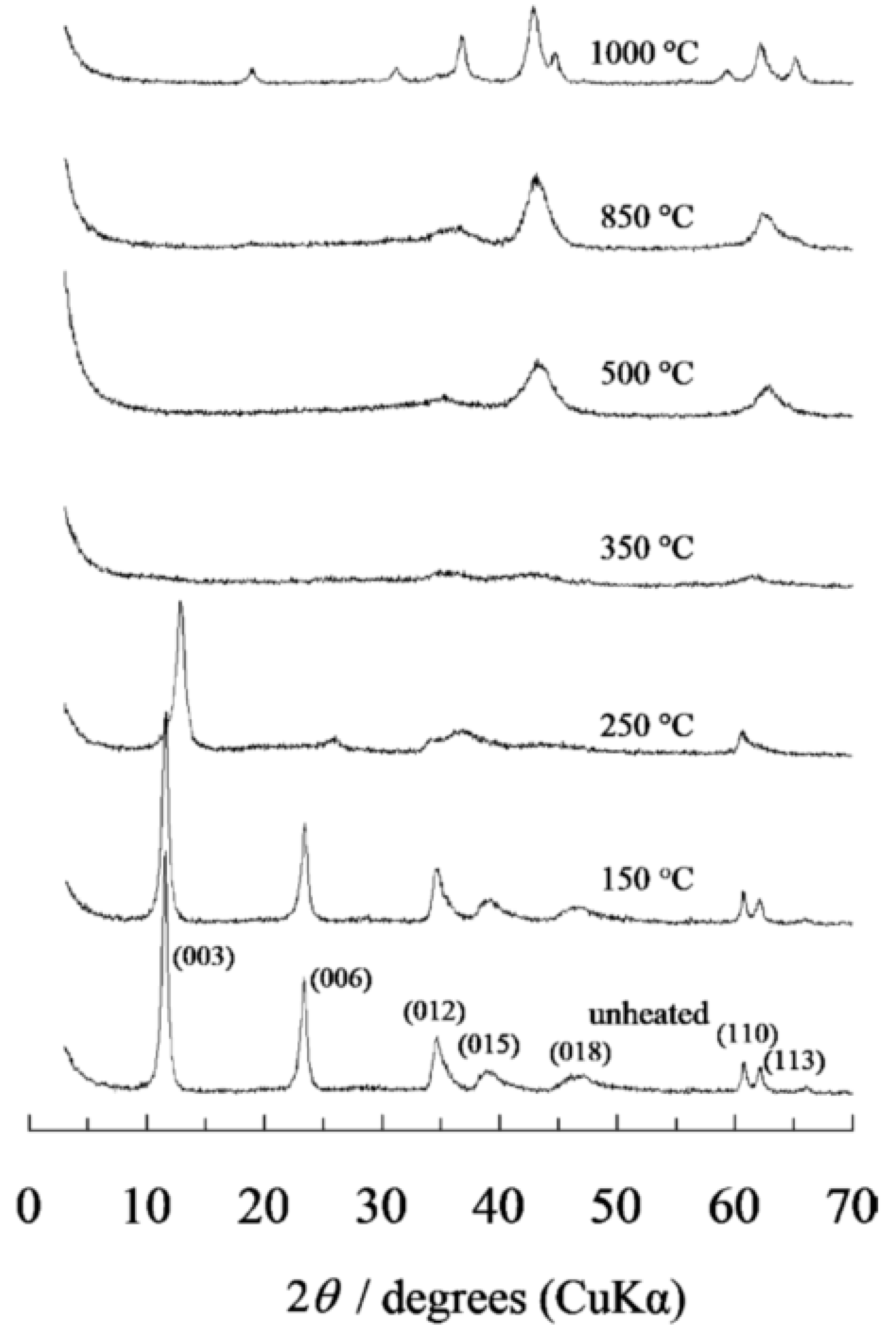
Figure 3.
XRD of MgAlCO
21.3. Thermal Behaviour of Hydrotalcite by SEM/TEM
Figure 5a shows SEM images of commercial LDH and CLDH Mg-Al from W.J. Long et al. [77][2]. After 3 h at 500 °C, the structure collapses. A decrease in size was also observed. P. Cai et al. [95][21] obtained similar results (Figure 5b) with the same temperature and time of calcination. After 4 h at 450 °C on Mg-Al LDH, C. Geng et al. [96][22] observed a decrease in particle size, and the hexagonal shape was hardly noticeable (Figure 5c). No other studies using different temperatures (different at 500 °C) and calcination times have been found that show SEM images of LDH and CLDH. Studies along these lines could fill this information gap.
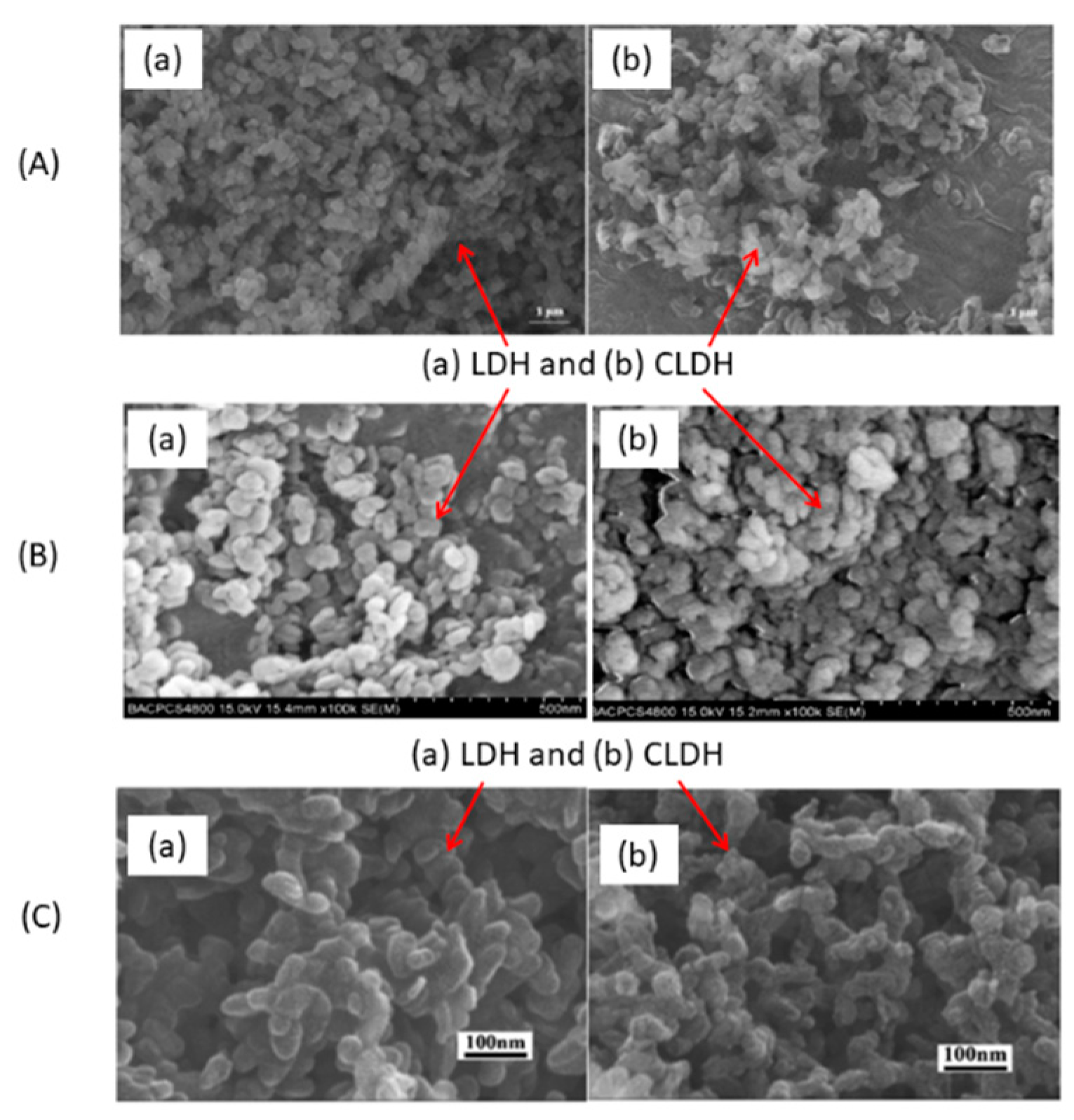
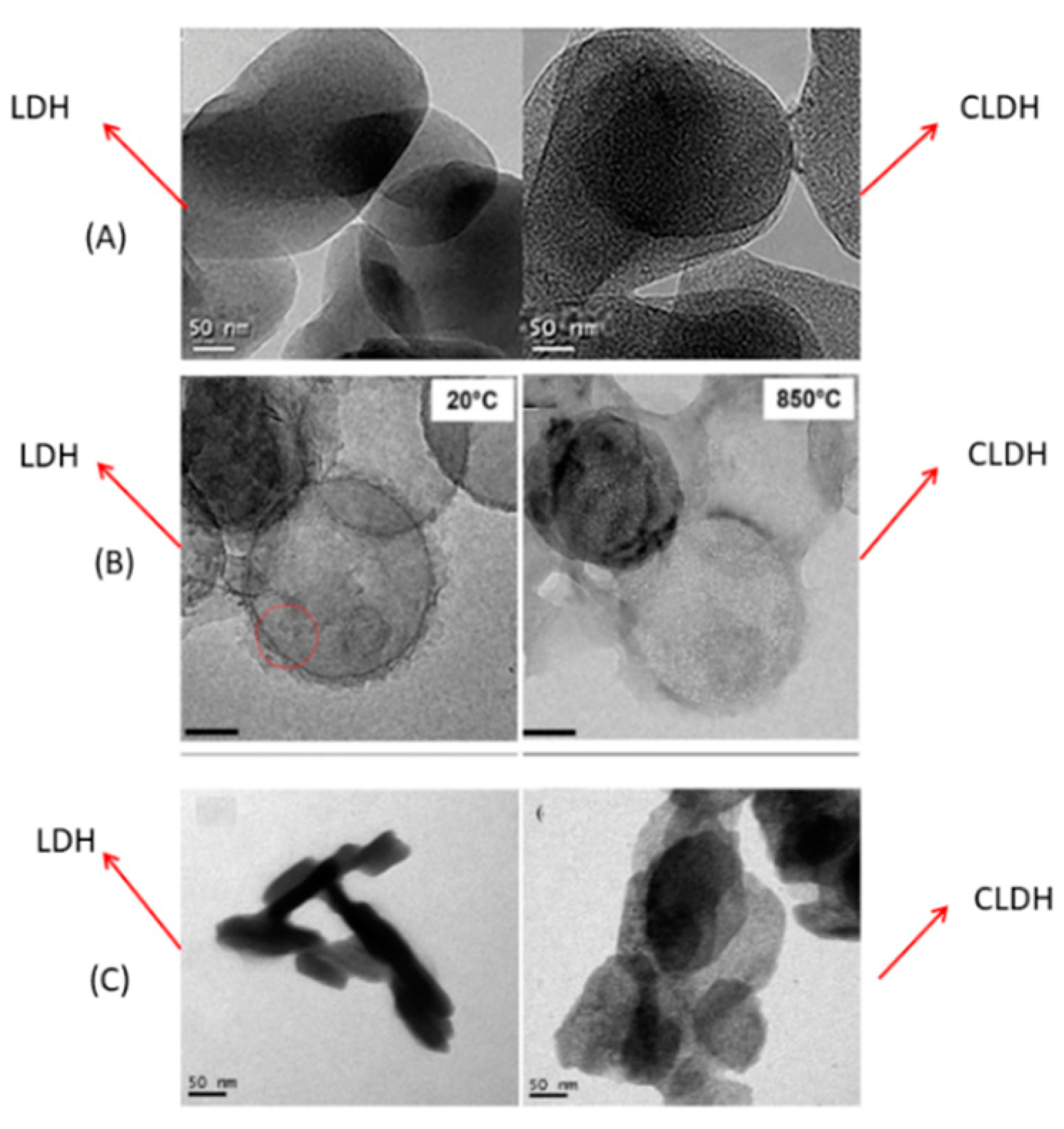
Figure 6A shows the TEM images of LDH and CLDH, after 2 h at 500 °C, obtained by Suescum-Morales et al. [33][6]. In CLDH, small pores were formed, attributable to the dehydration process, dehydroxilation of the OH− groups, and to the decomposition of the interlayer carbonate. C Hobbs et al. [97][23] studied the evolution of a Mg-Al LDH under different temperatures using a rate of 10 °C/min (Figure 6B). At a temperature of 20 °C (LDH), they have a well-defined platelet shape; the porous structure is clearly visible at 850 °C. S Luo et al. [98][24] also obtained the same porous structure in CLDH, as shown in Figure 6C.
Different heating rates have been used in LDH calcination; a heating rate of 10 °C/min was used in Mg/Al LDH by several authors [99,100,101,102][25][26][27][28] and a heating rate of 5 °C /min was used by [103][29]. It is known that if the heating rate is slower, the thermal effects are better observed (better porous structure). However, if the ramp is slower, it takes more time and wastes more energy; if the heating is too fast, the porous structure will be worse. These differences should be extensively studied, both from an energy point of view (higher consumption and, therefore, higher carbon footprint produced by the kiln when the larger ramp is used), and from the point of view of the efficiency of the CLDH itself. Another very important aspect is the kinetics of LDH, which has been extensively discussed in other research [104,105][30][31].
72. Combined Use of Hydrotalcites and Cement-Based Materials
The good and promising results obtained by the scientific community as adsorbents of hydrotalcites suggest the idea of incorporating them into construction materials, such as cement-based materials [66,67,68,69,70][15][32][33][34][35]. The ion exchange is the key feature that makes the use of LDH/CLDH in building materials attractive. These, together with the memory effect of CLDH, are the two suitable factors for the ion exchange and capture mechanism shown in Figure 107 [75][36].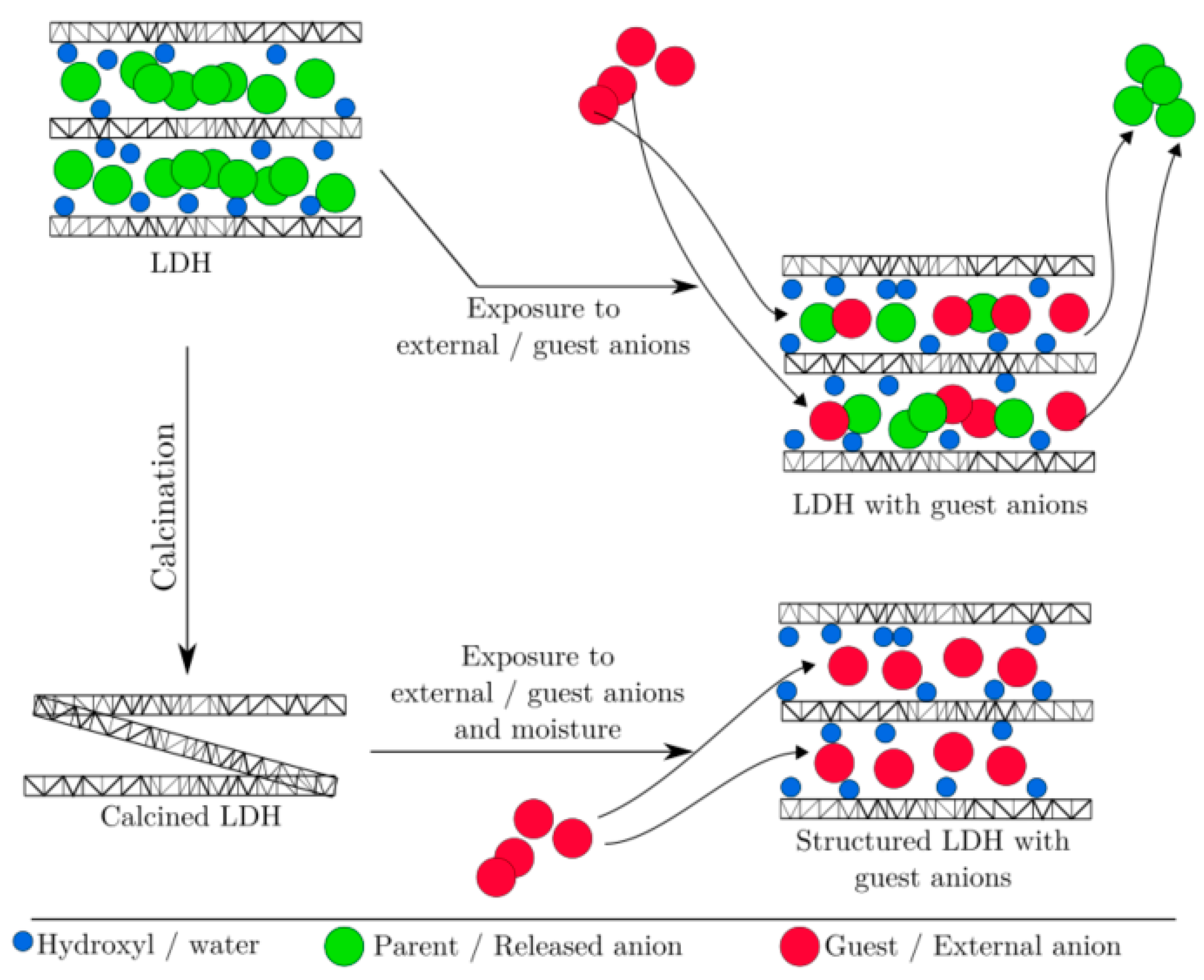
References
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Olivera, S.; Venkatesh, K. Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: A review. J. Clean. Prod. 2015, 103, 171–196.
- Long, W.-J.; Xie, J.; Zhang, X.; Fang, Y.; Khayat, K.H. Hydration and microstructure of calcined hydrotalcite activated high-volume fly ash cementitious composite. Cem. Concr. Compos. 2021, 123, 104213.
- Lauermannová, A.-M.; Paterová, I.; Patera, J.; Skrbek, K.; Jankovský, O.; Bartůněk, V. Hydrotalcites in Construction Materials. Appl. Sci. 2020, 10, 7989.
- Vágvölgyi, V.; Palmer, S.J.; Kristóf, J.; Frost, R.L.; Horváth, E. Mechanism for hydrotalcite decomposition: A controlled rate thermal analysis study. J. Colloid Interface Sci. 2007, 318, 302–308.
- Yahyaoui, R.; Jimenez, P.E.S.; Maqueda, L.A.P.; Nahdi, K.; Luque, J.M.C. Synthesis, characterization and combined kinetic analysis of thermal decomposition of hydrotalcite (Mg6Al2(OH)16CO3·4H2O). Thermochim. Acta 2018, 667, 177–184.
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.; Fernández, J. Mitigation of CO2 emissions by hydrotalcites of Mg3Al-CO3 at 0 °C and high pressure. Appl. Clay Sci. 2020, 202, 105950.
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56.
- Kannan, V.R.S.; Velu, S. Synthesis and physicochemical properties of cobalt aluminium hydrotalcites. J. Mater. Sci. 1995, 30, 1462–1468.
- Cocheci, L.; Barvinschi, P.; Pode, R.; Popovici, E.; Seftel, E.M. Structural Characterization of Some Mg/Zn-Al Type Hy-drotalcites Prepared for Chromate Sorption from Wastewater. Chem. Bull. 2010, 55, 40–45.
- Palmer, S.J.; Spratt, H.J.; Frost, R.L. Thermal decompostition of hydrotalcites with variable cationic ratios. J. Therm. Anal. Calorim. 2009, 95, 123–129.
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Shaffer, M.S.P. Graphene Oxide as Support for Layered Double Hydroxides: Enhancing the CO2 Adsorption Capacity. Chem. Mater. 2012, 24, 4531–4539.
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509.
- Wang, Q.; Wu, Z.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. High temperature adsorption of CO2 on Mg–Al hydrotalcite: Effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203.
- Hibino, T. Decarbonation Behavior of Mg-Al-CO3 Hydrotalcite-like Compounds during Heat Treatment. Clays Clay Miner. 1995, 43, 427–432.
- Yang, Z.; Fischer, H.; Cerezo, J.; Mol, J.M.C.; Polder, R. Aminobenzoate modified MgAAl hydrotalcites as a novel smart additive of reinforced concrete for anticorrosion applications. Constr. Build. Mater. 2013, 47, 1436–1443.
- Tao, Q.; Zhang, Y.; Zhang, X.; Yuan, P.; He, H. Synthesis and characterization of layered double hydroxides with a high aspect ratio. J. Solid State Chem. 2006, 179, 708–715.
- Garcés-Polo, S.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.; Gil, A. Adsorption of CO2 on mixed oxides derived from hydrotalcites at several temperatures and high pressures. Chem. Eng. J. 2018, 332, 24–32.
- Martunus; Othman, M.R.; Fernando, W.J.N. Elevated temperature carbon dioxide capture via reinforced metal hydrotalcite. Microporous Mesoporous Mater. 2011, 138, 110–117.
- Aschenbrenner, O.; McGuire, P.; Alsamaq, S.; Wang, J.; Supasitmongkol, S.; Al-Duri, B.; Styring, P.; Wood, J. Adsorption of carbon dioxide on hydrotalcite-like compounds of different compositions. Chem. Eng. Res. Des. 2011, 89, 1711–1721.
- Forano, C.T.-G.C.; Hibino, T.; Leroux, F. Layered double hydroxides. In Hand-Book of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Newnes, Australia, 2006; ISBN 978-0-08-044183-2.
- Cai, P.; Zheng, H.; Wang, C.; Ma, H.; Hu, J.; Pu, Y.; Liang, P. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg–Al–CO3 layered double hydroxides. J. Hazard. Mater. 2012, 213–214, 100–108.
- Geng, C.; Xu, T.; Li, Y.; Chang, Z.; Sun, X.; Lei, X. Effect of synthesis method on selective adsorption of thiosulfate by calcined MgAl-layered double hydroxides. Chem. Eng. J. 2013, 232, 510–518.
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 4.
- Luo, S.; Guo, Y.; Yang, Y.; Zhou, X.; Peng, L.; Wu, X.; Zeng, Q. Synthesis of calcined La-doped layered double hydroxides and application on simultaneously removal of arsenate and fluoride. J. Solid State Chem. 2019, 275, 197–205.
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48.
- Harizi, I.; Chebli, D.; Bouguettoucha, A.; Rohani, S.; Amrane, A. A New Mg–Al–Cu–Fe-LDH Composite to Enhance the Adsorption of Acid Red 66 Dye: Characterization, Kinetics and Isotherm Analysis. Arab. J. Sci. Eng. 2018, 44, 5245–5261.
- Chebli, D.; Bouguettoucha, A.; Reffas, A.; Tiar, C.; Boutahala, M.; Gulyas, H.; Amrane, A. Removal of the anionic dye Biebrich scarlet from water by adsorption to calcined and non-calcined Mg–Al layered double hydroxides. Desalin. Water Treat. 2016, 57, 22061–22073.
- Li, R.; Zhan, W.; Song, Y.; Lan, J.; Guo, L.; Zhang, T.C.; Du, D. Template-free synthesis of an eco-friendly flower-like Mg/Al/Fe-CLDH for efficient arsenate removal from aqueous solutions. Sep. Purif. Technol. 2021, 282, 120011.
- Liu, T.; Chen, Y.; Yu, Q.; Fan, J.; Brouwers, H. Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends. Constr. Build. Mater. 2020, 250, 118865.
- Singh, R.; Reddy, M.R.; Wilson, S.; Joshi, K.; da Costa, J.C.D.; Webley, P. High temperature materials for CO2 capture. Energy Procedia 2009, 1, 623–630.
- Ebner, A.D.; Reynolds, S.P.; Ritter, J.A. Nonequilibrium Kinetic Model That Describes the Reversible Adsorption and Desorption Behavior of CO2 in a K-Promoted Hydrotalcite-like Compound. Ind. Eng. Chem. Res. 2007, 46, 1737–1744.
- Qu, Z.; Yu, Q.; Brouwers, H. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem. Concr. Res. 2018, 105, 81–90.
- Yang, Z.; Fischer, H.; Polder, R. Synthesis and characterization of modified hydrotalcites and their ion exchange characteristics in chloride-rich simulated concrete pore solution. Cem. Concr. Compos. 2014, 47, 87–93.
- Yang, Z.; Fischer, H.; Polder, R. Modified hydrotalcites as a new emerging class of smart additive of reinforced concrete for anticorrosion applications: A literature review. Mater. Corros. 2013, 64, 1066–1074.
- Lozano-Lunar, A.; Álvarez, J.I.; Navarro-Blasco, Í.; Jiménez, J.R.; Fernández-Rodriguez, J.M. Optimisation of mortar with Mg-Al-Hydrotalcite as sustainable management strategy lead waste. Appl. Clay Sci. 2021, 212, 106218.
- Mir, Z.M.; Bastos, A.; Höche, D.; Zheludkevich, M.L. Recent Advances on the Application of Layered Double Hydroxides in Concrete—A Review. Materials 2020, 13, 1426.
- Raki, L.; Beaudoin, J.; Mitchell, L. Layered double hydroxide-like materials: Nanocomposites for use in concrete. Cem. Concr. Res. 2004, 34, 1717–1724.
- Hu, X.; Zhu, X.; Sun, Z. Fireproof performance of the intumescent fire retardant coatings with layered double hydroxides additives. Constr. Build. Mater. 2020, 256, 119445.
- Wu, Y.; Duan, P.; Yan, C. Role of layered double hydroxides in setting, hydration degree, microstructure and compressive strength of cement paste. Appl. Clay Sci. 2018, 158, 123–131.
- Gomes, C.; Mir, Z.; Sampaio, R.; Bastos, A.; Tedim, J.; Maia, F.; Rocha, C.; Ferreira, M. Use of ZnAl-Layered Double Hydroxide (LDH) to Extend the Service Life of Reinforced Concrete. Materials 2020, 13, 1769.
- Cao, L.; Guo, J.; Tian, J.; Xu, Y.; Hu, M.; Wang, M.; Fan, J. Preparation of Ca/Al-Layered Double Hydroxide and the influence of their structure on early strength of cement. Constr. Build. Mater. 2018, 184, 203–214.
- Long, W.; Xie, J.; Zhang, X.; Kou, S.; Xing, F.; He, C. Accelerating effect of calcined hydrotalcite-Na2SO4 binary system on hydration of high volume fly ash cement. Constr. Build. Mater. 2022, 328, 127068.
- Ma, J.; Duan, P.; Ren, D.; Zhou, W. Effects of layered double hydroxides incorporation on carbonation resistance of cementitious materials. J. Mater. Res. Technol. 2019, 8, 292–298.
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Potential CO2 capture in one-coat limestone mortar modified with Mg3Al–CO3 calcined hydrotalcites using ultrafast testing technique. Chem. Eng. J. 2021, 415, 129077.
- Suescum-Morales, D.; Fernández, D.C.; Fernández, J.M.; Jiménez, J.R. The combined effect of CO2 and calcined hydrotalcite on one-coat limestone mortar properties. Constr. Build. Mater. 2021, 280, 122532.
More
