Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Francesco Cacciola.
Bioemulsifiers have a larger molecular weight than biosurfactants, because they are complex mixes of lipopolysaccharides, lipoproteins, heteropolysaccharides, and proteins. Due to their functional capabilities and eco-friendly properties, bioemulsifiers (BE) are regarded as multifunctional biomolecules of the twenty-first century. Numerous microorganisms produce bioemulsifiers under a variety of diverse and extreme environmental conditions. Bioemulsifiers are widely used in a variety of industries, including medicine, petroleum, food, pharmaceuticals, chemicals, textiles, and cosmetics.
- emulsifiers
- food
- microbial surfactants
- biodegradable
- non-toxic
- fungi
1. Bioemulsifiers
Emulsifiers can be synthesized chemically or via microbial metabolism (bioemulsifiers). Bioemulsifiers are versatile chemical compounds that are capable of stabilizing oil-in-water emulsions and are critical in a variety of industrial applications [16][1]. They are also referred to as biopolymers or polysaccharides with a high molecular weight. Even at low concentrations, these molecules emulsify two immiscible liquids efficiently but are less effective at reducing surface tension. Combining polysaccharides, fatty acids, and protein components in bioemulsifiers enhances their emulsifying capacity [17][2]. Liposan, produced by Candida lipolytica, is the most studied bioemulsifier [18][3]. It is roughly 17% protein and 83% carbohydrate (polysaccharide–protein complex). The carbohydrate portion contains glucose, galactose, galactosamine, and galacturonic acid.
Emulsan is an extracellular heteropolysaccharide composed of two biopolymers: 20% exopolysaccharide and 80% lipopolysaccharide with a high molecular weight. It was extracted in the late 1970s from a hydrocarbon-degrading Arthrobacter sp. RAG-1 (later renamed Acinetobacter venetianus RAG-1) [19][4]. Emulsan addition improved the stability of alginate microspheres, allowing for the fine-tuning of biological molecule release by using different emulsan concentrations. The authors concluded that emulsan is an excellent candidate for protein and pharmaceutical delivery. Specific emulsan–alginate formulations have been granted patents as medication delivery methods and vehicles for the removal of protein-based toxins from food and/or other items [20,21][5][6]. Acinetobacter radioresistens was successfully used by Navon-Venezia et al. to produce Alasan [22][7]. Alasan is a compound of covalently bonded anionic polysaccharides that contain alanine-rich proteins. The emulsifying and surface activities of Alasan have been related to the compound’s three main proteins, which have molecular weights of 16, 31, and 45 kDa. According to Toren et al., the protein with a molecular mass of 45 kDa exhibited the highest emulsifying activity, exceeding even the intact alasan complex [23][8].
Mannoproteins are a class of glycoproteins isolated from the cell walls of a variety of yeasts. According to their chemical composition and specific functions in living systems, these molecules are classified as structural and enzymatic mannoproteins. The most abundant type of mannoprotein is structural, which consists of a small protein portion linked to a larger carbohydrate portion (mannopyranosyl), whereas enzymatic mannoproteins contain more protein moieties. Not only are these molecules effective emulsifiers, but they have also been linked to the stimulation of host immunity via the activation of immune cells and proteins as well as the induction of antibody production [24,25][9][10]. Figure 21 depicts the structure and mechanism of action of a number of significant emulsifiers produced by microorganisms through biotechnology processes.
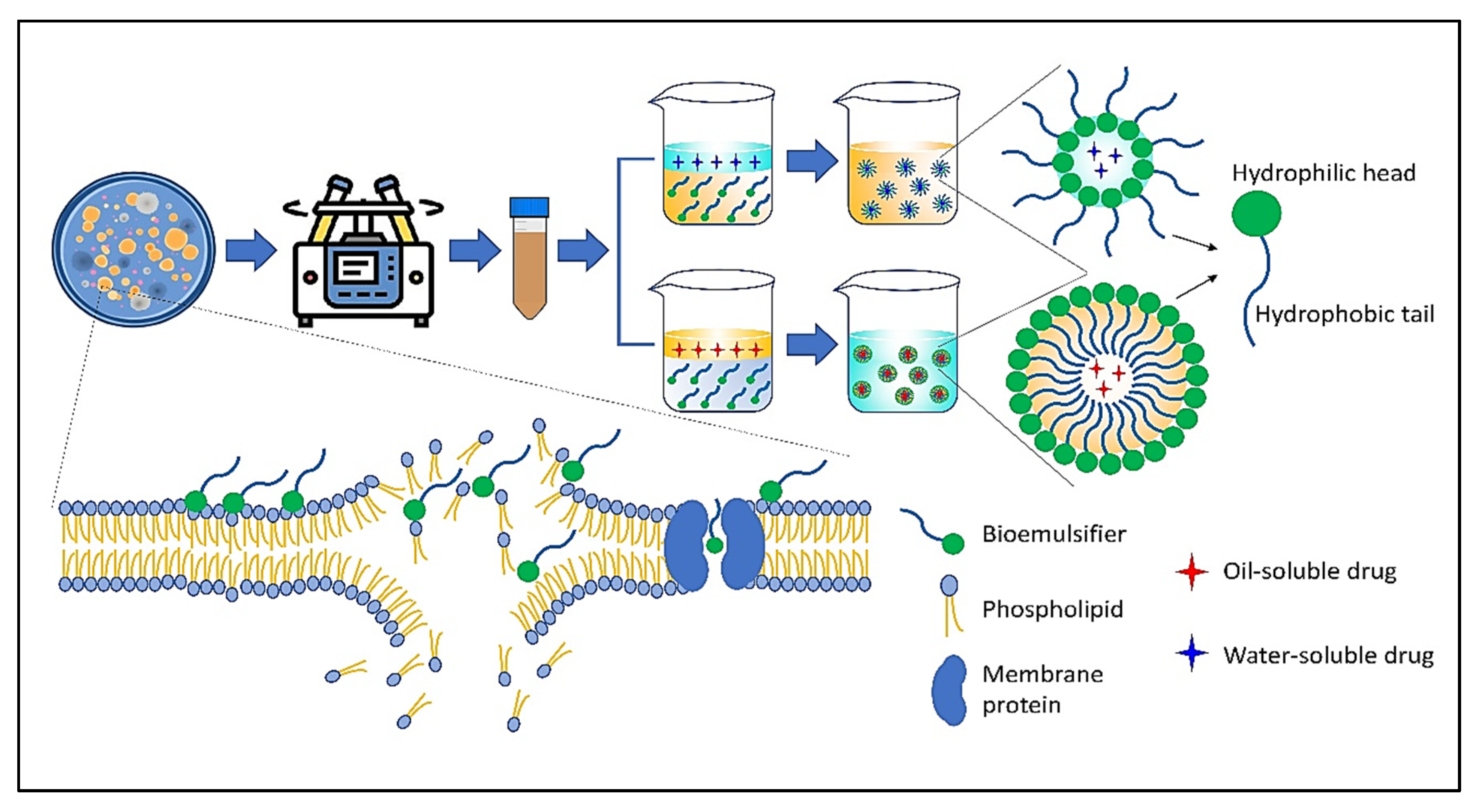
Figure 21.
The schematic and action mechanism of bioemulsifiers in emulsion systems.
2. Applications of Bioemulsifiers in Food Industry
The marketing of emulsifiers is expected to reach a value of USD 17.53 billion by 2027, while registering this growth at a rate of 6.90% for the forecast period of 2020 to 2027 [94][11]. Growing global demand for packaged foods worldwide is expected to create a new business opportunity for the market (Figure 32) [88][12]. The increasing use of emulsifiers in food products such as infant, child nutrition products and snacks are expected to enhance the market growth. Other factors such as increasing population health consciousness, rising disposable income, expansion in the cosmetics and personal care industry, and increasing concern about the food safety and quality will further provide the emulsifiers market in the forecast period of 2020 to 2027. However, these chemical emulsifiers cause negative impacts on gut health through impaired intestinal barrier function and increasing the incidence of inflammatory bowel disease (IBD). Researchers have produced emulsifiers using natural resources and the availability of a minor or non-toxic alternative, especially microorganisms due to restricted resources and high costs [95,96][13][14].
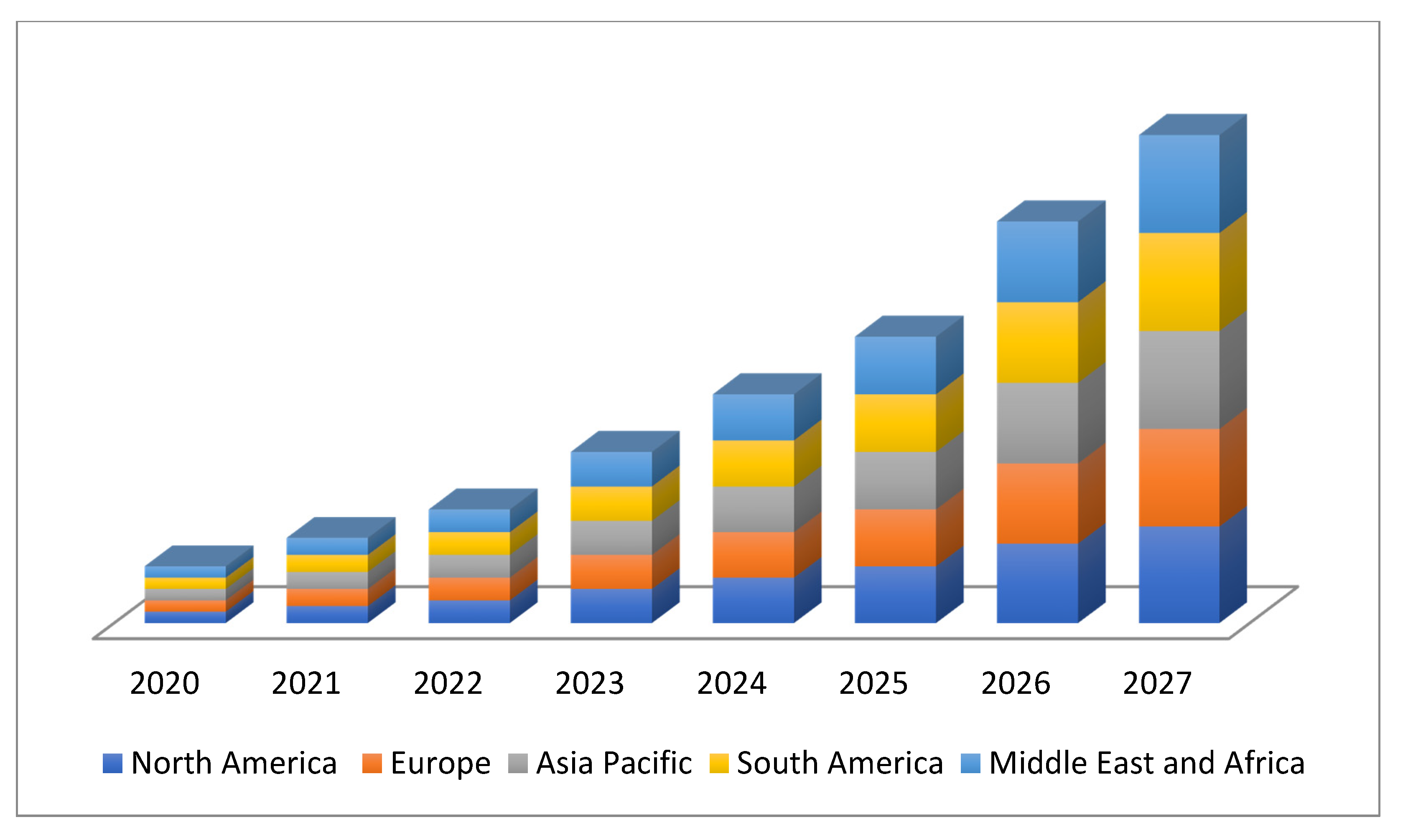
Figure 32.
Worldwide emulsifiers market size.
The unique natural properties of bioemulsifiers are the amphiphilicity (hydrophilic and hydrophobic) and their ability to reduce interfacial tension and surface area. Other interesting properties viz., coagulation, emulsification, cleansing, wetting, foaming ability, phase separation, surface activity and reduction in the oil viscosity permit their exploitation in many industries. Bioemulsifiers have a wide range of structural, compositional, and functional features due to the variety of their microbial origins, which include fungi [49[15][16],97], bacteria [98][17], and actinomycetes [99][18]. Figure 43 shows the main characteristics most bioemulsifiers may have to be considered as “emulsifier”. The bioemulsifiers such as liposan from Candida lipolytica were able to stabilize the emulsions of vegetable oils and water. It was also able to stabilize the corn oil, cottonseed oil, peanut oil, and soybean oil emulsions [100][19].

Figure 43.
Various biological and functional properties of bioemulsifiers.
The formulation of food determines several phases among particles [101][20]. Figure 32 shows basically the main types of emulsions that are important in a variety of foods. This precise structural organization of bioemulsifier molecules allows surface-active agents/emulsifiers to quintessence at the O/W interphase, leading to boosting the modynamic stability of an unstable system [102][21]. Because of their amphiphilic nature, emulsifiers have significant emulsifying powers and may be molded with starches and protein fractions of food items. Additionally, the partly digested fatty components are adequately emulsified/homogenized by bioemulsifiers. The emulsifier binds to protein portions of food items, causing them to aggregate together [103][22]. Mannor protein producing Saccharomyces cerevisiae facilitates the stabilization of W/O emulsions for products such as mayonnaise and ice creams [104][23]. Water in oil in water (W/O/W) and oil in water in oil (O/W/O) are two more sophisticated types of duplex emulsions (multiple) (Figure 54).
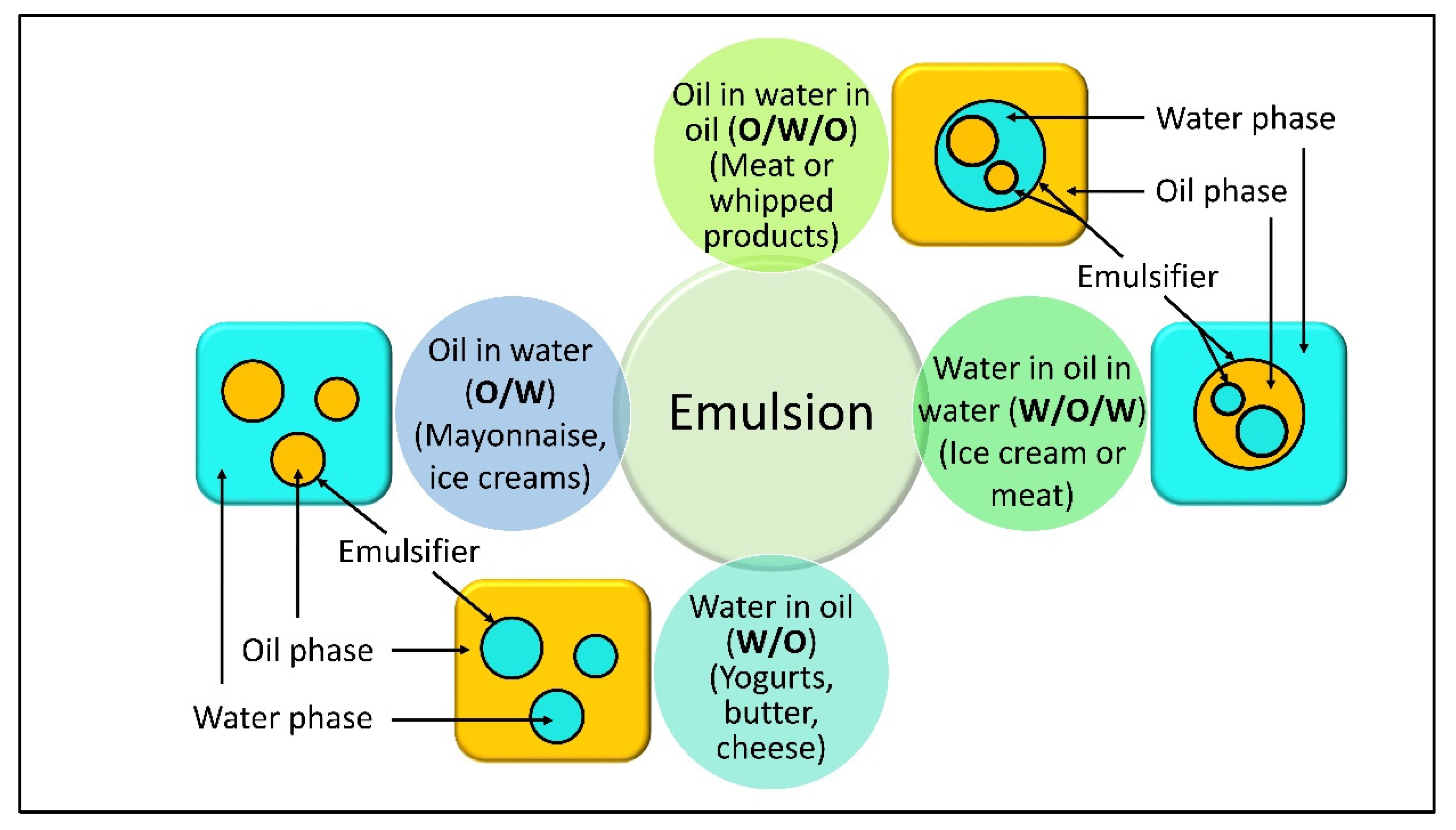
Figure 54.
Three main forms of emulsions important in a variety of foods.
Lipopolysaccharides, heteropolysaccharides, lipoproteins, glycoproteins, and proteins are regarded as beneficial for commercial applications as bioemulsifiers. A variety of new uses of new and well-known bioemulsifiers have been described in the recent three years. The excellent properties of both microbial produced biosurfactants and bioemulsifiers have features that make them desirable as natural emulsifiers for foods. Different studies have described the use of glycolipids to stabilize fat emulsions as well as glycolipids and lipopeptides as rheology modifiers in cookie and muffin dough [3,105][24][25]. Other studies have found that bioemulsifiers (such as exopolysaccharides and mannoproteins) have a high potential for aroma emulsification [106][26].
Incorporation of Bioemulsifiers in Food Formulations
-
Salad dressing formulation was prepared using sunflower oil, vinegar, water, egg powder, sugar, salt, starch, etc. with Candida-derived bioemulsifier (C. utilis 0.2–0.8% (w/v) combined with guar gum/carboxymethyl cellulose. The consistency and texture was improved using 0.7% of bioemulsifier [107][27
- 1
- summarizes some of the most interesting findings.
Table 51.
The latest (2015–2022) findings on some bioemulsifiers exhibiting potential activity.
| Bioemulsifiers | Microorganisms | Activity | Application | Reference |
|---|---|---|---|---|
| Lipopeptide | Bacillus licheniformis MS48 | Improving textural and sensorial properties | Yogurt | [109][29] |
| Glycolipoprotein | Acinetobacter indicus M6 | Antibacterial | Food control | [110][30] |
| Proteoglycan | Meyerozyma caribbica | Emulsifiers | Food industry | [111][31] |
| Exopolysaccharides (EPS) | Rhodobacter johrii CDR-SL 7 Cii | Emulsifier Emulsion Stabilizer | Food industry | [112][32] |
| Carbohydrate–lipid–protein complex | Candida utilis | Emulsifiers | Corn oil and Sunflower oil | [108][28] |
| Succinoglycan exopolysaccharide | Rhizobium radiobacter CAS | emulsion stabilization | Soybean oil | [113][33] |
| EPS | Pseudomonas fluorescens | Emulsifier | Food industry | [114][34] |
| EPS | Chromohalobacter canadensis 28 | Emulsifier Emulsion Stabilizer Foamer | Food industry | [108][28] |
| Glycoprotein | Lactobacillus plantarum subsp. | Emulsifiers | Food industry | [115][35] |
| Lipopeptide | Nesterenkonia sp. MSA31 | Antioxidant, Emulsifier, Emulsion Stabilizer | Food industry | [106][26] |
| emulsan-alginate | Pseudomonas stutzeri 273 | Removing protein-based toxins from food products | Food-processing contamination | [116][36] |
| Polyketide derivative | Penicillium chrysogenum | Emulsifiers | Oil | [117][37] |
- ].
-
Cookie dough formulation incorporated bioemulsifier from S. cerevisiae URM 6770, partially (2% (w/v)) or completely (4% (w/v)) substituting egg yolk in the existing formulation, and it showed similar physicochemical properties along with increasing the energy value of the cookies by providing fatty acids in the end product [3][24]. Table 5
References
- Alvarez, V.M.; Jurelevicius, D.; Serrato, R.V. Chemical characterization and potential application of exopolysaccharides produced by Ensifer adhaerens JHT2 as a bioemulsifier of edible oils. Int. J. Biol. Macromol. 2018, 114, 18–25.
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245.
- Pessoa, M.G.; Vespermann, K.A.C.; Paulino, B.N. Newly isolated microorganisms with potential application in biotechnology. Biotechnol. Adv. 2019, 37, 319–339.
- Mercaldi, M.P.; Dams-Kozlowska, H.; Panilaitis, B.; Joyce, A.P.; Kaplan, D.L. Discovery of the dual polysaccharide composition of emulsan and the isolation of the emulsion stabilizing component. Biomacromolecules 2008, 9, 1988–1996.
- Castro, G.R.; Kamdar, R.R.; Panilaitis, B.; Kaplan, D.L. Triggered release of proteins from emulsan–alginate beads. J. Control. Release 2005, 109, 149–157.
- Castro, G.R.; Panilaitis, B.; Kaplan, D.L. Emulsan, a tailorable biopolymer for controlled release. Biores. Technol. 2008, 99, 4566–4571.
- Navon-Venezia, S.; Zosim, Z.; Gottlieb, A.; Legmann, R.; Carmeli, S.; Ron, E.Z.; Rosenberg, E. Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl. Environ. Microbiol. 1995, 61, 3240–3244.
- Toren, A.; Navon-Venezia, S.; Ron, E.Z.; Rosenberg, E. Emulsifying activities of purified alasan proteins from Acinetobacter radioresistens KA53. Appl. Environ. Microbiol. 2001, 67, 1102–1106.
- Oliveira, M.C.; Figueiredo-Lima, D.F.; Faria Filho, D.E.; Marques, R.H.; Moraes, V.M.B.D. Effect of mannanoligosaccharides and/or enzymes on antibody titers against infectious bursal and Newcastle disease viruses. Arq. Bras. Med. Vet. Zootec. 2009, 61, 6–11.
- Snyman, C.; Mekoue Nguela, J.; Sieczkowski, N.; Marangon, M.; Divol, B. Optimised extraction and preliminary characterisation of mannoproteins from non-saccharomyces wine yeasts. Foods 2021, 10, 924.
- Emulsifiers Market Future on Recent Innovation 2026 Key Players|Corbion, BASF SE, Lonza., Stepan Company, Akzo Nobel N.V.; Estelle Chemicals Pvt. Ltd. 2020. Available online: https://www.openpr.com/news/2063526/emulsifiers-market-future-on-recent-innovation-2026-key-players (accessed on 14 March 2022).
- Haba, E.; Espuny, M.J.; Busquets, M.; Manresa, A. Screening and production of rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from waste frying oils. J. Appl. Microbiol. 2000, 88, 379–387.
- Emulsifiers Market Analysis. Available online: https://www.coherentmarketinsights.com/market-insight/emulsifiers-market-3850 (accessed on 16 March 2022).
- Data Bridge Market Research. Available online: https://www.databridgemarketresearch.com/reports/global-food-emulsifiers-market (accessed on 8 April 2022).
- Cameron, D.R.; Cooper, D.G.; Neufeld, R.J. The mannoprotein of Saccharomyces cerevisiae is an effective bioemulsifier. Appl. Environ. Microbiol. 1998, 54, 1420–1425.
- Zijarde, S.S.; Pant, A. Emulsifier from a tropical marine yeast, Yarrowia lipolytica NCIM 3589. J. Basic Microbiol. 2002, 42, 67–73.
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158.
- Zambry, N.S.; Ayoib, A.; Md Noh, N.A.; Yahya, A.R.M. Production and partial characterization of biosurfactant produced by Streptomyces sp. R1. Bioprocess Biosyst. Eng. 2017, 40, 1007–1016.
- Adamczak, M. Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnol. Lett. 2000, 22, 313–316.
- Kralova, I.; Sjöblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383.
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977.
- Hamzah, A.F.; Al Tamimi, W.H. Enhanced oil recovery by sand packed column supplemented with biosurfactants produced by local oil fields bacteria. Marsh Bull. 2021, 16, 135–143.
- Moreira, T.C.P.; da Silva, V.M.; Gombert, A.K.; da Cunha, R.L. Stabilization mechanisms of oil-in-water emulsions by Saccharomyces cerevisiae. Colloids Surf. B Biointerfaces 2016, 143, 399–405.
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Potential food application of a biosurfactant produced by Saccharomyces cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020, 8, 434.
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of lipopeptide biosurfactant by a marine Nesterenkonia sp. and its application in food industry. Front. Microbiol. 2017, 8, 1138.
- Kavitake, D.; Kalahasti, K.K.; Devi, P.B.; Ravi, R.; Shetty, P.H. Galactan exopolysaccharide based flavour emulsions and their application in improving the texture and sensorial properties of muffin. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100248.
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Characterization and application of a biosurfactant isolated from Candida utilis in salad dressings. Biodegradation 2019, 30, 313–324.
- Ravindran, A.; Kiran, G.S.; Selvin, J. Revealing the effect of lipopeptide on improving the probiotics characteristics: Flavor and texture enhancer in the formulated yogurt. Food Chem. 2022, 375, 131718.
- Karlapudi, A.P.; Venkateswarulu, T.C.; Srirama, K.; Kota, R.K.; Mikkili, I.; Kodali, V.P. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ. Sci. 2020, 32, 223–227.
- Bhaumik, M.; Dhanarajan, G.; Chopra, J.; Kumar, R.; Hazra, C.; Sen, R. Production, partial purification and characterization of a proteoglycan bioemulsifier from an oleaginous yeast. Bioprocess Biosyst. Eng. 2020, 43, 1747–1759.
- Sran, K.S.; Sundharam, S.S.; Krishnamurthi, S.; Choudhury, A.R. Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 2019, 127, 240–249.
- Kavitake, D.; Marchawala, F.Z.; Delattre, C.; Shetty, P.H.; Pathak, H.; Andhare, P. Biotechnological potential of exopolysaccharide as a bioemulsifier produced by Rhizobium radiobacter CAS isolated from curd. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100202.
- Vidhyalakshmi, R.; Nachiyar, C.V.; Kumar, G.N.; Sunkar, S.; Badsha, I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of Pseudomonas fluorescens. Biocatal. Agric. Biotechnol. 2018, 16, 320–325.
- Radchenkova, N.; Boyadzhieva, I.; Atanasova, N.; Poli, A.; Finore, I.; Di Donato, P.; Nicolaus, B.; Panchev, I.; Kuncheva, M.; Kambourova, M. Extracellular polymer substance synthesized by a halophilic bacterium Chromohalobacter canadensis 28. Appl. Microbiol. Biotechnol. 2018, 102, 4937–4949.
- Bakhshi, N.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S. Production and Partial Characterization of a Glycoprotein Bioemulsifier Produced by Lactobacillus plantarum subsp. plantarum PTCC 1896. J. Agric. Sci. Technol. 2018, 20, 37–49.
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and Anti-Infection of a Marine Bacterial Exopolysaccharide Against Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 102.
- Salo, O.V.; Ries, M.; Medema, M.H.; Lankhorst, P.P.; Vreeken, R.J.; Bovenberg, R.A.; Driessen, A.J. Genomic mutational analysis of the impact of the classical strain improvement program on β–lactam producing Penicillium chrysogenum. BMC Genom. 2015, 16, 937.
More
