Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 4 by Camila Xu.
It quickly became obvious that emergency ultrasound, or point of care ultrasound (POCUS), has some very interesting aspects in the context of the emergency room (ER), allowing the physician to immediately obtain images of the patient during the first visit and thus rule out major pathologies immediately. Chest pain often is associated with dyspnea or shortness of breath (SOB), which is in itself one of the most common causes of emergency room access. The origin of these symptoms is often sought in heart and lung diseases.
- POCUS
- point of care ultrasound
- emergency medicine
- ultrasound
1. POCUS in Chest Pain: What Role Could It Have?
Over the last twenty years, ultrasound has gained growing importance in the emergency department. Initially, ultrasounds were usually performed on trauma patients to evaluate whether patients needed surgery when CT (computed tomography) scans were not as widely spread as they are now [1].
It quickly became obvious that emergency ultrasound, or point of care ultrasound (POCUS), has some very interesting aspects in the context of the emergency room (ER), allowing the physician to immediately obtain images of the patient during the first visit and thus rule out major pathologies immediately [2].
Ultrasound has gained popularity in many different conditions and clinical presentations, from critically ill patients [3] to patients with musculoskeletal pain [4].
While on the one hand ultrasound presents a number of advantages, it also presents some potential pitfalls, particularly because it is highly influenced by the operator’s ability to perform it [5].
However, particularly in overcrowded ERs, ultrasound can aid the emergency physician in the diagnosis and stratification of patients.
Chest pain is one of the main causes of admission to the emergency room, and it has been estimated that about 1 million people refer to the ER for this reason in Italy every year. It has been estimated that in about half of the cases the cause is cardiac, yet in the other half it is not [6]. While acute coronary syndrome (ACS) is the first cause that needs to be ruled out, there are a number of other conditions that can present with chest pain [7]. Additionally, the characterization of the pain, which should in theory aid the staff towards the correct diagnosis, is not reliable in a large number of patients due to a number of psychosocial and physiological factors [6].
2. Cardiac Causes
As discussed above, chest pain is the second most frequent cause of accessing the emergency room [7]. In case of suspicion of ischemic chest pain, the patient is evaluated with a 12-lead electrocardiogram and troponin dosage, which are necessary for the diagnosis of ACS [8]. In the event of major changes in the ECG (electrocardiogram) or troponin values, the patient is urgently evaluated by the cardiologist. Once the diagnosis of ACS has been ruled out, there are many other cardiac clinical pictures in which the help of ultrasound can be decisive for the emergency room physician. There are, indeed, many other heart diseases that can occur in the emergency room with chest pain, such as aortic dissection, pericarditis, and pulmonary embolism. The use of echocardiography by the emergency physician has several advantages, such as a targeted and early evaluation, and is rapid and non-invasive. However, this method, which has been spreading rapidly for several years, does not yet have a good degree of overall accuracy [9]. Another condition in which echocardiography can help the physician is the finding of hypoechoic material between the two layers of the pericardium, which allows researchers to make a diagnosis of pericardial effusion (Figure 1). Another advantage is that the ultrasound performed in the emergency room by the emergency doctor has a very high sensitivity (96%) and specificity (98%) towards this pathology [10].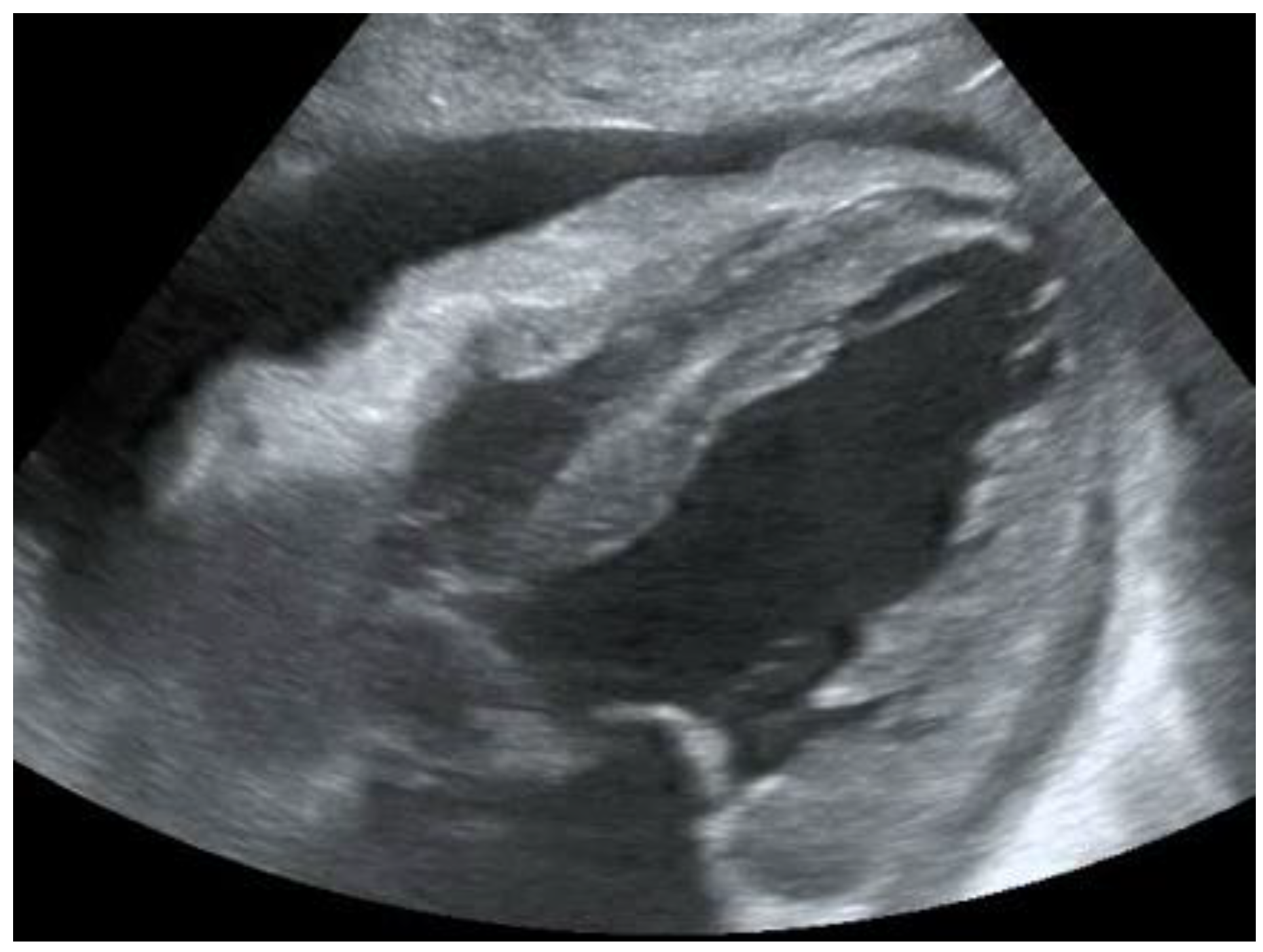 Figure 1. POCUS echocardiography: subcostal projection with pericardial effusion.
Figure 1. POCUS echocardiography: subcostal projection with pericardial effusion.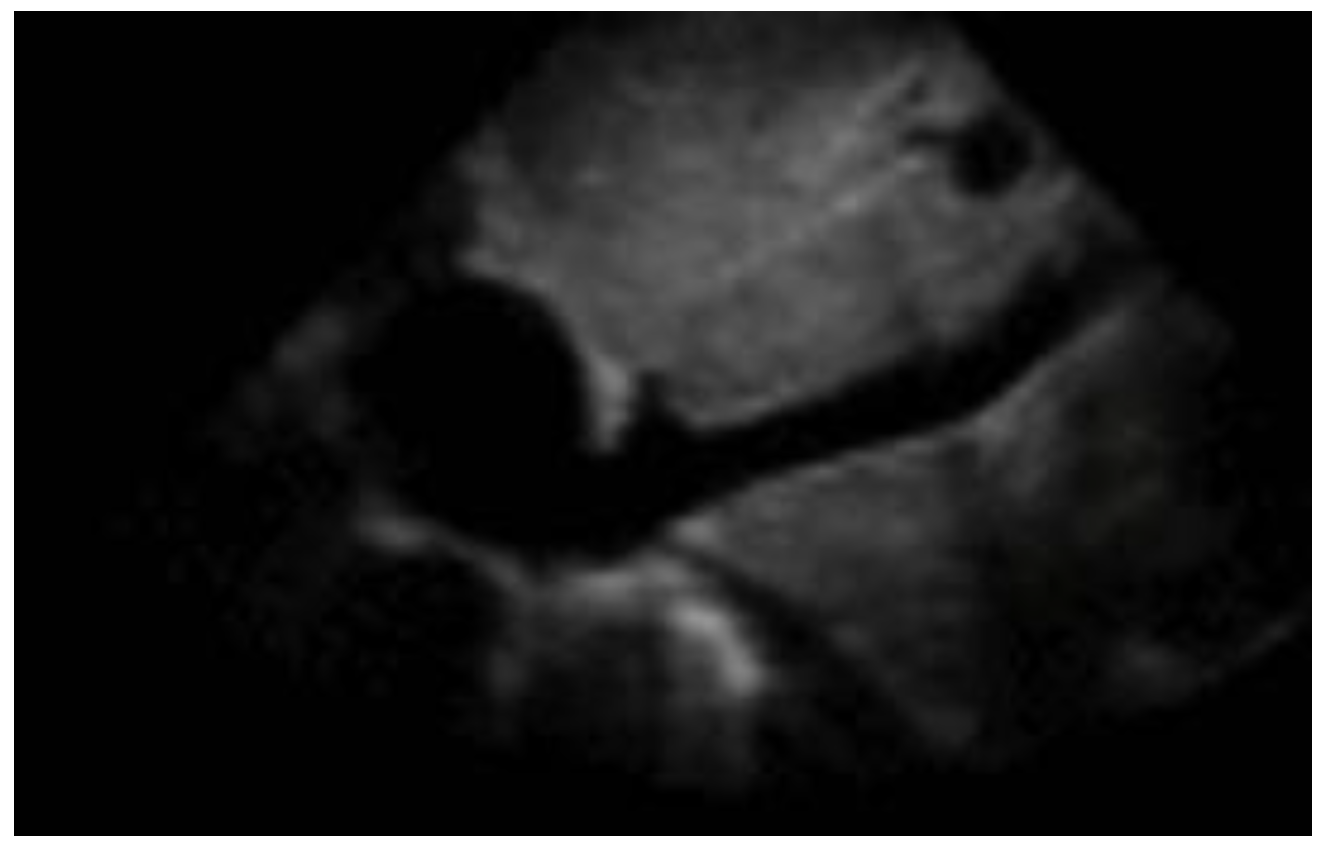 Figure 2. Subcostal longitudinal view: normal IVC.
Figure 2. Subcostal longitudinal view: normal IVC.Table 1. Relation between inferior vena cava diameter, inspiratory collapse and right atrium pressure.
| IVC Diameter | Inspiratory Collapse | Right Atrium Pressure |
|---|---|---|
| <2.1 cm | >50% | 3 mm Hg (range 0–5 mm Hg) |
| >2.1 cm | <50% | 15 mm Hg (range 10–20 mm Hg) |
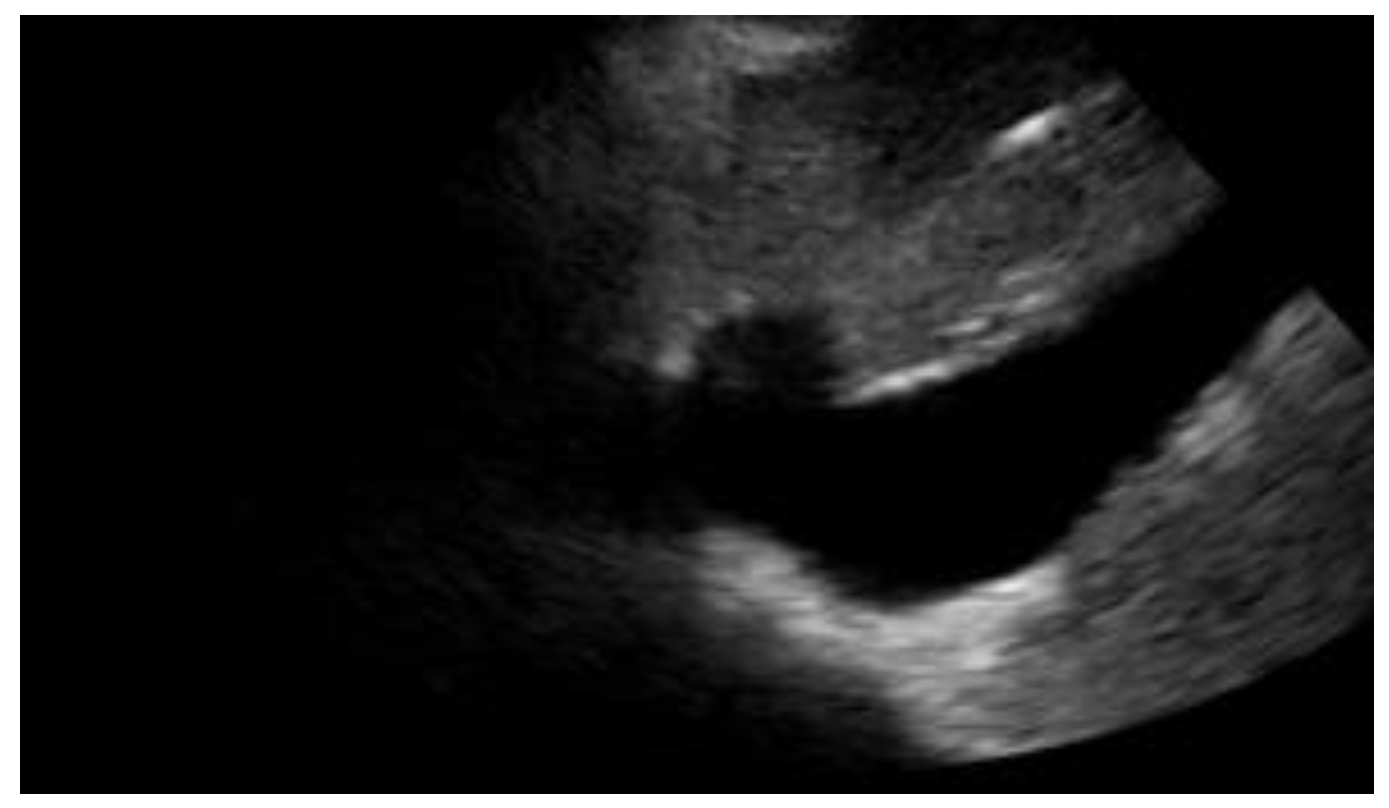 Figure 3. Subcostal longitudinal view: plethoric IVC. This finding suggests a state of fluid overload.
Figure 3. Subcostal longitudinal view: plethoric IVC. This finding suggests a state of fluid overload.Table 2. POCUS application in cardiac clinical pictures.
| Uses of Cardiac POCUS in the Emergency Department | |
|---|---|
| Disease | Assessment |
| Pulmonary embolism | Ejection fraction |
| Heart failure | Inferior vena cava filling |
| Aortic pathologies | |
| Pericardial effusion and cardiac tamponade | |
3. Chest Pain and Its Respiratory Causes
Chest pain often is associated with dyspnea or shortness of breath (SOB), which is in itself one of the most common causes of emergency room access. The origin of these symptoms is often sought in heart and lung diseases [21]. Patients presenting with these symptoms are routinely subjected to a chest X-ray examination, although in recent years researchers have witnessed the rapid growth and spread of thoracic POCUS, in which the physician himself, no longer the radiologist, performs the diagnostic examination. One of the advantages includes knowing first-hand the clinical picture and, consequently, the symptoms reported by the patient. For this reason, it is easier to orientate towards the correct differential diagnosis [22]. It has been known for some time now that the application of this method also presents many advantages for the patient when it is applied for the research of the thorax, including a lower exposure to ionizing radiation, a greater speed of execution, and a reduction in cost [23]. One of the most well-established aspects of this approach is that integrating ultrasound into the normal diagnostic tests for patients who come to the emergency room for dyspnoea and chest pain improves diagnostic accuracy [24].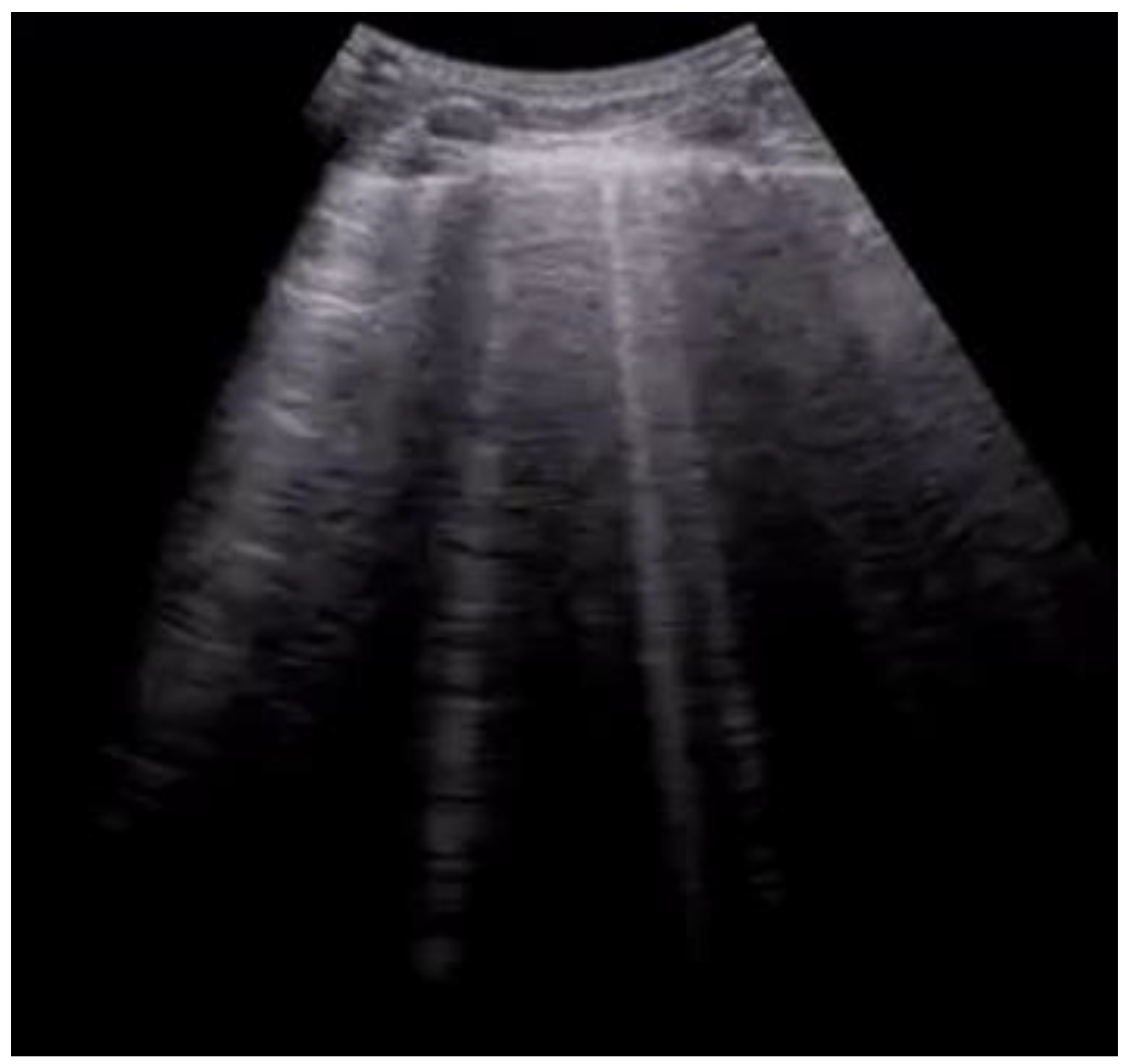 Figure 4. Longitudinal scan with evidence of B-lines: vertical artifacts perpendicular to the pleural line are indicative of inflammation or interstitial edema.
Figure 4. Longitudinal scan with evidence of B-lines: vertical artifacts perpendicular to the pleural line are indicative of inflammation or interstitial edema.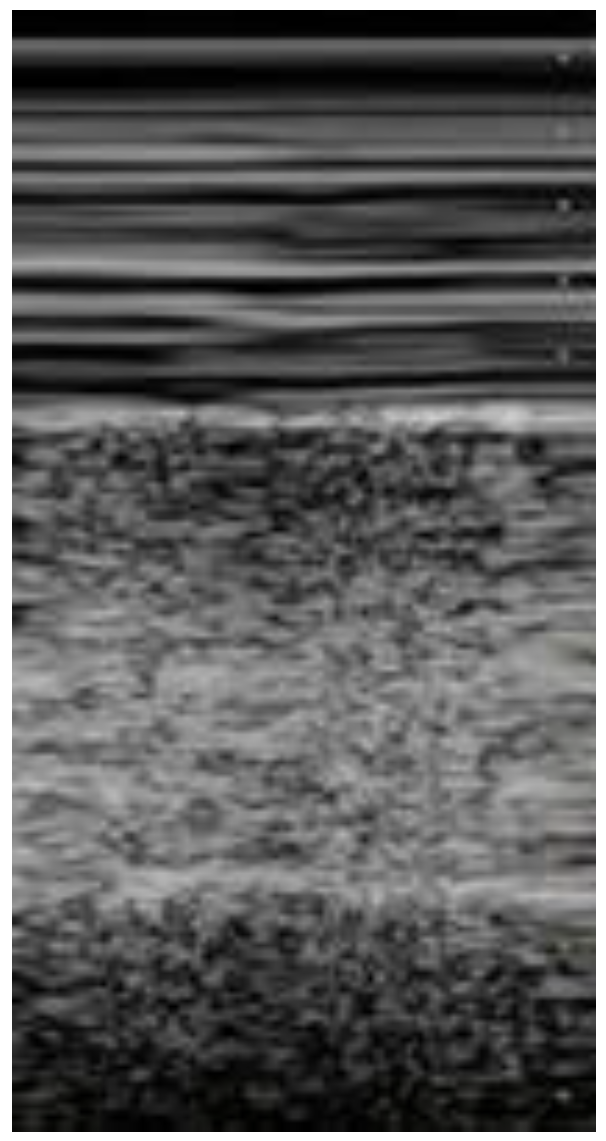 Figure 5. Lung ultrasonography, signs in motion mode (M-mode): seashore sign, indicative of the physiological sliding of the pleural line.
Figure 5. Lung ultrasonography, signs in motion mode (M-mode): seashore sign, indicative of the physiological sliding of the pleural line. Figure 6. Lung ultrasonography, signs in motion mode (M-mode): barcode sign (sign of the stratosphere), no evidence of pleural sliding, a sign suggestive of pneumothorax.
Figure 6. Lung ultrasonography, signs in motion mode (M-mode): barcode sign (sign of the stratosphere), no evidence of pleural sliding, a sign suggestive of pneumothorax.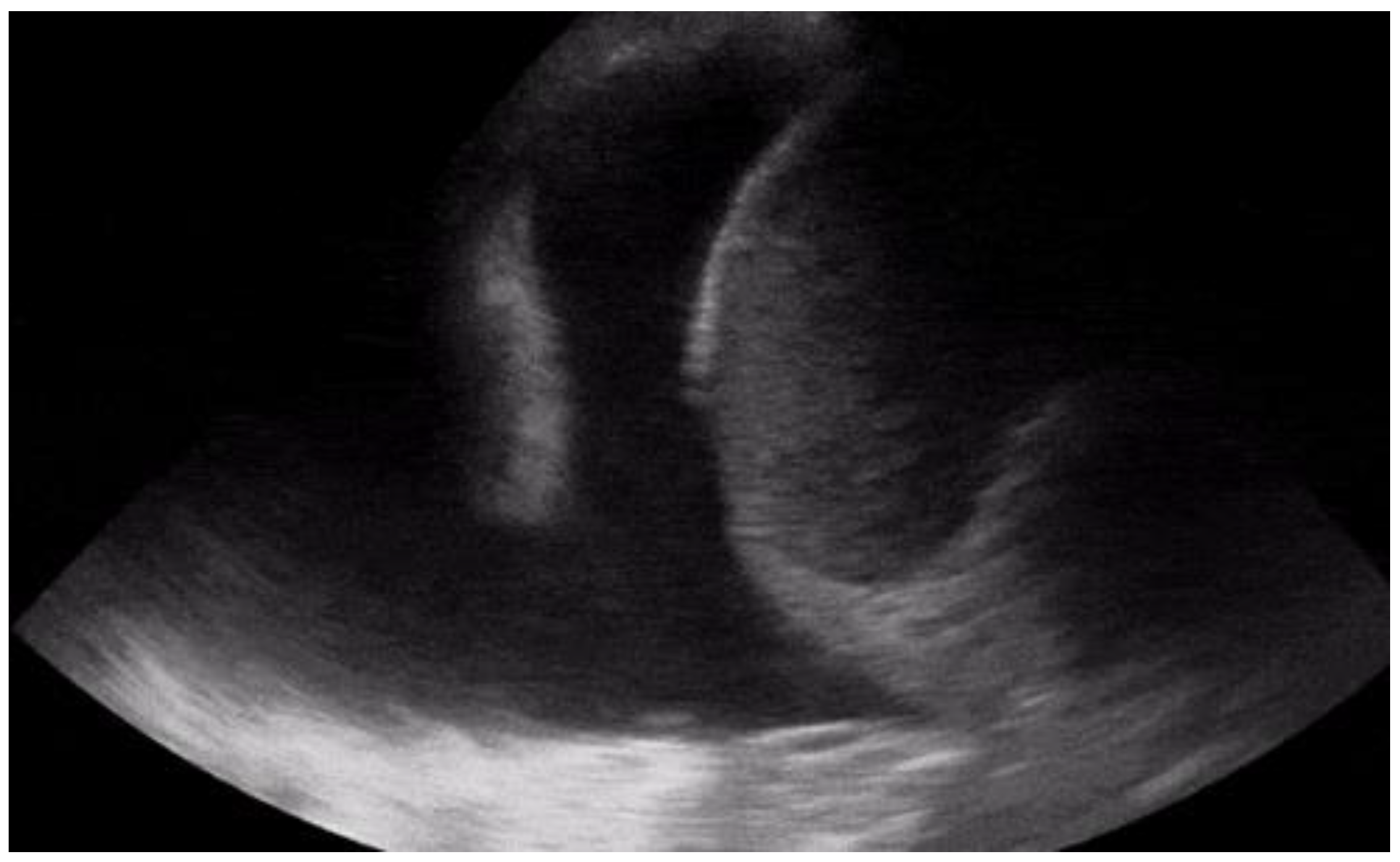 Figure 7. Longitudinal scan with presence of massive pleural effusion above the diaphragmatic line in pleural cavity, with atelectasis of adjacent lung parenchyma.
Figure 7. Longitudinal scan with presence of massive pleural effusion above the diaphragmatic line in pleural cavity, with atelectasis of adjacent lung parenchyma.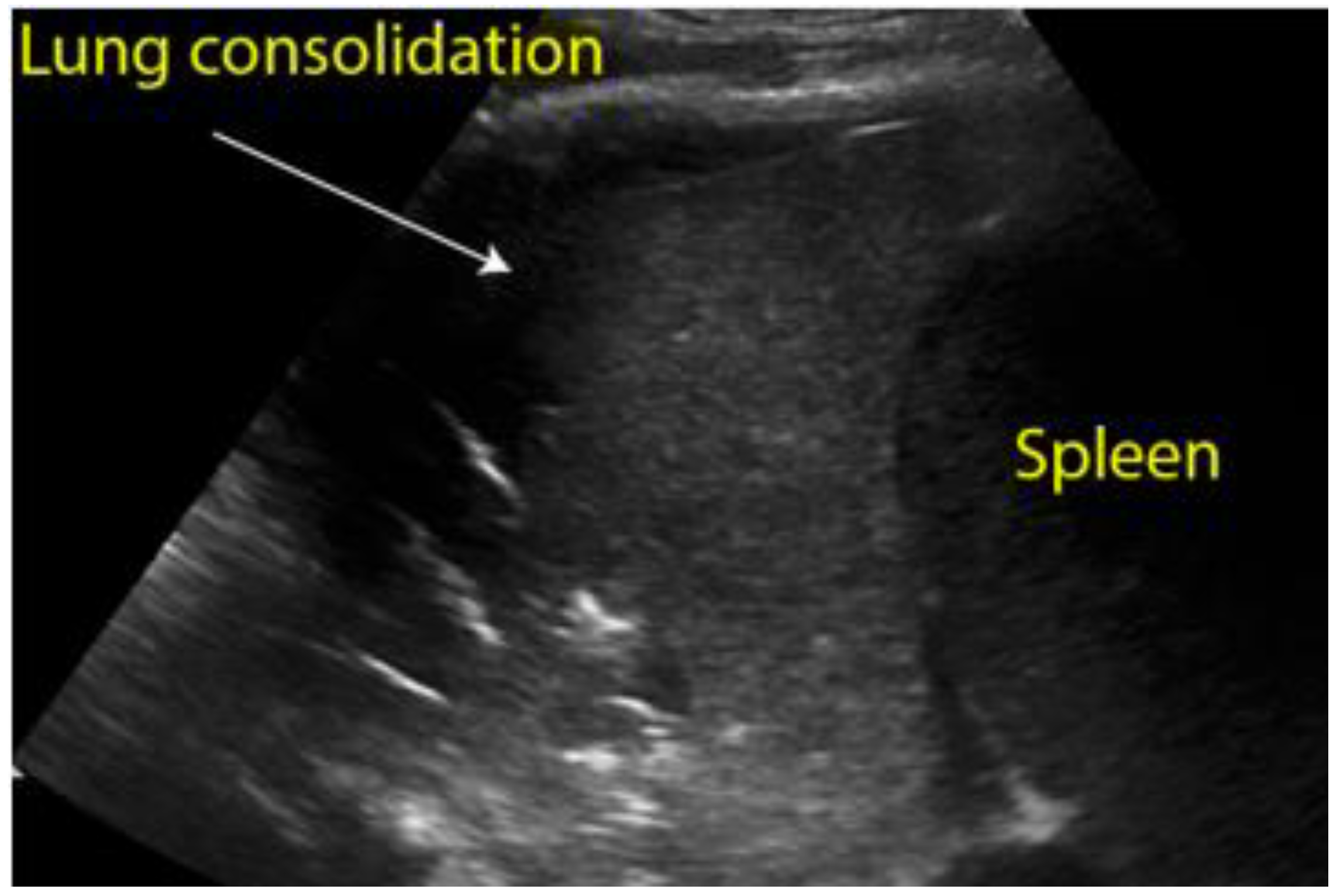
Figure 8.
Longitudinal scan of left hypochondrium with presence of lung consolidation suggestive of pneumonia.
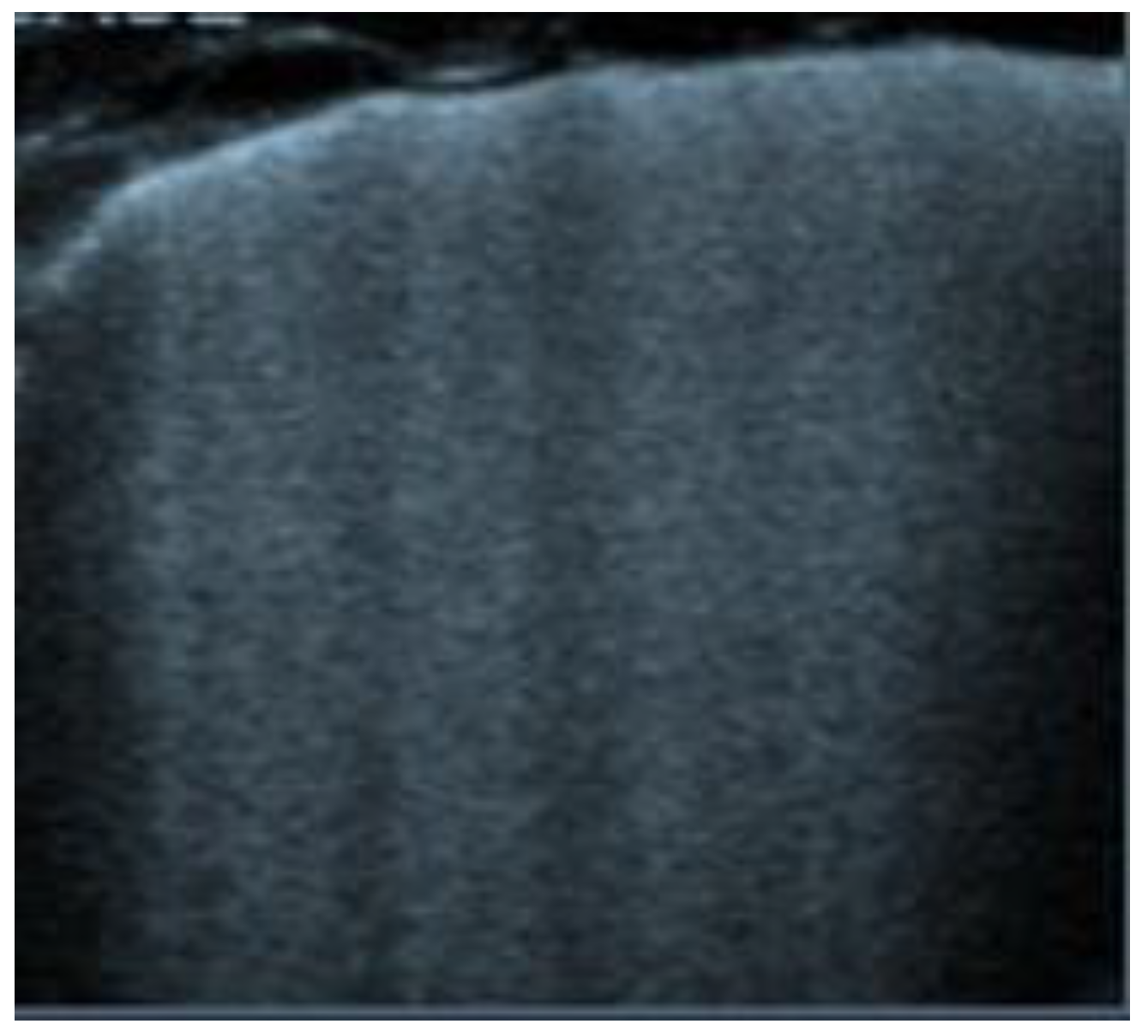
Figure 9.
Transverse scan, presence of diffuse B-lines over all fields (white lung) indicative of acute pulmonary edema.
4. Abdominal Causes
Chest pain can sometimes be a manifestation of abdominal pathology. A common example of this is gastritis, which can present itself in a similar fashion to cardiac ischaemia, as epigastric tenderness or as pain resolution after the administration of proton pump inhibitors (PPIs). In association with a lack of risk factors, POCUS is often used to determine whether gastric disturbances can be the cause of chest pain. However, although uncommon, there have been cases of patients suffering from more severe diseases and conditions who were discharged with the diagnosis of gastritis, only to return shortly after to the attention of the emergency physicians [40][41]. Another condition in which chest pain can be the main symptom is esophagitis. The most common cause is gastroesophageal reflux disease (GERD), but infections and autoimmune diseases can also determine this disease. Similarly to gastritis, endoscopy cannot be replaced by ultrasound in the diagnosis, nor can it rule out the diagnosis; however, the presence of a thickened oesophageal wall can support the diagnosis [42]. Ultrasound may not be conclusive for the diagnosis of inflammatory disorders of the oesophagus and the stomach, but it can offer significant help in other more severe disorders; in particular, oesophageal perforation can result in the non-visualization of the heart on an ultrasound due to the presence of air, and free fluid may also be present in the upper abdominal quadrants [43]. A similar presentation may also be present in the case of gastric rupture, in which the hyper echogenicity of the right anterior extrarenal tissue (renal rind sign) may also be present [44].References
- Gleeson, T.; Blehar, D. Point-of-Care Ultrasound in Trauma. Semin. Ultrasound CT MR 2018, 39, 374–383.
- Sahlani, L.; Thompson, L.; Vira, A.; Panchal, A.R. Bedside ultrasound procedures: Musculoskeletal and non-musculoskeletal. Eur. J. Trauma Emerg. Surg. 2016, 42, 127–138.
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg. Med. Clin. N. Am. 2010, 28, 29–56.
- Arnold, M.J.; Jonas, C.E.; Carter, R.E. Point-of-Care Ultrasonography. Am. Fam. Physician 2020, 101, 275–285.
- Wong, A.; Vieillard-Baron, A.; Malbrain, M. Emergency bedside ultrasound: Benefits as well as caution—Part 1. General. Curr. Opin. Crit. Care 2019, 25, 613–621.
- Lenfant, C. Chest pain of cardiac and noncardiac origin. Metabolism 2010, 59 (Suppl. 1), S41–S46.
- Chang, A.M.; Fischman, D.L.; Hollander, J.E. Evaluation of Chest Pain and Acute Coronary Syndromes. Cardiol. Clin. 2018, 36, 1–12.
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177.
- Tang, A.; Euerle, B. Emergency department ultrasound and echocardiography. Emerg. Med. Clin. N. Am. 2005, 23, 1179–1194.
- Chenkin, J.; Atzema, C.L. Contemporary Application of Point-of-Care Echocardiography in the Emergency Department. Can. J. Cardiol. 2018, 34, 109–116.
- Azarbal, A.; LeWinter, M.M. Pericardial Effusion. Cardiol. Clin. 2017, 35, 515–524.
- Ciozda, W.; Kedan, I.; Kehl, D.W.; Zimmer, R.; Khandwalla, R.; Kimchi, A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc. Ultrasound 2016, 14, 33.
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14.
- Darwish, O.S.; Mahayni, A.; Kataria, S.; Zuniga, E.; Zhang, L.; Amin, A. Diagnosis of Acute Heart Failure Using Inferior Vena Cava Ultrasound: Systematic Review and Meta-analysis. J. Ultrasound Med. 2020, 39, 1367–1378.
- Orso, D.; Paoli, I.; Piani, T.; Cilenti, F.L.; Cristiani, L.; Guglielmo, N. Accuracy of Ultrasonographic Measurements of Inferior Vena Cava to Determine Fluid Responsiveness: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2020, 35, 354–363.
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151.
- Balderston, J.R.; You, A.X.; Evans, D.P.; Taylor, L.A.; Gertz, Z.M. Feasibility of focused cardiac ultrasound during cardiac arrest in the emergency department. Cardiovasc. Ultrasound 2021, 19, 19.
- Whitson, M.R.; Mayo, P.H. Ultrasonography in the emergency department. Crit. Care 2016, 20, 227.
- Amini, R.; Stolz, L.A.; Kartchner, J.Z.; Thompson, M.; Stea, N.; Hawbaker, N.; Joshi, R.; Adhikari, S. Bedside echo for chest pain: An algorithm for education and assessment. Adv. Med. Educ. Pract. 2016, 7, 293–300.
- Kennedy Hall, M.; Coffey, E.C.; Herbst, M.; Liu, R.; Pare, J.R.; Andrew Taylor, R.; Thomas, S.; Moore, C.L. The “5Es” of emergency physician-performed focused cardiac ultrasound: A protocol for rapid identification of effusion, ejection, equality, exit, and entrance. Acad. Emerg. Med. 2015, 22, 583–593.
- Shrestha, A.P.; Shrestha, R.; Shrestha, S.K.; Pradhan, A. Prevalence of Dyspnea among Patients Attending the Emergency Department of a Tertiary Care Hospital: A Descriptive Cross-sectional Study. JNMA J. Nepal. Med. Assoc. 2019, 57, 302–306.
- Bowra, J.; Duong, M.T. Point-of-care ultrasound in emergency department: Tips, tricks and controversies. Emerg. Med. Australas. 2020, 32, 155–157.
- De Luca, C.; Valentino, M.; Rimondi, M.R.; Branchini, M.; Baleni, M.C.; Barozzi, L. Use of chest sonography in acute-care radiology. J. Ultrasound 2008, 11, 125–134.
- Pirozzi, C.; Numis, F.G.; Pagano, A.; Melillo, P.; Copetti, R.; Schiraldi, F. Immediate versus delayed integrated point-of-care-ultrasonography to manage acute dyspnea in the emergency department. Crit. Ultrasound J. 2014, 6, 5.
- Wallbridge, P.; Steinfort, D.; Tay, T.R.; Irving, L.; Hew, M. Diagnostic chest ultrasound for acute respiratory failure. Respir. Med. 2018, 141, 26–36.
- Chan, K.K.; Joo, D.A.; McRae, A.D.; Takwoingi, Y.; Premji, Z.A.; Lang, E.; Wakai, A. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst. Rev. 2020, 7, Cd013031.
- Ebrahimi, A.; Yousefifard, M.; Kazemi, H.M.; Rasouli, H.R.; Asady, H.; Jafari, A.M.; Hosseini, M. Diagnostic Accuracy of Chest Ultrasonography versus Chest Radiography for Identification of Pneumothorax: A Systematic Review and Meta-Analysis. Tanaffos 2014, 13, 29–40.
- Eibenberger, K.L.; Dock, W.I.; Ammann, M.E.; Dorffner, R.; Hörmann, M.F.; Grabenwöger, F. Quantification of pleural effusions: Sonography versus radiography. Radiology 1994, 191, 681–684.
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.F.; Moss, A.; Juarez-Colunga, E.; Soni, N.J.; Miglioranza, M.H.; Platz, E.; DeSanto, K.; et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults with Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e190703.
- Ibitoye, B.O.; Idowu, B.M.; Ogunrombi, A.B.; Afolabi, B.I. Ultrasonographic quantification of pleural effusion: Comparison of four formulae. Ultrasonography 2018, 37, 254–260.
- Montoya, J.; Stawicki, S.P.; Evans, D.C.; Bahner, D.P.; Sparks, S.; Sharpe, R.P.; Cipolla, J. From FAST to E-FAST: An overview of the evolution of ultrasound-based traumatic injury assessment. Eur. J. Trauma Emerg. Surg. 2016, 42, 119–126.
- Staub, L.J.; Biscaro, R.R.M.; Kaszubowski, E.; Maurici, R. Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and haemothorax: A systematic review and meta-analysis. Injury 2018, 49, 457–466.
- Wang, Y.; Shen, Z.; Lu, X.; Zhen, Y.; Li, H. Sensitivity and specificity of ultrasound for the diagnosis of acute pulmonary edema: A systematic review and meta-analysis. Med. Ultrason. 2018, 1, 32–36.
- Estrada, Y.M.R.M.; Oldham, S.A. CTPA as the gold standard for the diagnosis of pulmonary embolism. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 557–563.
- Nazerian, P.; Vanni, S.; Volpicelli, G.; Gigli, C.; Zanobetti, M.; Bartolucci, M.; Ciavattone, A.; Lamorte, A.; Veltri, A.; Fabbri, A.; et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest 2014, 145, 950–957.
- Orso, D.; Guglielmo, N.; Copetti, R. Lung ultrasound in diagnosing pneumonia in the emergency department: A systematic review and meta-analysis. Eur. J. Emerg. Med. 2018, 25, 312–321.
- Giannella, L.; Catania, A.; Provaroni, A.; Cerami, L.B.; Chesi, G. The value of chest ultrasound along with inflammatory biomarkers in the management of pneumonia in a non-compliant pregnant woman. J. Matern. Fetal Neonatal Med. 2012, 25, 1830–1832.
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 75, 1096–1104.
- Jackson, K.; Butler, R.; Aujayeb, A. Lung ultrasound in the COVID-19 pandemic. Postgrad. Med. J. 2021, 97, 34–39.
- Boccardi, L.; Bisconti, C.; Camboni, C.; Chieffi, M.; Putini, R.L.; Macali, L.; Spina, A.; Lukic, V.; Ciferri, E. Chest pain in women: A multicenter study of the National Association of Hospital Cardiologists (ANMCO) of the Lazio Region. Ital. Heart J. Suppl. 2002, 3, 1034–1041.
- Ohtani, N.; Kiyokawa, K.; Asada, H.; Kawakami, T. Stanford type A acute dissection developing acute myocardial infarction. Jpn. J. Thorac. Cardiovasc. Surg. 2000, 48, 69–72.
- Mohammadi, A.; Sadreddini, M.; Sepehrvand, N.; Pedram, A.; Yarmohammadi, N.; Mladkova, N.; Ghasemi-Rad, M. Lack of utility of transabdominal ultrasound in the detection of gastroesophageal reflux disease-induced esophagitis in comparison with endoscopy. Ultrasound Q. 2011, 27, 121–125.
- Derr, C.; Drake, J.M. Esophageal rupture diagnosed with bedside ultrasound. Am. J. Emerg. Med. 2012, 30, 2093.e1–2093.e3.
- Coppolino, F.F.; Gatta, G.; Di Grezia, G.; Reginelli, A.; Iacobellis, F.; Vallone, G.; Giganti, M.; Genovese, E.A. Gastrointestinal perforation: Ultrasonographic diagnosis. Crit. Ultrasound J. 2013, 5, S4.
More
