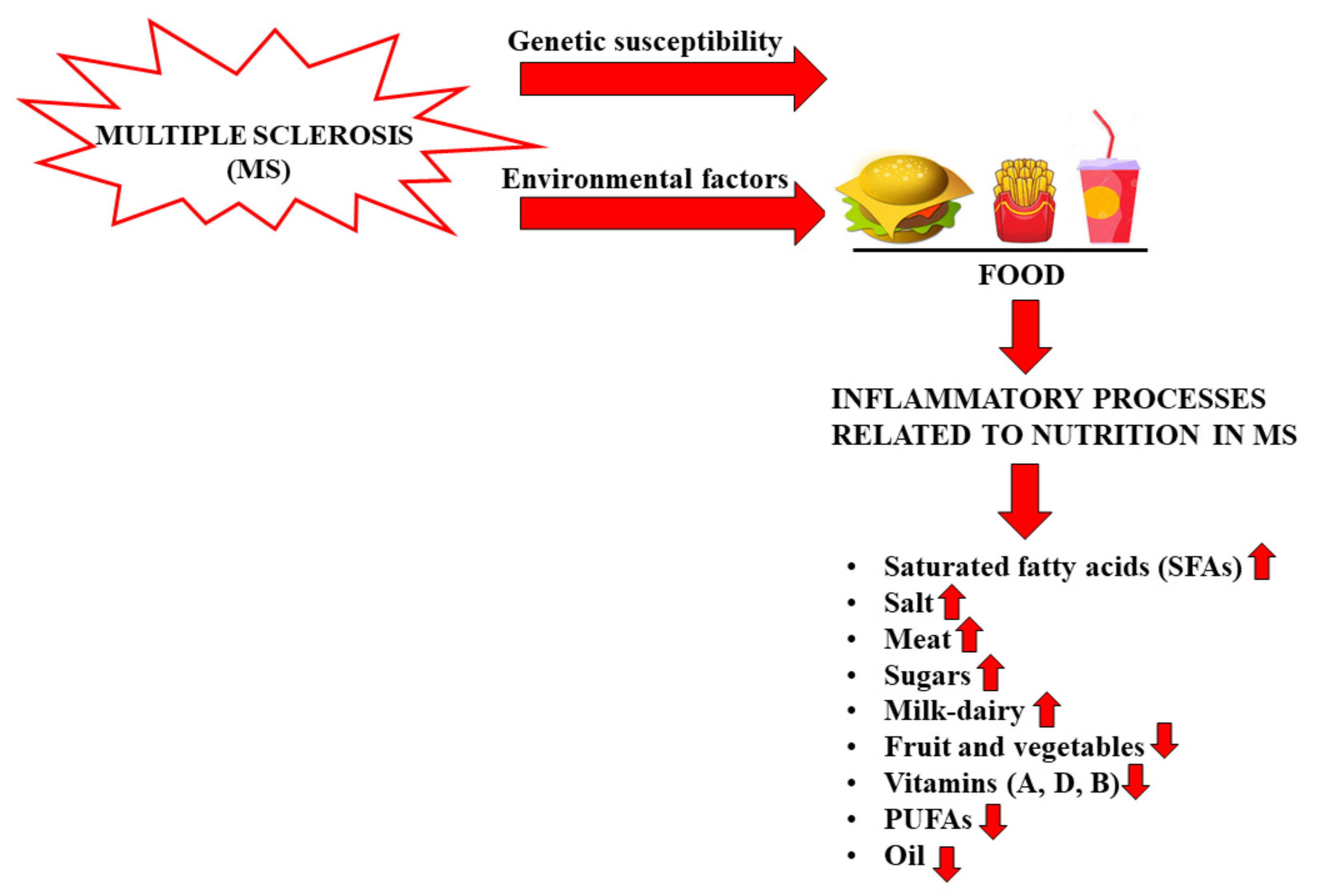
Multiple sclerosis (MS) is a neurological and inflammatory autoimmune disease of the Central Nervous System in which selective activation of T and B lymphocytes prompts a reaction against myelin, inducing demyelination and axonal loss. Although MS is recognized to be an autoimmune pathology, the specific causes are many; thus, it has been considered a disorder resulting from environmental factors in genetically susceptible individuals. Among the environmental factors hypothetically involved in MS, nutrition seems to be well related, although the role of nutritional factors is still unclear. The gut of mammals is home to a bacterial community of about 2000 species known as the “microbiota”, whose composition changes throughout the life of each individual. There are five bacterial phylas that make up the microbiota in healthy adults: Firmicutes (79.4%), Bacteroidetes (16.9%), Actinobacteria (2.5%), Proteobacteria (1%) and Verrucomicrobia (0.1%). The diversity and abundance of microbial populations justifies a condition known as eubiosis. On the contrary, the state of dysbiosis refers to altered diversity and abundance of the microbiota. Many studies carried out have demonstrated that there is a relationship between the intestinal microflora and the progression of multiple sclerosis. This correlation was also demonstrated by the discovery that patients with MS, treated with specific prebiotics and probiotics, have greatly increased bacterial diversity in the intestinal microbiota, which might be otherwise reduced or absent. In particular, natural extracts of Aloe vera and bergamot fruits, rich in polyphenols and with a high percentage of polysaccharides (mostly found in indigestible and fermentable fibers), appear to be potential candidates to re-equilibrate the gut microbiota in MS patients.
- multiple sclerosis
- gut microbiota
- nutrition
- milk
- SCFAs
- microbiota–brain communication
- Aloe vera
- Citrus bergamia
- acemannans
1. Introduction
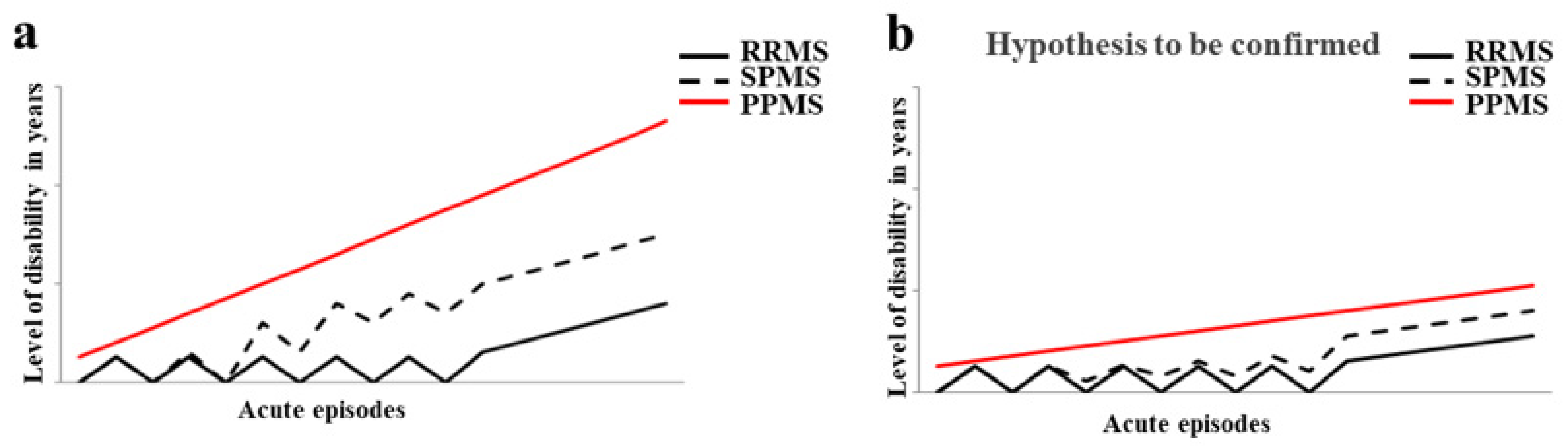
2. Nutrition and MS
3. Gut Microbiota and Brain
4. The Interaction of Plant Fibers with the Intestinal Microbiota
The ability of the diet to modify the gastrointestinal microbiota of humans and other mammals has been extensively investigated. In addition to a description of the effects of the main macronutrients on the gut microbiota, a further study should concern the intake of fiber and how quickly the composition of the microbiota changes. For example, a major change in bacterial diversity has already been demonstrated in humans 24 h after the intake of a fiber-rich (>30 g/day) agrarian diet instead of a fiber-free, meat-based diet [113][76]. The Codex Alimentarius Commission defines dietary fiber as “carbohydrate polymers with 10 or more monomeric units, which are neither digested nor absorbed in the human small intestine.” The role of dietary fiber in the gut microbiota depends on several factors including the origin, chemical makeup, physical structure, and degree of polymerization (chain length) [114][77]. The physicochemical properties of fiber include fermentability, solubility, and viscosity. Insoluble fiber, such as cellulose, is generally poorly fermented by intestinal microbes. This fiber increases the rate of intestinal transit and thus reduces the amount of time available for the bacterial fermentation of undigested food [115][78]. However, their high solubility and viscosity results in unique therapeutic effects, including the ability to improve glycemic control and lower blood cholesterol levels [116][79]. Among the highly soluble, fermentable, and viscous fibers are β-glucan and pectins [117][80]. Soluble and nonviscous fibers are readily fermented from the gastrointestinal microbiota and include inulin, resistant maltodextrins, resistant starch, polydextrose, and soluble corn fiber [118][81]. The different solubility of complex carbohydrates affects the location of fermentation in the human gastrointestinal tract: for example, soluble fiber and pectin are metabolized by bacteria in the ileum and ascending colon; fiber that is less soluble, such as cellulose, can be partially fermented in the distal colon [119][82]. In general, fermentable fiber behaves as a prebiotic [120][83], although it is appropriate to add that not all fiber can be classified as a prebiotic. To date, we know that the consumption of prebiotics is a dietary strategy that allows the intestinal microbiota to be modified to improve health. The definition of a prebiotic has evolved over the years; the currently accepted and most comprehensive one is “a non-digestible food ingredient which selectively stimulates its fermentation by the microbiota, increases the growth and/or activity of a limited number of bacteria in the gastrointestinal tract, and positively affects the host. It must also be resistant to gastric acidity and hydrolysis by enzymes during gastrointestinal absorption” [121][84]. Numerous scientific evidences have highlighted the role of glyconutrients such as dietary fibers, useful to improve the composition of the intestinal microbiota [122][85]. In particular, Ambratose is a combination of eight sugars (acemannan), that the body uses to produce glycoproteins, and has been used in humans for many years, given its antioxidant effects [123][86], the well-known immune boosting benefits and the improvement of cognitive performance [124][87]. It has been demonstrated in vitro that Ambratose is a very promising prebiotic and clinical data, conducted on 350 participants, demonstrated that treatment with Ambratose (2–4 g per day for 6 months) did not produce adverse events, improving overall intestinal health [125,126][88][89]. Given their high proportion of acemannans and fibers, Aloe vera and Citrus bergamia are proposed to be hypothetical prebiotic intestinal remedies.References
- Díaz, C.; Zarco, L.A.; Rivera, D.M. Highly active multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2019, 30, 215–224.
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16 (Suppl. S6), s53–s59.
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424.
- Riccio, P.; Rossano, R. Nutrition Facts in Multiple Sclerosis. ASN Neuro 2015, 7, 1759091414568185.
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Multiple sclerosis—Etiology and diagnostic potential. Postepy Hig. Med. Dosw. 2017, 71, 551–563.
- Solomon, A.J. Diagnosis, Differential Diagnosis, and Misdiagnosis of Multiple Sclerosis. Continuum 2019, 25, 611–635.
- Naseri, A.; Nasiri, E.; Sahraian, M.A.; Daneshvar, S.; Talebi, M. Clinical Features of Late-Onset Multiple Sclerosis: A Systematic Review and Meta-analysis. Mult. Scler. Relat. Disord. 2021, 50, 102816.
- Brola, W.; Steinborn, B. Pediatric multiple sclerosis—Current status of epidemiology, diagnosis and treatment. Neurol. Neurochir. Pol. 2020, 54, 508–517.
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779.
- Simpson, A.; Mowry, E.; Newsome, S.D. Early Aggressive Treatment Approaches for Multiple Sclerosis. Curr. Treat. Options Neurol. 2021, 23, 19.
- Ziemssen, T.; Derfuss, T.; de Stefano, N.; Giovannoni, G.; Palavra, F.; Tomic, D.; Vollmer, T.; Schippling, S. Optimizing treatment success in multiple sclerosis. J. Neurol. 2016, 263, 1053–1065.
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. S1), 1–5.
- Bronwlee, W.S.; Swanton, J.K.; Altmann, D.R.; Ciccarelli, O.; Miller, D.H. Earlier and more frequent diagnosis of multiple sclerosis using the McDonald criteria. J. Neurol. Neurosurg. Psychiatry 2015, 86, 584–585.
- Baldassari, L.E.; Fox, R.J. Therapeutic Advances and Challenges in the Treatment of Progressive Multiple Sclerosis. Drugs 2018, 78, 1549–1566.
- Hemond, C.C.; Bakshi, R. Magnetic Resonance Imaging in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028969.
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40.
- Ochoa-Repáraz, J.; Kirby, T.O.; Kasper, L.H. The Gut Microbiome and Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029017.
- Ghareghani, M.; Reiter, R.J.; Zibara, K.; Farhadi, N. Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway. Front. Immunol. 2018, 9, 2484.
- Bagur, M.J.; Murcia, M.A.; Jiménez-Monreal, A.M.; Tur, J.A.; Bibiloni, M.M.; Alonso, G.L.; Martínez-Tomé, M. Influence of Diet in Multiple Sclerosis: A Systematic Review. Adv. Nutr. 2017, 8, 463–472.
- Sun, Y.; Zhang, Z.; Cheng, L.; Zhang, X.; Liu, Y.; Zhang, R.; Weng, P.; Wu, Z. Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota. Food Res. Int. 2021, 149, 110675.
- Constantin-Teodosiu, D.; Constantin, D. Molecular Mechanisms of Muscle Fatigue. Int. J. Mol. Sci. 2021, 22, 11587.
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of Two Popular Eating Plans within the Multiple Sclerosis Community: Low Saturated Fat and Modified Paleolithic. Nutrients 2019, 11, 352.
- Di Biase, A.; Salvati, S. Exogenous lipids in myelination and myelination. Kaohsiung J. Med. Sci. 1997, 13, 19–29.
- Ruiz-Núñez, B. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20.
- Yadav, V.; Bourdette, D. Complementary and alternative medicine: Is there a role in multiple sclerosis? Curr. Neurol. Neurosci. Rep. 2006, 6, 259–267.
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618.
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Oppedisano, F.; Macrì, R.; et al. Environmental and Nutritional “Stressors” and Oligodendrocyte Dysfunction: Role of Mitochondrial and Endoplasmatic Reticulum Impairment. Biomedicines 2020, 8, 553.
- Tettey, P.; Simpson, S.; Taylor, B.; Ponsonby, A.L.; Lucas, R.M.; Dwyer, T. An adverse lipid profile and increased levels of adiposity significantly predict clinical course after a first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2017, 88, 395–401.
- Uher, T.; Fellows, K.; Horakova, D.; Zivadinov, R.; Vaneckova, M.; Sobisek, L. Serum lipid profile changes predict neurodegeneration in interferon-beta1a-treated multiple sclerosis patients. J. Lipid Res. 2017, 58, 403–411.
- Haghikia, A.; Jorg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 43, 817–829.
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.M.; Mar, S.; Hart, J.; et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33.
- Neate, S.L.; Donald, A.; Jelinek, G.A.; Nag, N. Experiences of and attitudes to lifestyle modification for the management of multiple sclerosis: A qualitative analysis of free-text survey data. Health Expect. 2022, 25, 214–222.
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85.
- Von Geldern, G.; Mowry, E.M. The influence of nutritional factors on the prognosis of multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 678–869.
- De Carvalho, T.S. Calorie restriction or dietary restriction: How far they can protect the brain against neurodegenerative diseases? Neural Regen. Res. 2022, 17, 1640–1644.
- Parks, N.E.; Jackson-Tarlton, C.S.; Verdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 5, CD004192.
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330.
- Tan, G.A.; Furber, K.L.; Thangaraj, M.P.; Sobchishin, L.; Doucette, J.R.; Nazarali, A.J. Organotypic Cultures from the Adult CNS: A Novel Model to Study Demyelination and Remyelination Ex Vivo. Cell. Mol. Neurobiol. 2018, 38, 317–328.
- Penkert, H.; Bertrand, A.; Tiwari, V.; Breimann, S.; Müller, S.A.; Jordan, P.M.; Gerl, M.J.; Klose, C.; Cantuti-Castelvetri, L.; Bosch-Queralt, M.; et al. Proteomic and lipidomic profiling of demyelinating lesions identifies fatty acids as modulators in lesion recovery. Cell Rep. 2021, 37, 109898.
- Masullo, L.; Papas, M.A.; Cotugna, N.; Baker, S.; Mahoney, L.; Trabulsi, J. Complementary and alternative medicine use and nutrient intake among individuals with multiple sclerosis in the United States. J. Community Health 2015, 40, 153–160.
- Silbermann, E.; Senders, A.; Wooliscroft, L.; Rice, J.; Cameron, M.; Waslo, C.; Orban, A.; Chase, E.; Yadav, V.; Bourdette, D.; et al. Cross-sectional survey of complementary and alternative medicine used in Oregon and Southwest Washington to treat multiple sclerosis: A 17-Year update. Mult. Scler. Relat. Disord. 2020, 41, 102041.
- Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 616.
- Hadgkiss, E.J.; Jelinek, G.A.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr. Neurosci. 2015, 18, 125–136.
- Noormohammadi, M.; Ghorbani, Z.; Naser Moghadasi, A.; Saeedirad, Z.; Shahemi, S.; Ghanaatgar, M.; Rezaeimanesh, N.; Hekmatdoost, A.; Ghaemi, A.; Razeghi Jahromi, S. MIND Diet Adherence Might be Associated with a Reduced Odds of Multiple Sclerosis: Results from a Case-Control Study. Neurol. Ther. 2022, 11, 397–412.
- Sharifi, M.H.; Keshani, P.; Salehi, A.; Jaladat, A.M.; Mirzaei, Z.; Nikseresht, A. Association between multiple sclerosis and dietary patterns based on the traditional concept of food nature: A case-control study in Iran. BMC Neurol. 2021, 21, 453.
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160.
- Evans, E.; Levasseur, V.; Cross, A.H.; Piccio, L. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 36, 101393.
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11.
- Gu, Y.; Brickman, A.M.; Stern, Y.; Habeck, C.G.; Razlighi, Q.R.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Scarmeas, N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015, 85, 1744–1751.
- García-Casares, N.; Gallego Fuentes, P.; Barbancho, M.Á.; López-Gigosos, R.; García-Rodríguez, A.; Gutiérrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642.
- Yadav, V.; Marracci, G.; Kim, E.; Spain, R.; Cameron, M.; Overs, S.; Riddehough, A.; Li, D.K.; McDougall, J.; Lovera, J.; et al. Low-fat, plant-based diet in multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2016, 9, 80–90.
- Bisht, B.; Darling, W.G.; Grossmann, R.E.; Shivapour, E.T.; Lutgendorf, S.K.; Snetselaar, L.G.; Hall, M.J.; Zimmerman, M.B.; Wahls, T.L. A Multimodal Intervention for Patients with Secondary Progressive Multiple Sclerosis: Feasibility and Effect on Fatigue. J. Altern. Complement. Med. 2014, 20, 347–355.
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783.
- Redwan, E.M.; Al-Hejin, A.M.; Almehdar, H.A.; Elsaway, A.M.; Uversky, V.N. Prediction of Disordered Regions and Their Roles in the Anti-Pathogenic and Immunomodulatory Functions of Butyrophilins. Molecules 2018, 23, 328.
- Stefferl, A.; Schubart, A.; Storch, M.; Amini, A.; Mather, I.; Lassmann, H.; Linington, H. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J. Immunol. 2000, 165, 2859–2865.
- Guggenmos, J.; Schubart, A.S.; Ogg, S.; Andersson, M.; Olsson, T.; Mather, I.H.; Linington, C. Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. J. Immunol. 2004, 172, 661–668.
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlach, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human Milk Oligosaccharides and Bacterial Profile Modulate Infant Body Composit.ion during Exclusive Breastfeeding. Int. J. Mol. Sci. 2022, 23, 2865.
- Vantourout, P.; Laing, A.; Woodward, M.J.; Zlatareva, I.; Apolonia, L.; Jones, A.W.; Snijders, A.P.; Malim, M.H.; Hayday, A.C. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc. Natl. Acad. Sci. USA 2018, 115, 1039–1044.
- Wynford-Thomas, D.A.; Reith, W.; Trowsdale, J. Regulation of Immunity by Butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172.
- Chunder, R.; Weier, A.; Mäurer, H.; Luber, N.; Enders, M.; Luber, G.; Heider, T.; Spitzer, A.; Tacke, S.; Becker-Gotot, J.; et al. Antibody cross-reactivity between casein and myelin-associated glycoprotein results in central nervous system demyelination. Proc. Natl. Acad. Sci. USA 2022, 119, e2117034119.
- Mañá, P.; Goodyear, M.; Bernard, C.; Tomioka, R.; Freire-Garabal, M.; Liñares, D. Tolerance induction by molecular mimicry: Prevention and suppression of experimental autoimmune encephalomyelitis with the milk protein butyrophilin. Int. Immunol. 2004, 16, 489–499.
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 155.
- Na, S.; Na, L.; Xinwang, D.; Haitao, N. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14.
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520.
- Davenport, E.R.; Mizrahi-Man, O.; Michelini, K.; Barreiro, L.B.; Ober, C.; Gilad, Y. Seasonal Variation in Human Gut Microbiome Composition. PLoS ONE 2014, 9, e90731.
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12.
- Ron, R.; Fragman-Sapir, O.; Kadmon, R. Dispersal increases ecological selection by increasing effective community size. Proc. Natl. Acad. Sci. USA 2018, 115, 11280–11285.
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107.
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017, 79, 920–926.
- Belkaid, Y.; Harrison, O.J. Homeostatic immunity and the microbiota. Immunity 2017, 46, 562–576.
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatr. Obes. 2017, 12, 3–17.
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121.
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4.
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Hart, J.; Roalstad, S.; Graves, J.; Spencer, C.M.; Lynch, S.V.; Zamvil, S.S.; Waubant, E. Associations between the gut microbiota and host immune markers in pediatric multiple sclerosis and controls. BMC Neurol. 2016, 16, 182.
- Tsunoda, I. Lymphatic system and gut microbiota affect immunopathology of neuroinflammatory diseases, including multiple sclerosis, neuromyelitis optica and Alzheimer’s disease. Clin. Exp. Neuroimmunol. 2017, 8, 177–179.
- Zhang, Y.; Zhang, J.; Liu, H.; He, F.; Chen, A.; Yang, H.; Pi, B. Meta-analysis of FOXP3 gene rs3761548 and rs2232365 polymorphism and multiple sclerosis susceptibility. Medicine 2019, 98, e17224.
- Khan, U.; Ghazanfar, H.T. Lymphocytes and Autoimmunity. Int. Rev. Cell Mol. Biol. 2018, 341, 125–168.
- Farrokhi, V.; Nemati, R.; Nichols, F.C.; Yao, X.; Anstadt, E. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin. Transl. Immunol. 2013, 2, e8.
- Browne, R.W.; Jakimovski, D.; Ziliotto, N.; Kuhle, J.; Bernardi, F.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. High-density lipoprotein cholesterol is associated with multiple sclerosis fatigue: A fatigue-metabolism nexus? J. Clin. Lipidol. 2019, 13, 654–663.
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’Arcy, C. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. 2019, 25, 653–660.
- Savva, C.G.; Clark, A.R.; Naylor, C.E.; Popoff, M.R.; Moss, D.S. The pore structure of Clostridium perfringens epsilon toxin. Nat. Commun. 2019, 10, 2641.
- Anwar, Z.; Regan, S.B.; Linden, J. Enrichment and Detection of Clostridium perfringens Toxinotypes in Retail Food Samples. J. Vis. Exp. 2019, 152, e59931.
- Abdurasulova, I.N.; Tarasova, E.A.; Nikiforova, I.G.; Il’ves, A.G.; Ivashkova, V.; Matsulevich, A.V.; Tatarinov, A.E.; Shangina, L.V.; Ermolenko, E.I.; Suvorov, A.N. The intestinal microbiota composition in patients with multiple sclerosis receiving different disease-modifying therapies DMT. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2018, 118, 62–69.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563.
- Kataoka, K.J. The intestinal microbiota and its role in human health and disease. Med. Investig. 2016, 63, 27–37.
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152.
- McRorie, J.W. Psyllium is not fermented in the human gut. Neurogastroenterol. Motil. 2015, 27, 1681–2682.
