Cardiovascular disease (CVD) is one of the greatest public health concerns and is the leading cause of morbidity and mortality in the United States and worldwide. CVD is a broad yet complex term referring to numerous heart and vascular conditions, all with varying pathologies. Macrophages are one of the key factors in the development of these conditions. Macrophages play diverse roles in the maintenance of cardiovascular homeostasis, and an imbalance of these mechanisms contributes to the development of CVD. Here the researchers provide an overview of the diversity of macrophages, their roles in maintaining tissue homeostasis within the heart and vasculature, and potential mechanisms through which macrophages may be targeted in the treatment of CVD.
- macrophages
- cardiovascular disease
- CVD
- atherosclerosis
- heart failure
1. Introduction
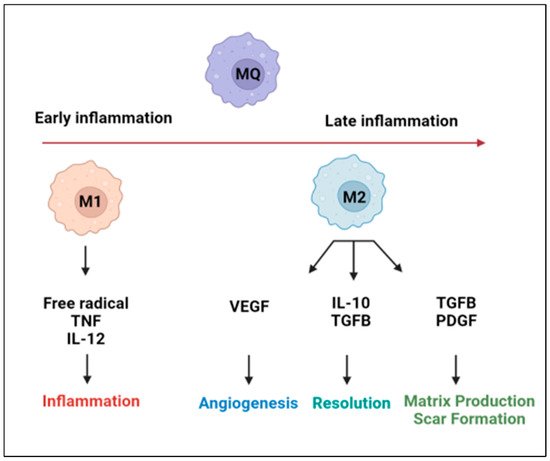
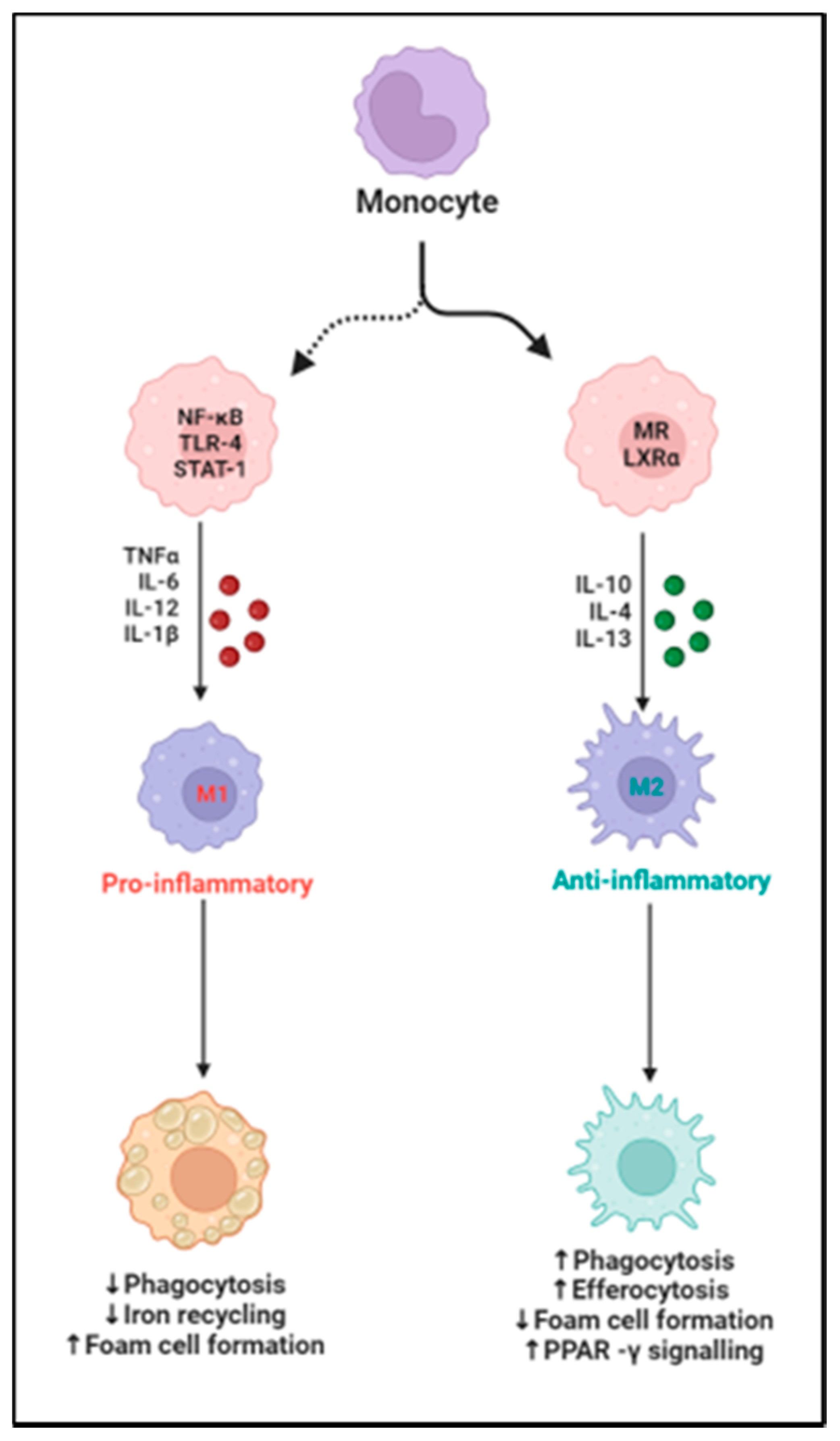
2. Targeting Macrophages in the Treatment of CVD
References
- Kddcup1999. 1991. Available online: http://kdd.ics.uci.edu/databases/ (accessed on 28 October 1999).
- Bhatnagar, P.; Wickramasinghe, K.; Williams, J.; Rayner, M.; Townsend, N. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015, 101, 1182–1189.
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212.
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635.
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114.
- Liu, M.; Li, X.-C.; Lu, L.; Cao, Y.; Sun, R.-R.; Chen, S.; Zhang, P.-Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharm. Sci. 2014, 18, 2918–2926.
- Dube, P.; DeRiso, A.; Patel, M.; Battepati, D.; Khatib-Shahidi, B.; Sharma, H.; Gupta, R.; Malhotra, D.; Dworkin, L.; Haller, S.; et al. Vascular calcification in chronic kidney disease: Diversity in the vessel wall. Biomedicines 2021, 9, 404.
- Lioufas, N.M.; Pedagogos, E.; Hawley, C.M.; Pascoe, E.M.; Elder, G.J.; Badve, S.V.; Valks, A.; Toussaint, N.D. Aortic calcification and arterial stiffness burden in a chronic kidney disease cohort with high cardiovascular risk: Baseline characteristics of the impact of phosphate reduction on vascular end-points in chronic kidney disease trial. Am. J. Nephrol. 2020, 51, 201–215.
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522.
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030.
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528.
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440.
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090.
- Lavine, K.J.; Pinto, A.R.; Epelman, S.; Kopecky, B.J.; Clemente-Casares, X.; Godwin, J.; Rosenthal, N.; Kovacic, J.C. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4). J. Am. Coll. Cardiol. 2018, 72, 2213–2230.
- Wang, Z.; Koenig, A.L.; Lavine, K.J.; Apte, R.S. Macrophage plasticity and function in the eye and heart. Trends Immunol. 2019, 40, 825–841.
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131.
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019, 139, 1289–1299.
- Hanna, A.; Frangogiannis, N.G. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863.
- Ceneri, N.; Zhao, L.; Young, B.D.; Healy, A.; Coskun, S.; Vasavada, H.; Yarovinsky, T.O.; Ike, K.; Pardi, R.; Qin, L.; et al. Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1β production. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 328–340.
- DeBerge, M.; Shah, S.J.; Wilsbacher, L.; Thorp, E.B. Macrophages in heart failure with reduced versus preserved ejection fraction. Trends Mol. Med. 2019, 25, 328–340.
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Oddi Erdle, C.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 blockade in recently decompensated systolic heart failure: Results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 2017, 10, e004373.
- Buckley, L.F.; Carbone, S.; Trankle, C.R.; Canada, J.M.; Erdle, C.O.; Regan, J.A.; Viscusi, M.M.; Kadariya, D.; Billingsley, H.; Arena, R.; et al. Effect of Interleukin-1 Blockade on Left Ventricular Systolic Performance and Work: A Post-Hoc Pooled Analysis of Two Clinical Trials. J. Cardiovasc. Pharmacol. 2018, 72, 68.
- Yokoe, I.; Kobayashi, H.; Giles, J.; Yoneyama, K.; Kitamura, N.; Takei, M. Impact of tocilizumab on N-terminal pro-brain natriuretic peptide levels in patients with active rheumatoid arthritis without cardiac symptoms. Scand. J. Rheumatol. 2018, 47, 364–370.
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016, 37, 2406–2413.
- Zheng, Y.; Qin, L.; Zacarías, N.V.O.; De Vries, H.; Han, G.W.; Gustavsson, M.; Dabros, M.; Zhao, C.; Cherney, R.J.; Carter, P.; et al. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature 2016, 540, 458–461.
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic valve stenosis: From basic mechanisms to novel therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900.
- Johnson, T.A.; Singla, D.K. PTEN inhibitor VO-OHpic attenuates inflammatory M1 macrophages and cardiac remodeling in doxorubicin-induced cardiomyopathy. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1236–H1249.
- Munjal, C.; Jegga, A.; Opoka, A.M.; Stoilov, I.; Norris, R.A.; Thomas, C.J.; Smith, J.M.; Mecham, R.P.; Bressan, G.M.; Hinton, R.B. Inhibition of MAPK-Erk pathway in vivo attenuates aortic valve disease processes in Emilin1-deficient mouse model. Physiol. Rep. 2017, 5, e13152.
- Xu, M.; Li, X.; Song, L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm. Biol. 2020, 58, 655–663.
- Zhao, M.; Li, F.; Jian, Y.; Wang, X.; Yang, H.; Wang, J.; Su, J.; Lu, X.; Xi, M.; Wen, A.; et al. Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced glycolysis. Eur. J. Pharmacol. 2020, 871, 172916.
- Kim, A.; Xu, N.; Umeyama, K.; Hulin, A.; Ponny, S.R.; Vagnozzi, R.J.; Green, E.A.; Hanson, P.; McManus, B.M.; Nagashima, H.; et al. Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation 2020, 141, 132–146.
- Choi, B.; Kim, E.-Y.; Kim, J.-E.; Oh, S.; Park, S.-O.; Kim, S.-M.; Choi, H.; Song, J.-K.; Chang, E.-J. Evogliptin suppresses calcific aortic valve disease by attenuating inflammation, fibrosis, and calcification. Cells 2021, 10, 57.
- Nguyen, P.A.; Won, J.S.; Rahman, M.K.; Bae, E.J.; Cho, M.K. Modulation of Sirt1/NF-κB interaction of evogliptin is attributed to inhibition of vascular inflammatory response leading to attenuation of atherosclerotic plaque formation. Biochem. Pharmacol. 2019, 168, 452–464.
- Kain, D.; Amit, U.; Yagil, C.; Landa, N.; Naftali-Shani, N.; Molotski, N.; Aviv, V.; Feinberg, M.S.; Goitein, O.; Kushnir, T.; et al. Macrophages dictate the progression and manifestation of hypertensive heart disease. Int. J. Cardiol. 2016, 203, 381–395.
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211.
