Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Mohd Imran.
Diabetic nephropathy (DN) is a serious kidney illness characterized by proteinuria, glomerular enlargement, reduced glomerular filtration, and renal fibrosis. DN is the most common cause of end-stage kidney disease, accounting for nearly one-third of all cases of diabetes worldwide.
- diabetic nephropathy
- streptozotocin
- antioxidant
- medicinal plants
1. Introduction
Diabetes mellitus is a set of metabolic disorders marked by hyperglycemia due toa defect in insulin secretion or insulin action or both. In 2021, as per the International Diabetic Federation, approximately 10.5% (537 million people) of adults aged between 20 to 79 years were affected with diabetes [1,2][1][2]. By 2030, it is expected that this number will rise to over 643 million people. The incidence rate of diabetic nephropathy (DN) is high in the first 10 to 20 years after the onset of diabetes (three% per year) [3].
2. Pathogenesis of Diabetic Nephropathy
Hyperglycemia is a major risk factor for DN [4]. The pathophysiology of DN also involves the increased production of lipid peroxides, active carbonyl compounds and free radicals leading to oxidative stress and no enzymatic protein glycosylation. Antioxidant enzymes play a role in protecting cells and tissues from causing damage. The variation in the generation of free radicals and antioxidants is found to be the main reason for the development of disease. Nephropathy is caused primarily by the interaction of hemodynamic and metabolic systems. Metabolic pathways are also activated within the diabetic kidney, resulting in advanced glycosylated end products (AGEs) deposition, protein kinase C (PKC) activation, renal polyol synthesis, and enhanced oxidative stress. As a result, several cytokines and growth factors are activated. These processes eventually lead to changes in the renal histology of glomeruli, that is, mesangial expansion, glomerular basement membrane (GBM) thickening and glomerular sclerosis [5,6,7][5][6][7] (Figure 1).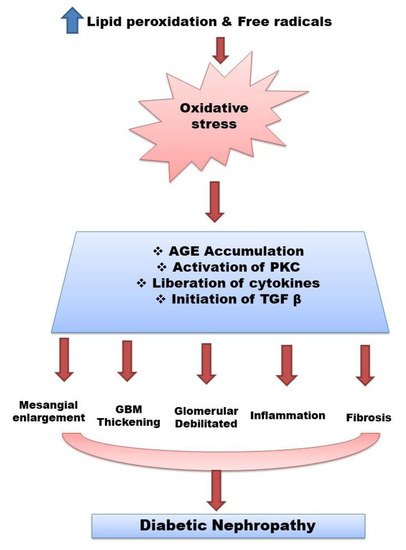
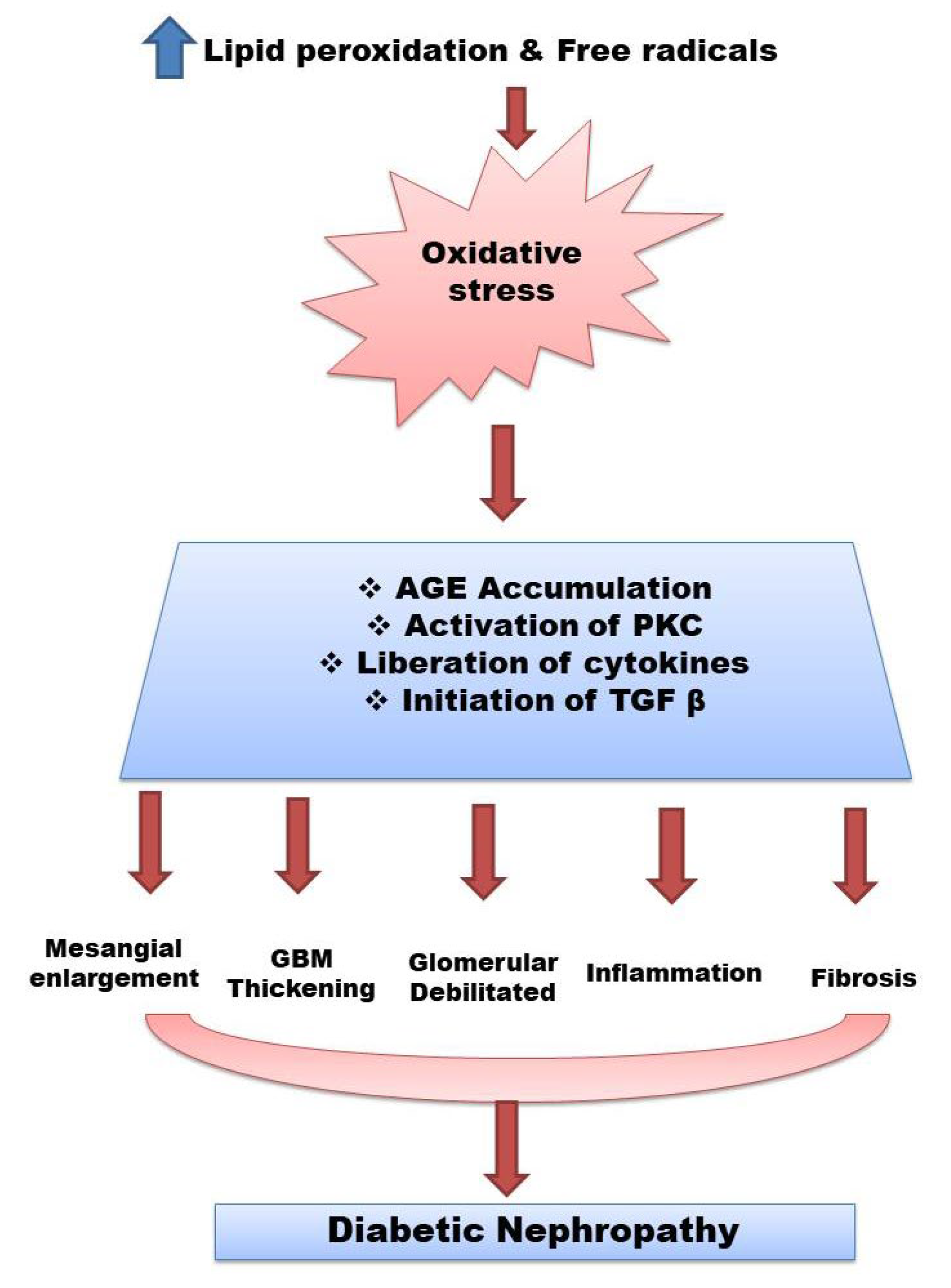 Figure 1. Pathophysiology of diabetes nephropathy. Abbreviations: Advanced glycosylated end products (AGEs), glomerular basement membrane (GBM), protein kinase C (PKC), transforming growth factor beta(TGF-β).
Figure 1. Pathophysiology of diabetes nephropathy. Abbreviations: Advanced glycosylated end products (AGEs), glomerular basement membrane (GBM), protein kinase C (PKC), transforming growth factor beta(TGF-β).3. Medicinal Plants and Diabetic Nephropathy
Natural remedies have received much interest in terms of medical research in recent years due to their capacity to heal a variety of ailments. Despite huge improvements in the pharmaceutical drug industry over the last two decades, the number of medications that can slow the progression of diabetes nephropathy is still restricted. Herbal treatments are popular because of their accessibility, cost effectiveness, lesser side effects, and high tolerance. Table 1 represents the list of a few medicinal plants and their action on DN.Table 1.
List of medicinal plants and their action on diabetic nephropathy.
| Botanical Name | Family | Common Name | Parts Used | Mechanism of Action | References |
|---|---|---|---|---|---|
| Zingiber officinale | Zingiberacae | Ginger | Rhizomes | Lowers lipid peroxidation and enhances plasma antioxidant capacity | [8] |
| Allium sativum | Amaryllidaceae | Garlic | Bulb | The anti-glycation and hypolipidemic properties of aged garlic extract may be responsible for its protection against DN. | [9] |
| Cinnamon cassia | Lauraceae | Cinnamon | Bark | Prevents AGE accumulation in vivo and reduced alteration in the renal function of diabetic rats. | [10] |
| Syzygiumaromaticum | Myrtaceae | Clove | Roots | Decreases lipid peroxidation and cytokine release. | [11] |
| Curcuma longa | Zingiberaceae | Turmeric | Seeds | Curcumin’s protective impact in DN may be due to the suppression of NLRP3 inflammasome activation. | [12] |
| Camellia sinensis | Theaceae, | Green tea | Leaves | Green tea protects the kidneys from DN by preventing glomerular hyperfiltration, hypertrophic alterations, and protein loss in the urine. | [13] |
| Psidium guajava | Myrtaceae | Guava | Fruits | Anti-inflammatory, antioxidant and anti-gylcative properties. | [14] |
| Trigonella foenumgraecum | Myrtaceae | Fenugreek | Seeds | By reducing renal oxidative stress and inhibiting the TGF-β1/CTGF signaling pathway. | [15] |
| Phyllanthus emblica | Grossulariaceae | Gooseberry | Fruits | Gallotanin, an important ingredient of gooseberry, was found to be efficient in lowering plasma creatinine levels and reducing apoptosis by blocking poly ADP-ribose polymerase cleavage. | [16] |
| Piper nigrum | Piperaceae | Pepper | Fruit | Inhibited NF-κB and NLRP3 activation. | [17] |
| Coriandrum sativum | Apiaceae | Coriander | Seed | Delayed progression of DN, by inhibiting AGE. | [18] |
| Ocimum sanctum | Labiatae | Tulasi | Leaves | Antioxidant and anti-inflammatory activity. | [19] |
| Pterocarpus santalinus | Fabaceae | Red sandalwood | Bark | Antioxidant activity. | [20] |
3.1. Ginger
Often known as Zingiber officinale, Zingiberacea is the most commonly used herbal supplement in the world. Patients seek therapy from it for a variety of ailments, though it is commonly utilized for culinary purposes. Many analytical methods identify approximately 115 components in fresh and dried ginger. Fresh ginger contains a large amount of gingerol. Some of the terpene components present in ginger includes β-bisabolene, zingiberene, α-farnesene, β-sesquiphellandrene and α-curcumene, which are also identified to be the main active components of ginger essential oil [7]. Plants possess many medicinal uses including anti-diabetic, nephroprotective, hepato-protective, anti-cancer, antioxidant, analgesic, immunomodulatory, and anti-inflammatory properties [21,22,23][21][22][23]. In ayurveda, ginger is recommended for its traditional properties such as cardio protection, appetite stimulant, anti-asthmatic, for constipation, pain reduction, for normalization of blood circulation etc. [24].3.2. Garlic
Garlic, also known as Allium sativum, belongs to the family of Amaryllidaceae. Due to its vast range of uses as a traditional spice and a key element in folk medicine prescriptions, garlic is considered an essential herb. A large amount has been learned regarding the various photochemical characteristics of garlic [26,27][25][26]. Many recent studieshave established garlic’s functional activity in cardiovascular disease [28,29][27][28] and cancer. It possesses anti-inflammatory, cardio-protective, anti-diabetic, antioxidant, reno-protective, anti-hypertensive, anti-microbial, immunomodulation, and antiviral effects [28,29,30][27][28][29]. According to a recent study by Arellano et al. [31][30], allicin’s anti-inflammatory mechanism is thought to be based on the reduction of NF-κB activation, which is involved in the synthesis of pro-inflammatory cytokines. Furthermore, allicin inhibits the activity of the NF-κB regulatory unit, which is essential for pro-inflammatory cytokine transcription activation and activation of TGF-β1 receptors. These inflammatory markers were found to be elevated in the diabetic group but controlled by treatment with allicin.3.3. Cinnamon
Cinnamon Cassia has been a popular spice in several cultures around the world for many years. Other than its use for culinary purposes, it is recommended as a therapy for treatment of digestive, respiratory, and gynecological disorders. Various parts of the cinnamon tree, such as leaves, flowers, fruits, bark, and roots, have culinary and medicinal properties. The volatile oils extracted from the bark, leaves, and roots have a wide range of chemical compositions, implying that their pharmacological effects may vary as well. Active constituents of this plant include cinnamaldehyde, eugenol, and camphor. Cinnamon is mainly used in the treatment of cancer, inflammation, for its cardio-protective effects, as an antioxidant, to prevent migraines, as a treatment for Alzheimer’s disease, and for its anti-microbial activities [33,34][31][32]. Cinnamon and its procyanidin-B2 (PCB2) enriched fraction have good inhibitory effects in vitro, inhibiting the generation of AGE in diabetic nephropathic rats. Streptozotocin induced diabetic rats were given 3 percent cinnamon or 0.002 percent PCB-fraction for 12 weeks. At the end of the experiment, biochemical analysis of urine and blood had been performed. The renal biomarkers were evaluated using immunohistochemistry, immunoblotting, and reverse transcription polymerase chain reaction (RT-PCR) as measures of renal function. Suppression of glycation-mediated red blood cells-immunoglobulin G (RBC-IgG) cross-links and HbA1c augmentation in cinnamon and PCB2 treated diabetic mice was observed. It also reduced the significantly advanced glycated end product, N-carboxylmethyl lysine (CML), from accumulating in diabetic kidneys. Treatment with cinnamon inhibited the advanced glycation end product mediated decrease of the expression of glomerular podocyte proteins, including nephrin and podocin.3.4. Clove
Commonly known as (Syzygiumaromaticum) clove, this precious spice belongs to the family of Mirtaceae which has been used for centuries as medicine and as a food preservative because of its antimicrobial and antioxidant properties. It contains a variety of volatile oil in it. Caffeic, ferulic, salicylic, and ellagic acids are some of the phenolic acids present in clove. It also contains some flavonoids, including quercetin and kaempferol. The antioxidant activities of ethanol and water extracts of a different spices, including pepper, onion, cinnamon, garlic, mint, ginger, and clove, were investigated, and it was discovered that clove has the most antioxidant properties of all the spices tested [36,37,38][33][34][35].3.5. Turmeric
Turmeric is a spice that has grabbed scientific and culinary attention, and it is a ginger related rhizomatous herbaceous perennial plant [39][36]. Most research concludes that the medicinal property of turmeric is mainly due to the active constituent named curcumin. It is basically grown in tropical and subtropical areas of the world and is mainly cultivated in countries such as India and China. Turmeric, also called curcumin, is the main natural polyphenol which is found in the rhizome of Curcuma longa (turmeric) and is called diferuloyl methane. It has a variety of medicinal properties, including treatment for diabetic wounds, rheumatism, anti-cancer, anti-hyperlipidemia, inflammation treatment, antimicrobial, anti-fertility, anti-venom, liver toxicity, renal injury, skin disease, and anti-platelets, among others. Due to the antioxidant, anti-mutagenic, anti-inflammatory, antibacterial, and anticancer characteristics, Curcuma longa has been used as a medicinal herb in Asian countries for centuries [40,41,42,43][37][38][39][40].3.6. Green Tea
Commonly known as Camellia sinensis and belonging to the Theaceae family, green tea is an ancient beverage with many therapeutic properties. It has anti-cancer, anti-inflammatory, anti-arthritic, anti-bacterial, anti-viral, anti-angiogenic, anti-oxidative, neuroprotective and anti-hyperlipidemic properties [49,50,51,52,53,54,55][41][42][43][44][45][46][47]. Green tea flavonoids have anti-inflammatory and anti-oxidative properties. This is due to the presence of polyphenols and flavonoids which protect the kidney from diabetes and hypertension-related renal oxidative stress. In diabetic patients who had been receiving the highest recommended dose of renin-angiotensin system inhibitor, there had been a reduction in albuminuria after treatment with green tea polyphenol. This activity may be due to the inhibition of podocyte apoptosis via activation of the wingless-related integration site (WNT) pathway. The result of the restudyearch confirms in a clinical context that activating the WNT pathway and lowering podocyte apoptosis with green tea could reduce albuminuria in DN [56][48].3.7. Guava
The guava tree (Psidium guajava Linn.), of the Myrtaceae family, primarily grows in tropical and subtropical regions. Its fruits and leaves have medicinal purposes, the fruit being used as a food source and processed into juice as well as jam [61][49]. Aside from these applications, Gutiérrez et al. [62][50] described the medicinal properties of guava, such as its use in liver disorders, allergy, its free radical scavenging property, its toxicity to normal cells, and its use for diabetes, cough, inflammation, cardiac disorders, pain, etc. Lin C.Y., et al. [14] assessed the renal protective effects of guava aqueous extract (GAE) and ethanol extract (GEE) in diabetic mice by analyzing the concentration of phenolic acid and flavonoids in extracts of guava fruits. Myricetin, caffeic acid, and quercetin were present in higher quantities in GAE, and the results indicated that GAE reduced interleukin (IL)-6, reactive oxygen species, tumor necrosis factor, and IL-1 levels in the kidney. Fructose, N-(carboxymethyl) lysine, and pentosidine levels in the kidneys were reduced by 2% GAE and GEE treatments. These findings demonstrate that it has renal protective action through its anti-oxidative properties.3.8. Fenugreek
It is commonly known as Trigonella foenumgraecum L., belonging to the family Fabaceae, and is one of the oldest plants found in India and Northern Africa. Powders and extracts are prepared from its leaves and seeds, which have medicinal value. It has been used for bread production, as a supplement to wheat and maize flour, and as part of the general population’s daily diet [69,70][51][52]. Many pre-clinical and clinical studies have reported that extracts of fenugreek seed have been used to remedy anti-diabetic, hypo-cholesterolemic, and antioxidant properties. The ability of fenugreek seed powder (FSP) to reverse oxidative damage induced by oxygen-free radicals has been demonstrated as an indication of antioxidant enzymes, implying that it may have antioxidant properties. Because of diminished ROS generation and antioxidant enzyme stimulation, AGE production has been reduced and NF-kB has been activated. There is a significant reduction in IL-6 and inflammation in FSP treated diabetic rats, which could be associated with an elevation in antioxidant enzymes [71][53].3.9. Gooseberry
Indian gooseberry or Amla is familiarly known as Phyllanthus emblica andbelongs to the family of Grossulariaceae. It is mainly grown in tropical parts of Southeast Asia. The fruit and its extract have been used for the treatment of diabetes, pain, cancer, obesity, constipation, and tumor treatment [73][54]. It is also commonly used as a gentle laxative. Amla powder has a lot of phenolic antioxidants in it. According to the high performance liquid chromatography (HPLC) study, the ethanol extract of Amla contains a large amount of gallic acid, ellagic acid, and catechin hydrate. These natural antioxidants have the potential to effectively scavenge free radicals and reactive oxygen species (ROS). In sodium arsenate induced rats with renal disease, the 2K1C rats had considerably higher uric acid and creatinine levels than the control group in this investigation. After the treatment with amla powder, creatinine and uric acid in the plasma of 2K1C rats were normalized [74][55].3.10. Oats
A high intake of whole-grain foods, such as oats, is mainly associated with a lower risk of cardiovascular complications and type-2 diabetes. Al Maliki et al. conducted an anti-diabetic study on oats. In this restudyearch, streptozotocin (STZ) induced diabetic rats developed DN as determined by elevated serum blood urea nitrogen (BUN), creatinine, creatinine clearance, and 24-h urine albumin. Oats supplementation for 21 weeks considerably improved renal function and had a hypoglycemic impact, which contributed to the reversal of DN [75][56].3.11. Pepper
Piper nigrum belongs to the family Piperaceae and is the most prominent species of this genus. Piperine is also called the "father of spices.” It has beneficial health and disease-inhibiting properties and possesses antiviral, immunomodulatory, anti-inflammatory, anti-pyretic, and bioavailability enhancement [76,77,78][57][58][59]. The main alkaloidal phenolic component of black pepper is piperine, which possesses different medicinal uses, including antioxidant properties, and it activates digestive enzymes in pancreas and reduces lipid peroxidation. Samra Y.A., et al. [17] described the study on the combination of cepharanthine and piperine on the progression of diabetes and concluded that animal groups after treatment showed a significant decrease in kidney weight. Serum creatinine was increased in the animal group treated with streptozotocin, which was significantly reduced after treating with piperine and cepharanthin and their combination. Piperine is an antioxidant that prevents lipid peroxidation and quenches free radicals and ROS, thus protecting against oxidative damage.3.12. Coriander
Commonly known as coriandrum sativum L., coriander belongs to the family of Apiaceae. The main phytoconstituents of coriander are alkaloids, flavones, tannins, resins, sugars, alkaloids, anthraquinones, and fixed oil sterols, [79,80][60][61]. The chief components of coriander fruit are essential oil and fatty oil. Fatty acids present in coriander include petroselinic acid (cis-6-octadecenoic acid, 18:1), linoleic acid (18:2), oleic acid (18:1), and palmitic acid (16:0). Like other green leafy vegetables, coriander is also a good source of vitamins, minerals, and iron, with the least saturated fat and cholesterol, in addition to zinc, thiamine, and dietary fiber [81,82][62][63].3.13. Silymarin
Silymarin increases protein synthesis and cellular regeneration in the kidney epithelium via stimulating ribonucleic acid (RNA) polymerase I. These effects are thought to be mostly caused by silybin, Lysiuk, and silichristin, while silidianin has little effect. According to the restudyearch, silymarin may be especially beneficial in cases when the renal epithelium is necrotic [83][64]. Silymarin bioflavonoid has antioxidant and anti-inflammatory properties, and it induces protein synthesis, suppresses lipid peroxidation, leukotriene and prostaglandin formation, and neutrophil migration [84,85,86][65][66][67]. Silymarin may have beneficial effects in the treatment of patients with renal insufficiency. Recent studies show that treating hemodialysis patients with silymarin alone or in combination with vitamin-E lowers plasma MDA levels while increasing blood glutathione peroxidase and haemoglobin levels [87][68]. In alloxan-induced diabetes rats, treatment with silymarin lowered the damage to the kidney and restored superoxide dismutase, catalase enzyme activity and glutathione peroxidase. Extract of milk thistle inhibits diabetic renal damage in streptozotocin induced diabetes rats, most possibly via elevating glutathione peroxidase and catalase activity and lowering lipid peroxidation in renal tissue [88][69].3.14. Tulasi
The scientific name of tulasi is Ocimum sanctum (family of Labiatae). This plant is also known for its medicinal properties. The medicinal herb is employed in indigenous medicine. Eugenol, carvacrol, urosolic acid, rosmarinic acid, linalool, β-caryophyllene, eugenic acid, geraneol, and ocimene are among the principal chemical elements in the aqueous extract of the leaves [19]. This plant possesses anti-stress, anti-asthmatic, anti-fungal, anti-bacterial, anti-oxidant, anti-viral, anti-tumor, gastric anti-ulcer activity, immunostimulant and anti-mutagenic activities. Additionally, these plants have been used to treat diabetes, cataracts, hypertension, diarrhea, cardiac toxicity, allergic hypercholesterolemia, depression, thyroid, neurotoxicity, and rheumatoid arthritis. Other medicinal properties include the chemo-preventive, anti-microbial, anti-inflammatory, radio protective, anti-carcinogenic, analgesic, anti-pyretic, memory enhancement, anti-tussive, anti-fertility, anti-emetic, anti-spasmodic, anti-stress, and the anti-coagulant [91][70].3.15. Red Sandal Wood
Belonging to the family Fabaceae, treatment with Pterocarpus santalinus resulted in a considerable reduction in blood sugar levels as well as an improvement in glucose tolerance tests. The extract of red sandalwood showed an antioxidant effect, as it reduced MDA levels. The extract also increased antioxidants, catalase superoxide dismutase and lowered lipid peroxidase synthesis, as determined by a thiobarbituric acid reactive substance. Serum creatinine and urine albumin were reduced after the treatment. The result was supported by histological report of the kidney for DN, and it indicated that combination therapy for 16 weeks indicated a reduction in levels of lipid profiles and an increase in the high density lipoprotein cholesterol of diabetes treated rats [20,93][20][71].References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109–119.
- Zhang, S.; Ge, Q.; Chen, L.; Chen, K. Studies of the Anti-Diabetic Mechanism of Puerarialobata Based on Metabolomics and Network Pharmacology. Processes 2021, 9, 1245.
- Magee, C.; Grieve, D.J.; Watson, C.J.; Brazil, D.P. Diabetic nephropathy: A tangled web tounweave. Cardiovasc. Drugs Ther. 2017, 31, 579–592.
- Schena, F.P.; Gesualdo, L. Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 2005, 16, S30–S33.
- Vinod, P.B. Pathophysiology of diabetic nephropathy. Clin. Queries Nephrol. 2012, 1, 121–126.
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011, 2, 243–247.
- Ayse, N.; Duygu, A.T.; Hakký, A.I.; Tansel, O.Y.; Ýsmet, D.G.; Ismail, K. Antimicrobial and cytotoxic activities of Zingiber officinalis extracts. FABAD J. Pharm. Sci. 2008, 33, 77–86.
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycaemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389.
- Shiju, T.M.; Rajesh, N.G.; Viswanathan, P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2013, 45, 18–23.
- Muthenna, P.; Raghu, G.; Kumar, P.A.; Surekha, M.V.; Reddy, G.B. Effect of cinnamon and its procyanidin-B2 enriched fraction on diabetic nephropathy in rats. Chem. Biol. Interact. 2014, 222, 68–76.
- Joshua, S.; Sweeten, O.; Ifunaya, C.C. Evaluation of Renal Function of Streptozotocin (STZ)-Induced Diabetic Albino Rats Treated with Syzygiumaromaticum (Clove). Int. J. Nephrol. 2021, 8, 17–26.
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin ameliorates diabetic nephropathy by suppressing NLRP3 inflammasome signalling. BioMed. Res. Int. 2017, 2017, 1516985.
- Kang, M.Y.; Park, Y.H.; Kim, B.S.; Seo, S.Y.; Jeong, B.C.; Kim, J.I.; Kim, H.H. Preventive effects of green tea (Camellia sinensis var. assamica) on diabetic nephropathy. Yonsei Med. J. 2012, 53, 138–144.
- Lin, C.Y.; Yin, M.C. Renal Protective Effects of Extracts from Guava Fruit (Psidium guajava L.) in Diabetic Mice. Plant Foods Hum. Nutr. 2012, 67, 303–308.
- Jin, Y.; Shi, Y.; Zou, Y.; Miao, C.; Sun, B.; Li, C. Fenugreek prevents the development of STZ-induced diabetic nephropathy in a rat model of diabetes. Evid. Based Complement. Altern. Med. 2014, 2014, 259368.
- Chandak, P.G.; Gaikwad, A.B.; Tikoo, K. Gallotannin ameliorates the development of streptozotocin-induced diabetic nephropathy by preventing the activation of PARP. Phytother. Res. 2009, 23, 72–77.
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199.
- Kajal, A.; Singh, R. Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS ONE 2019, 14, e0213147.
- Pandiri, I.; Moni, A. Ocimum herb species: A potential treatment strategy for diabetic kidney disease. J. Adv. Biotechnol. Exp. Ther. 2018, 1, 88–91.
- Halim, M.E.; Misra, A. The effects of the aqueous extract of Pterocarpus santalinus heartwood and vitamin E supplementation in streptozotocin-induced diabetic rats. J. Med. Plants Res. 2011, 5, 398–409.
- Al-Tahtawy, R.H.M.; El-Bastawesy, A.M.; Monem, M.G.A.; Zekry, Z.K.; Al-Mehdar, H.A.; El-Merzabani, M.M. Antioxidant activity of the volatile oils of Zingiber officinale (ginger). Spatula DD 2011, 1, 1–8.
- Dissanayake, K.G.; Waliwita, W.A.; Liyanage, R.P. A review on medicinal uses of Zingiber officinale (ginger). Int. J. Health Sci. 2020, 10, 142–148.
- Mishra, S. Bhavaprakasha Nighantu Vidyodhinihindi Commentary, 10th ed.; Chaukhamba Orientalia: Varanasi, India, 2002; p. 15.
- Sun, J.; Zhao, Y.; Hu, J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS ONE 2013, 8, e67078.
- Asdaq, S.M.B.; Challa, O.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Asad, M. The Potential Benefits of Using Garlic Oil and Its Active Constituent, Dially Disulphide, in Combination with Carvedilol in Ameliorating Isoprenaline-Induced Cardiac Damage in Rats. Front. Pharmacol. 2021, 12, 739758.
- Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M. Cardioprotective Potential of Garlic Oil and Its Active Constituent, Diallyl Disulphide, in Presence of Carvedilol during Chronic Isoprenaline Injection-Mediated Myocardial Necrosis in Rats. Molecules 2021, 26, 5137.
- Rahman, K. Historical perspective on garlic and cardiovascular disease. J. Nutr. 2001, 131, 977S–979S.
- Asdaq, S.M.B.; Lokaraja, S.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Almutiri, A.H.; Nagaraja, S.; Imran, M. Potential Interaction of Fresh Garlic with Metformin during Ischemia-Reperfusion Induced Cardiac Injury in Diabetic Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 9739089.
- Davis, S.R. An overview of the antifungal properties of allicin and its breakdown products-the possibility of a safe and effective antifungal prophylactic. Mycoses 2005, 48, 95–100.
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory effects of the nutraceutical garlic derivative allicin in the progression of diabetic nephropathy. Int. J. Mol. Sci. 2018, 19, 3107.
- Lee, S.H.; Lee, S.Y.; Son, D.J.; Lee, H.; Yoo, H.S.; Song, S.; Oh, K.W.; Han, D.C.; Kwon, B.M.; Hong, J.T. Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem. Pharmacol. 2005, 69, 791–799.
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942.
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506.
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759.
- Hussain, S.; Rahman, R.; Mushtaq, A.; Zerey-Belaskri, A.E. Clove: A review of a precious species with multiple uses. Int. J. Chem. Biochem. 2017, 11, 129–133.
- Jonah, A.O.; Enoh, E. In vitro Anti-trypanosomal activity of curcumin isolated from Curcuma longa (Turmeric) rhizomes. J. Entomol. Zool. Stud. 2020, 8, 729–731.
- Sahebkar, A.; Serbanc, M.C.; Ursoniuc, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909.
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474.
- Lysiuk, R.; Mansuetusmboya, J. Herbal drugs for the treatment of diabetic nephropathy: Current status and prospects for the application. J. Kidney Treat. Diagn. 2020, 3, 3–4.
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945.
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13.
- Huang, Y.C.; Zhu, H.M.; Cai, J.Q.; Huang, Y.Z.; Xu, J.; Zhou, Y.; Chen, X.H.; Li, X.Q.; Yang, Z.M.; Deng, L. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1407–1415.
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341.
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529.
- Roccaro, S.A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973.
- Sartippour, M.R.; Shao, Z.M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311.
- Weber, J.M.; Ruzindana-Umunyana, A.; Imbeault, L.; Sircar, S. Inhibition of adenovirus infection and adenain by green tea catechins. Antivir. Res. 2003, 58, 167–173.
- Ahn, T.G.; Kim, H.K.; Park, S.W.; Kim, S.A.; Lee, B.R.; Han, S.J. Protective effects of green tea polyphenol against cisplatin-induced nephrotoxicity in rats. Obstet. Gynecol. Sci. 2014, 57, 464–470.
- Deguchi, Y.; Miyazaki, K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr. Metab. 2010, 7, 9.
- Gutiérrez, R.M.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27.
- Morcos, S.R.; Elhawary, Z.; Gabrial, G.N. Protein rich food mixtures for feeding the young in Egypt. 1. Formulation. Z. Ernährungswissenschaft 1981, 20, 275–282.
- Bdel-Bary, J.A.; Abdel Hassan, I.A.; Al-Hakiem, M.H.H. Hypoglycemic and antihyperglycemic effect of Trigonellafoenumgraecum leaf in normal and alloxan induced diabetic rats. J. Ethnopharmacol. 1997, 58, 149–155.
- Sayed, A.A.; Khalifa, M.; Abd el-Latif, F.F. Fenugreek attenuation of diabetic nephropathy in alloxan-diabetic rats. J. Physiol. Biochem. 2012, 68, 263–269.
- Unander, D.W.; Webster, G.L.; Blumberg, B.S. Records of usage or assays in Phyllanthus (Euphorbiaceae) I. Subgenera Isocladus, Kirganelia, Cicca and Emblica. J. Ethnopharmacol. 1990, 30, 233–264.
- Zhao, T.; Sun, Q.; Marques, M.; Witcher, M. Anticancer properties of Phyllanthus emblica (Indian gooseberry). Oxidative Med. Cell Longev. 2015, 2015, 950890.
- Al-Malki, A.L. Oat protects against diabetic nephropathy in rats via attenuating advanced glycation end products and nuclear factor kappa B. Evid. Based Complement. Altern. Med. 2013, 2013, 609745.
- Arcaro, C.A.; Gutierres, V.O.; Assis, R.P.; Moreira, T.F.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin-diabetic rats. PLoS ONE 2014, 9, e113993.
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food. sci. Nutr. 2007, 47, 735–748.
- Lee, E.B.; Shin, K.H.; Woo, W.S. Pharmacological study on piperine. Arch. Pharm. Res. 1984, 7, 127–132.
- Önder, A. Coriander and its Phytoconstituents for the Beneficial Effects. Potential of Essential Oils; IntechOpen: London, UK, 2018; Volume 26, pp. 165–185.
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem. 2012, 132, 841–848.
- Uitterhaegen, E.; Sampaio, K.A.; Delbeke, E.I.P.; Greyt, W.D.; Cerny, M.; Evon, P.; OthmaneMerah, O.; Talou, T.; Stevens, C.V. Characterization of French coriander oil as a source of petroselinic acid. Molecules 2016, 21, 1202.
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428.
- Hahbazi, F.; Dashti-Khavidaki, S.; Khalili, H.; Lessan-Pezeshki, M. Potential renoprotective effects of silymarin against nephrotoxic drugs: A review of literature. J. Pharm. Sci. 2012, 15, 112–123.
- Loguerico, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301.
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063.
- Dixit, N.; Baboota, S.; Kohli, K.; Ahmad, S.; Ali, J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharmacol. 2007, 39, 172–179.
- Roozbeh, J.; Shahriyari, B.; Akmali, M.; Vessal, G.; Pakfetrat, M.; Jalali, G.A.R.; Afshariani, R.; Hasheminasab, M.; Ghahramani, N. Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis. Ren. Fail. 2011, 33, 118–123.
- Vessal, G.; Akmali, M.; Najafi, P.; Moein, M.R.; Sagheb, M.M. Silymarin and milk thistle may prevent the progression of dibatic nephropathy in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 733–739.
- Cohen, M. Tulsi—Ocimum sanctum: An herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259.
- Alanazi, A.Z.; Mohany, M.; Alasmari, F.; Mothana, R.A.; Alshehri, A.O.; Alhazzani, K.; Ahmed, M.M.; Al-Rejaie, S.S. Amelioration of Diabetes-Induced Nephropathy by Loranthusregularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia. Appl. Sci. 2021, 11, 4548.
More
