EAF slag is a non-metallic by-product that consists mainly of silicates and oxides formed during the process of refining the molten steel. Raw EAF slag often appears as grey or black colored lumps, depending on its ferrous oxide content. This type of slag generally has a rough surface texture, with a surface pore diameter of 0.01–10 μm.
- EAF steel slag waste
- Steel making industry
1. Introduction
Steel is one of the most popular construction materials in the world. This alloy is typically used as support for structural frameworks of all sorts of constructions, ranging from skyscrapers to highway construction. The main reason why steel is so commonly used is simply due to its unique combination of strength, durability, workability, and cost. However, while being one of the world's largest industries, the steelmaking industry is known to have significant negative impacts on the environment [1][2]. Although steel can be produced through recycling scrap iron, researchers have estimated that about two billion tons of iron ore and one billion ton of metallurgical coal are used in the global steel industry every year [3]. In addition to the raw material requirement, there are also issues regarding the steel slag by-product disposal. Based on previous reports, around 190–290 million tons of steel slag are generated every year [4]. Most of the global steel slag ends up being disposed of, with only a small portion recycled [5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20]. Studies have also shown that the recycling rate of steel slag is still generally much lower in Asian countries [2][21][22][23][24][25][26][27][28][29][30][31][32][33][34].
With the Earth’s ability to sustain life eroding every day, there is an urgent need to reduce the waste produced and preserve the non-renewable resources. Thus, the present review’s main goal was to assess the EAF steel slag's recycling potential, especially for the Malaysian steelmaking industry. Possible steel slag recycling options were evaluated based on the engineering properties. Moreover, as sustainable development has been highlighted these past few years, it is generally known that countries with higher human development tend to have extreme environmental problems [35].
2. Characterization of Steel Slag
Generally, the properties of the steel slag generated varies depending on the manufacturer, types of steel produced as well as cooling conditions of the slag. Therefore, before steel slag can be recycled into greener products, it is essential to investigate and understand the slag properties. This includes how steel slag is formed, its chemical compositions, mineralogical behavior, and hazardous concerns. The scope of discussion in the present review will focus on EAF steel slag, which is the most common steel by-product in Malaysia.
2.1. Formation of Electric Arc Furnace (EAF) Slag
EAF slag is a non-metallic by-product that consists mainly of silicates and oxides formed during the process of refining the molten steel. As mentioned previously, the feed materials for EAF are mainly steel scrap and pig iron. It is generally known that steel scrap contains impurities such as phosphorus (P), aluminum (Al), manganese (Mn), and silicon (Si). The presence of these impurities deteriorates the mechanical properties of the steel product. Thus, it is essential to perform additional refining or treatment processes to remove the impurities from the molten steel. During this refining process, oxygen gas is injected directly into the molten steel to oxidize the impurities that are present. These oxidized impurity compounds combine with the lime added during the refining process, forming a layer of molten slag. The molten slag has a lower density than the molten steel and will remain on top of the molten steel [36][37]. Upon completion of the melting process and the steel has achieved its desired chemical composition, the slag and molten steel will be discharged separately [19]. Thus, the EAF slag might contain a low amount of iron oxide that originates from iron oxidation when oxygen is injected.
The EAF slag actually plays several other roles in refining the molten steel, in addition to its main function of absorbing deoxidation products and impurities from the molten steel [38]. One of these is to protect the electrodes and refractories in the furnace from thermal radiation by inhibiting oxidation [39]. The slag also protects the molten steel against re-oxidation and acts as a heat insulator to prevent heat loss to the surroundings. This would serve to increase the thermal efficiency in EAF by refining the furnace heat-up rate, maximizing the active power of the transformer and better electrical stability.
2.2. Properties of EAF Slag
Raw EAF slag often appears as grey or black colored lumps, depending on its ferrous oxide content [40][41]. This type of slag generally has a rough surface texture, with a surface pore diameter of 0.01–10 μm [42]. Examples of EAF slag from different countries is shown in Figure 31. EAF slag is generally categorized as aggregates with a particle size range of 5–40 mm, and has a similar appearance to aggregates that are commonly used in the construction industry [43]. It is known that EAF slag from different regions and different manufacturers can exhibit a different appearance and physical properties, depending on the composition of steel scrap that is used as feed materials, the type of furnace, steel grades and refining processes. Nonetheless, EAF slag typically has Mohs hardness values in the range of 6–7, regardless of the differences in chemical compositions [44].
The water absorption and density of EAF slag from different sources (including Malaysia) are presented in Table 1. From this table, the water absorption of EAF slag is around 0.5–4.0%, while its density is in the range of 2.8–3.9 g/cm3. There is no clear connection between the water absorption and density of EAF slag. Water absorption is an essential property for the EAF slag, as it represents the ability of fluids penetrating the slag body and causes degradation. It is reported that degradation such as carbonation occurs when EAF slag with high basic (alkaline) contents (i.e., Ca and Mg oxides) is exposed to an acidic source (i.e., CO2 or H2CO3). The process of acidic source neutralization forms a decomposed carbonate phase [45][46]. Although it is generally agreed that more porous EAF slag would have higher water absorption values, this does not necessarily translate to a higher density. Instead, EAF slag with a higher iron content could potentially have a higher density.
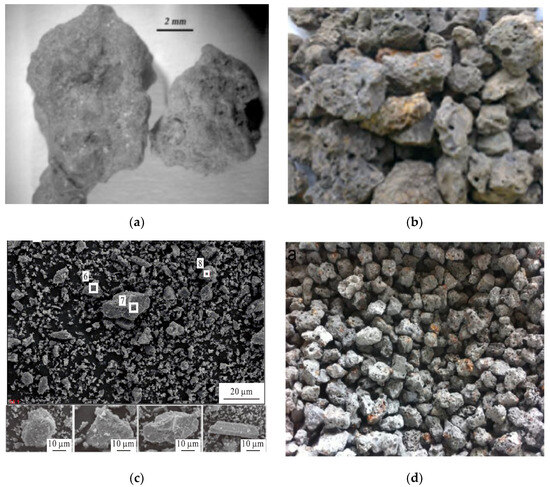
Figure 31. EAF slag samples from different countries. (a) Italy [43]; (b) Malaysia [47]; (c) China [27]; and (d) Spain [11]. Color variation of EAF steel slag is due to the different chemical compositions and oxidation conditions.
Table 1. Water absorption and density of electric arc furnace (EAF) slag as reported in various studies.
|
Water absorption (%) |
Density (g/cm3) |
Sources |
References |
|
– |
3.30–3.60 |
China |
[33] |
|
2.00 |
1.54–2.90 |
India |
[23] |
|
2.60 |
2.80 |
France |
[10] |
|
3.90 |
2.82–3.05 |
Malaysia |
[48] |
|
2.30–3.40 |
3.63–3.76 |
Italy |
[42] |
|
0.950 |
3.85 |
Italy |
[49] |
|
<4.00 |
3.40 |
Spain |
[11] |
|
1.12–3.55 |
3.51–3.64 |
Spain |
[12] |
|
1.12 |
3.42 |
Spain |
[13] |
|
2.93 |
3.40 |
Vietnam |
[28] |
|
– |
2.84 |
China |
[27] |
|
1.50 |
3.56 |
Malaysia |
[50] |
2.3. Chemical Composition and Mineralogy of EAF Slag
The chemical composition of EAF slag is most commonly analyzed using x-ray fluorescence (XRF) spectroscopy. The weight percentage of each element present in the EAF slag, as reported by several sources including Malaysia, is presented in Table 2. In this table, the different types of iron oxides (i.e., FeO, Fe2O3, or Fe3O4) in EAF slag are represented as total Fe (FeOx). The main elements in the EAF slag are iron (Fe), calcium (Ca), silicon (Si), and aluminum (Al) oxides, while the minor elements in the EAF slag are magnesium (Mg) and manganese (Mn) oxides, although it should be mentioned that trace elements in the EAF slag with wt.% of <1.0% such as lead (Pb), phosphorus (P), and fluoride (F) were not included in Table 2. It is believed that the compositions of EAF slag from different sources would vary often, based on the composition of the scrap steel materials used for the steel production, the grade of steel produced, and the condition of the EAF refractory lining [19][38][51]. The inconsistent chemical composition issue is one of the main reasons that prevent EAF slag from being effectively recycled into new products at an industrial scale. Thus, in order to ensure a thorough representative of the slag sample and reproducibility of the analysis, a proper sampling method has to be performed prior to its recycling.
Table 2. Typical chemical composition of EAF slag reported by several researchers.
|
EAF slag Source |
Type of steel |
Chemical composition |
Reference |
|||||
|
CaO |
Total Fe |
SiO2 |
Al2O3 |
MgO |
MnO |
|||
|
Iran |
– |
34.0 |
25.0 |
14.0 |
5.00 |
14.0 |
2.00 |
[6] |
|
India |
– |
22.8 |
42.4 |
20.3 |
7.30 |
8.00 |
– |
[21] |
|
China |
|
30.0–50.0 |
5.00–22.0 |
11.0–20.0 |
10.0–18.0 |
8.00–13.0 |
5.00–10.0 |
[33] |
|
Malaysia |
– |
27.5 |
33.3 |
19.3 |
9.40 |
3.07 |
3.55 |
[52] |
|
Egypt |
– |
33.0 |
36.8 |
13.1 |
5.51 |
5.03 |
– |
[7] |
|
Malaysia |
– |
29.0–29.5 |
31.7–32.5 |
19.7–20.5 |
8.83–8.58 |
2.60–3.13 |
3.94–3.95 |
[22] |
|
Italy |
|
27.9 |
37.5 |
9.71 |
8.21 |
2.17 |
4.68 |
[9] |
|
Malaysia |
Carbon steel |
16.9 |
43.4 |
26.4 |
4.84 |
1.86 |
2.66 |
[47] |
|
Malaysia |
– |
27.2 |
33.3 |
20.8 |
9.19 |
2.06 |
3.98 |
[29] |
|
France |
Stainless steel |
41.7 |
0.540 |
34.7 |
6.26 |
9.06 |
2.15 |
[10] |
|
Italy |
– |
26.0 |
35.0 |
14.0 |
12.0 |
5.00 |
6.00 |
[42] |
|
Spain |
Carbon steel |
27.7 |
26.8 |
19.1 |
13.7 |
2.50 |
5.30 |
[12] |
|
Spain |
Carbon steel |
25.0–35.0 |
17.0–50.0 |
10.0–20.0 |
3.00–10.0 |
2.00–9.00 |
<6.00 |
[11] |
|
Spain |
– |
32.9 |
22.3 |
20.3 |
12.2 |
3.00 |
5.10 |
[13] |
|
Malaysia |
– |
29.9 |
22.0 |
21.4 |
9.60 |
4.89 |
– |
[53] |
|
Vietnam |
Carbon steel |
25.9 |
34.7 |
16.3 |
8.31 |
6.86 |
5.18 |
[28] |
|
China |
Stainless steel |
43.2 |
7.54 |
27.8 |
2.74 |
7.35 |
0.680 |
[27] |
|
Spain |
– |
26.7 |
24.5 |
20.9 |
12.1 |
3.20 |
4.60 |
[15] |
|
Malaysia |
– |
26.2 |
28.6 |
18.1 |
5.88 |
5.80 |
4.14 |
[54] |
|
Malaysia |
– |
20.9 |
43.0 |
10.8 |
6.86 |
1.65 |
– |
[30] |
|
Malaysia |
|
30.0 |
27.3 |
17.3 |
4.67 |
5.39 |
5.03 |
[50] |
|
Iran |
– |
33.3 |
25.9 |
19.5 |
4.88 |
4.25 |
– |
[17] |
EAF slag is well known for its highly complex crystallinity due to the presence of several mineral phases [9]. The mineral phases present in EAF slag from different sources, identified through x-ray diffraction (XRD) analysis, are shown in Table 3. According to Chiang and Pan (2017), the crystalline phases in EAF slag can be divided into those that consist of iron oxides (i.e., wustite, magnesioferrite, magnetite, and hematite), silicates (i.e., larnite, bregedite/merwinite, and gehlenite), and manganese oxides (i.e., birnessite, hausmannite, rutile/hollandite, and groutellite) [55]. In most cases, each of these minerals’ XRD patterns overlaps with the other and needs to be appropriately identified [9][19][55]. The mineralogy and crystalline phases in the EAF slag are dependent on the chemical compositions of the molten slag and the cooling process. Both of these factors will result in the variation of crystalline phases being formed in the slag. It is known that each chemical composition in the EAF slag exhibits specific properties that would serve to provide different functions when recycled into new products, as described in Table 4 [26][33].
Table 3. Typical crystalline phases present in EAF slag proposed by various researchers.
|
EAF slag source |
Crystalline phases |
Reference |
||||||
|
Bredigite |
Gehlenite |
Larnite |
Merwinite |
Hematite |
Wustite |
Magnetite |
||
|
Sweden |
|
|
✓ |
✓ |
✓ |
|
|
[5] |
|
Iran |
✓ |
|
|
|
|
✓ |
|
[6] |
|
India |
✓ |
✓ |
|
|
|
✓ |
|
[21] |
|
UAE |
✓ |
|
✓ |
|
|
✓ |
✓ |
[41] |
|
Italy |
|
|
✓ |
|
✓ |
✓ |
|
[56] |
|
Malaysia |
|
✓ |
✓ |
|
|
✓ |
|
[22] |
|
Italy |
|
|
✓ |
|
✓ |
✓ |
|
[9] |
|
Malaysia |
|
✓ |
✓ |
|
✓ |
✓ |
|
[57] |
|
Malaysia |
|
✓ |
|
|
|
|
|
[53] |
|
Malaysia |
|
|
✓ |
|
✓ |
✓ |
✓ |
[50] |
Table 4. Chemical composition in the EAF slag and possible recycle functions.
|
Chemical composition |
Possible recycle functions |
|
FeOx |
Iron reclamation |
|
CaO, MgO, FeO, MgO, MnO |
Fluxing agent |
|
C3S, C2S, and C4AF |
Cementation composition in cement and concrete production |
|
CaO, MgO |
Carbon dioxide capture and flue gas desulfurization |
|
FeO, CaO, SiO2 |
Raw material for cement clinker |
|
CaO, SiO2, MgO, P2O5, and FeO |
Fertilizer and soil improvement |
2.4. Leaching Behavior and Hazardous Concerns of EAF Slag
The leaching behavior assessment is often conducted to measure the amount of heavy metals released when EAF slag is exposed to a water source. This assessment would serve as a guideline to ensure that EAF slag is non-hazardous, environmentally friendly, and safe to be incorporated into new products. The experimental methodology for the leaching assessment of EAF slag reported in most literature is based on the EN 12457 European Standard [9][40][52][57][58][59].
Through the leaching assessment, concentrations of toxic heavy metals leached from the EAF slag are evaluated and benchmarked with the regulations specified by the Department of Environment of respective countries [40]. Some of the known heavy metals that might be present in EAF slag are Cd, Cr, Cu, Mn, Pb, and Zn. Although these heavy metals often appear only as trace elements, they serve as key factors in pollution and toxicity [60]. Researchers have suspected that the presence of these heavy metal impurities originated from the scrap steel feed materials for steel production. However, most literature has reported that the concentrations of these heavy metals are kept within the safety limit as regulated by the respective country, and therefore, are safe to be recycled.
EAF slag is considered basic (alkaline) by nature, mainly due to its large amount of CaO. When exposed to a water source, EAF slag undergoes a hydrolysis reaction, forming a Ca–CO3–OH ion matrix solution that exhibits a pH of 10.0–12.5 [61][62]. The basicity of EAF slag is comparable to concrete and can be used to increase soil pH. Some researchers have also suggested using EAF slag to neutralize environmental issues such as acid mine drainage [63][64][65]. Nonetheless, the relatively high basicity of EAF slag could cause several complications. First, it is reported that the high pH condition stimulates the mobility of various oxyanion-forming contaminations such as vanadium, chromium, barium, and molybdenum [62][66]. Chaurand et al. (2007) reported the presence of vanadate (oxyanion of vanadium) under highly basic conditions [66]. Second, the basic condition also leads to the precipitation of carbonate minerals in water sources such as rivers and lakes. Long-term effects in the increment of pH in these water sources would lead to water quality deterioration, eventually diminishing the diversity of the invertebrate and fish [62][67]. This would further highlight the drawbacks of EAF slag landfilling and the necessity to recycle the slag into new products.
References
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour. Conserv. Recycl. 2006, 48, 301–314, doi:10.1016/j.resconrec.2006.03.003.
- Schino, A.D. Environmental Impact of Steel Industry. In Handbook of Environmental Materials Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21.
- World Steel Association. Steel Stastical Yearbook. Available online: https://www.worldsteel.org/steel-by-topic/statistics/steel-statistical-yearbook.html. (accessed on 11 September 2020).
- USGS Iron and Steel Slag Data Sheet - Mineral Commodity Summaries 2020 Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-iron-steel-slag.pdf (accessed on 11 September 2020).
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344, doi:10.1016/j.wasman.2006.08.002.
- Badiee, H.; Maghsoudipour, A.; Raissi Dehkordi, B. Use of Iranian steel slag for production of ceramic floor tiles. Adv. Appl. Ceram. 2008, 107, 111–115, doi:10.1179/174367608X263377.
- Hekal, E.E.; Abo-El-Enein, S.A.; El-Korashy, S.A.; Megahed, G.M.; El-Sayed, T.M. Hydration characteristics of Portland cement – Electric arc furnace slag blends. HBRC J. 2013, 9, 118–124, doi:10.1016/j.hbrcj.2013.05.006.
- Andreas, L.; Diener, S.; Lagerkvist, A. Steel slags in a landfill top cover – Experiences from a full-scale experiment. Waste Manag. 2014, 34, 692–701, doi:10.1016/j.wasman.2013.12.003.
- Cornacchia, G.; Agnelli, S.; Gelfi, M.; Ramorino, G.; Roberti, R. Reuse of EAF Slag as Reinforcing Filler for Polypropylene Matrix Composites. JOM 2015, 67, 1370–1378, doi:10.1007/s11837-015-1396-6.
- Adegoloye, G.; Beaucour, A.-L.; Ortola, S.; Noumowe, A. Mineralogical composition of EAF slag and stabilised AOD slag aggregates and dimensional stability of slag aggregate concretes. Constr. Build. Mater. 2016, 115, 171–178, doi:10.1016/j.conbuildmat.2016.04.036.
- Skaf, M.; Manso, J.M.; Aragón, Á.; Fuente-Alonso, J.A.; Ortega-López, V. EAF slag in asphalt mixes: A brief review of its possible re-use. Resour. Conserv. Recycl. 2017, 120, 176–185, doi:10.1016/j.resconrec.2016.12.009.
- Fuente-Alonso, J.A.; Ortega-López, V.; Skaf, M.; Aragón, Á.; San-José, J.T. Performance of fiber-reinforced EAF slag concrete for use in pavements. Constr. Build. Mater. 2017, 149, 629–638, doi:10.1016/j.conbuildmat.2017.05.174.
- Santamaría, A.; Orbe, A.; Losañez, M.M.; Skaf, M.; Ortega-Lopez, V.; González, J.J. Self-compacting concrete incorporating electric arc-furnace steelmaking slag as aggregate. Mater. Des. 2017, 115, 179–193, doi:10.1016/j.matdes.2016.11.048.
- Ortega-López, V.; Fuente-Alonso, J.A.; Santamaría, A.; San-José, J.T.; Aragón, Á. Durability studies on fiber-reinforced EAF slag concrete for pavements. Constr. Build. Mater. 2018, 163, 471–481, doi:10.1016/j.conbuildmat.2017.12.121.
- García-Cuadrado, J.; Santamaría-Vicario, I.; Rodríguez, A.; Calderón, V.; Gutiérrez-González, S. Lime-cement mortars designed with steelmaking slags as aggregates and validation study of their properties using mathematical models. Constr. Build. Mater. 2018, 188, 210–220, doi:10.1016/j.conbuildmat.2018.08.093.
- Lizárraga, J.M.; Gallego, J. Self-Healing Analysis of Half-Warm Asphalt Mixes Containing Electric Arc Furnace (EAF) Slag and Reclaimed Asphalt Pavement (RAP) Using a Novel Thermomechanical Healing Treatment. Materials (Basel). 2020, 13, 2502, doi:10.3390/ma13112502.
- Motevalizadeh, S.M.; Sedghi, R.; Rooholamini, H. Fracture properties of asphalt mixtures containing electric arc furnace slag at low and intermediate temperatures. Constr. Build. Mater. 2020, 240, 117965, doi:10.1016/j.conbuildmat.2019.117965.
- Kirschen, M.; Jung, I.-H.; Hackl, G. Phase Equilibrium Diagram for Electric Arc Furnace Slag Optimization in High Alloyed Chromium Stainless Steelmaking. Metals 2020, 10, 826, doi:10.3390/met10060826.
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 1–13, doi:10.1155/2011/463638.
- Chukwudi, B.C.; Ademusuru, P.O.; Okorie, B.A. Characterization of Sintered Ceramic Tiles Produced from Steel Slag. J. Miner. Mater. Charact. Eng. 2012, 11, 863–868.
- Sarkar, R.; Singh, N.; Das Kumar, S. Utilization of steel melting electric arc furnace slag for development of vitreous ceramic tiles. Bull. Mater. Sci. 2010, 33, 293–298, doi:10.1007/s12034-010-0045-5.
- Teo, P.-T.; Anasyida, A.S.; Basu, P.; Nurulakmal, M.S. Recycling of Malaysia’s electric arc furnace (EAF) slag waste into heavy-duty green ceramic tile. Waste Manag. 2014, 34, 2697–2708, doi:10.1016/j.wasman.2014.08.015.
- Sekaran, A.; Palaniswamy, M.; Balaraju, S. A Study on Suitability of EAF Oxidizing Slag in Concrete: An Eco-Friendly and Sustainable Replacement for Natural Coarse Aggregate. Sci. World J. 2015, 2015, 1–8, doi:10.1155/2015/972567.
- Lim, J.W.; Chew, L.H.; Choong, T.S.Y.; Tezara, C.; Yazdi, M.H. Utilizing steel slag in environmental application - An overview. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012067, doi:10.1088/1755-1315/36/1/012067.
- Zhao, J.; Yan, P.; Wang, D. Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J. Clean. Prod. 2017, 156, 50–61, doi:10.1016/j.jclepro.2017.04.029.
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330, doi:10.1016/j.wasman.2018.04.045.
- Saly, F.; Guo, L.; Ma, R.; Gu, C.; Sun, W. Properties of Steel Slag and Stainless Steel Slag as Cement Replacement Materials: A Comparative Study. J. Wuhan Univ. Technol. Sci. Ed. 2018, 33, 1444–1451, doi:10.1007/s11595-018-1989-3.
- Lam, M.N.-T.; Le, D.-H.; Jaritngam, S. Compressive strength and durability properties of roller-compacted concrete pavement containing electric arc furnace slag aggregate and fly ash. Constr. Build. Mater. 2018, 191, 912–922, doi:10.1016/j.conbuildmat.2018.10.080.
- Teo, P.T.; Anasyida, A.S.; Kho, C.M.; Nurulakmal, M.S. Recycling of Malaysia’s EAF steel slag waste as novel fluxing agent in green ceramic tile production: Sintering mechanism and leaching assessment. J. Clean. Prod. 2019, 241, 118144, doi:10.1016/j.jclepro.2019.118144.
- Omale, S.O.; Choong, T.S.Y.; Abdullah, L.C.; Siajam, S.I.; Yip, M.W. Utilization of Malaysia EAF slags for effective application in direct aqueous sequestration of carbon dioxide under ambient temperature. Heliyon 2019, 5, e02602, doi:10.1016/j.heliyon.2019.e02602.
- Li, C.-C.; Lin, C.-M.; Chang, Y.-E.; Chang, W.-T.; Wu, W. Stabilization and Crystal Characterization of Electric Arc Furnace Oxidizing Slag Modified with Ladle Furnace Slag and Alumina. Metals. 2020, 10, 501, doi:10.3390/met10040501.
- Bankole, L.K.; Rezan, S.A.; Sharif, N.M. Thermodynamic Modeling of Mineral Phases Formation in EAF Slag System and Its Application As Agricultural Fertilizer. SEAISI Q. J. 2011, 40, 26–32.
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801, doi:10.1016/j.proenv.2012.10.108.
- Yusuf, M.; Chuah, L.A.; Mohammed, M.A.; Shitu, A. Investigations of Nickel (II) removal from Aqueous Effluents using Electric Arc Furnace Slag. Res. J. Chem. Sci. 2013, 3, 29–37.
- Yumashev, A.; Ślusarczyk, B.; Kondrashev, S.; Mikhaylov, A. Global Indicators of Sustainable Development: Evaluation of the Influence of the Human Development Index on Consumption and Quality of Energy. Energies 2020, 13, 2768.
- Hosseini, S.; Soltani, S.M.; Fennell, P.S.; Choong, T.S.Y.; Aroua, M.K. Production and applications of electric-arc-furnace slag as solid waste in environmental technologies: A review. Environ. Technol. Rev. 2016, 5, 1–11.
- Ducman, V.; Mladenovič, A. The potential use of steel slag in refractory concrete. Mater. Charact. 2011, 62, 716–723.
- Shi, C. Steel Slag—Its Production, Processing, Characteristics, and Cementitious Properties. J. Mater. Civ. Eng. 2004, 16, 230–236.
- Luz, A.P.; Tomba Martinez, A.G.; López, F.; Bonadia, P.; Pandolfelli, V.C. Slag foaming practice in the steelmaking process. Ceram. Int. 2018, 44, 8727–8741.
- Pellegrino, C.; Gaddo, V. Mechanical and durability characteristics of concrete containing EAF slag as aggregate. Cem. Concr. Compos. 2009, 31, 663–671.
- Abu-Eishah, S.I.; El-Dieb, A.S.; Bedir, M.S. Performance of concrete mixtures made with electric arc furnace (EAF) steel slag aggregate produced in the Arabian Gulf region. Constr. Build. Mater. 2012, 34, 249–256.
- Monosi, S.; Ruello, M.L.; Sani, D. Electric arc furnace slag as natural aggregate replacement in concrete production. Cem. Concr. Compos. 2016, 66, 66–72.
- Suh, M.; Troese, M.J.; Hall, D.A.; Yasso, B.; Yzenas, J.J.; Proctor, D.M. Evaluation of electric arc furnace-processed steel slag for dermal corrosion, irritation, and sensitization from dermal contact. J. Appl. Toxicol. 2014, 34, 1418–1425.
- Grubeša, I.N.; Barisic, I.; Fucic, A.; Bansode, S.S. Characteristics and Uses of Steel Slag in Building Construction; Elsevier Woodhead Publishing: Chennai, India, 2016; ISBN 9780081003688.
- Suer, P.; Lindqvist, J.-E.; Arm, M.; Frogner-Kockum, P. Reproducing ten years of road ageing—Accelerated carbonation and leaching of EAF steel slag. Sci. Total Environ. 2009, 407, 5110–5118.
- Baciocchi, R.; Costa, G.; Di Bartolomeo, E.; Polettini, A.; Pomi, R. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valoriz. 2010, 1, 467–477.
- Roslan, N.H.; Ismail, M.; Khalid, N.H.A.; Muhammad, B. Properties of concrete containing electric arc furnace steel slag and steel sludge. J. Build. Eng. 2020, 28, 101060.
- Oluwasola, E.A.; Hainin, M.R.; Aziz, M.M.A. Comparative evaluation of dense-graded and gap-graded asphalt mix incorporating electric arc furnace steel slag and copper mine tailings. J. Clean. Prod. 2016, 122, 315–325.
- Faleschini, F.; Brunelli, K.; Zanini, M.A.; Dabalà, M.; Pellegrino, C. Electric Arc Furnace Slag as Coarse Recycled Aggregate for Concrete Production. J. Sustain. Metall. 2016, 2, 44–50.
- Lim, J.S.; Cheah, C.B.; Ramli, M.B. The setting behavior, mechanical properties and drying shrinkage of ternary blended concrete containing granite quarry dust and processed steel slag aggregate. Constr. Build. Mater. 2019, 215, 447–461.
- Sas, W.; Głuchowski, A.; Radziemska, M.; Dzięcioł, J.; Szymański, A. Environmental and Geotechnical Assessment of the Steel Slags as a Material for Road Structure. Materials 2015, 8, 4857–4875.
- Lateef, K.B.; Rezan, S.A.; Nurulakmal, M.S. Assessment of EAF Steel Slag Solubility by Statistical Design. Adv. Mater. Res. 2013, 858, 228–235.
- Lim, J.W.; Lee, K.F.; Chong, T.S.Y.; Abdullah, L.C.; Razak, M.A.; Tezara, C. Phosphorus removal by electric arc furnace steel slag adsorption. IOP Conf. Ser. Mater. Sci. Eng. 2017, 257, 012063.
- Cheah, C.B.; Jasme, N. Preliminary Study on Properties of Supersulfated Flowable Mortars Containing Electric Arc Furnace Slag as Fine Aggregate. Int. J. Eng. Technol. 2018, 7, 371–374.
- Chiang, P.-C.; Pan, S.-Y. Carbon Dioxide Mineralization and Utilization; Springer: Singapore, 2017; ISBN 978-981-10-3267-7.
- Pellegrino, C.; Cavagnis, P.; Faleschini, F.; Brunelli, K. Properties of concretes with Black/Oxidizing Electric Arc Furnace slag aggregate. Cem. Concr. Compos. 2013, 37, 232–240.
- Teo, P.T.; Abu Seman, A.; Basu, P.; Mohd Sharif, N. Chemical, Thermal and Phase Analysis of Malaysia’s Electric Arc Furnace (EAF) Slag Waste. Mater. Sci. Forum 2016, 840, 399–403.
- Barella, S.; Gruttadauria, A.; Magni, F.; Mapelli, C.; Mombelli, D. Survey about Safe and Reliable Use of EAF Slag. ISIJ Int. 2012, 52, 2295–2302.
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The efficiency of quartz addition on electric arc furnace (EAF) carbon steel slag stability. J. Hazard. Mater. 2014, 279, 586–596.
- Gurtubay, L.; Gallastegui, G.; Elias, A.; Rojo, N.; Barona, A. Accelerated ageing of an EAF black slag by carbonation and percolation for long-term behaviour assessment. J. Environ. Manag. 2014, 140, 45–50.
- Horii, K.; Kato, T.; Sugahara, K.; Tsutsumi, N.; Kitano, Y. Overview of Iron/Steel Slag Application and Development of New Utilization Technologies. Nippon Steel Sumimoto Met. Tech. Rep. 2015, 109, 5–11.
- Riley, A.L.; Mayes, W.M. Long-term evolution of highly alkaline steel slag drainage waters. Environ. Monit. Assess. 2015, 187, 463.
- Ziemkiewicz, P.; Skousen, J. The Use of Steel Slag in Acid Mine Drainage Treatment and Control. In Proceedings of the 19th West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 7–8 April 1998; p. 14.
- Hamilton, J.; Gue, J.; Socotch, C. The use of steel slag in passive treatment design for AMD discharge in the Huff Run watershed restoration. In Proceedings of the 24th ASMR, Gillette, WY, USA, 2–6 June 2007; pp. 272–282.
- Mack, B.; Gutta, B. An Analysis of Steel Slag and Its Use in Acid Mine Drainage (AMD) Treatment. In Proceedings of the America Society of Mining and Reclaimation, Billings, MT, USA, 30 May–5 June 2009; pp. 722–742.
- Chaurand, P.; Rose, J.; Briois, V.; Olivi, L.; Hazemann, J.-L.; Proux, O.; Domas, J.; Bottero, J.-Y. Environmental impacts of steel slag reused in road construction: A crystallographic and molecular (XANES) approach. J. Hazard. Mater. 2007, 139, 537–542.
- Koryak, M.; Stafford, L.J.; Reilly, R.J.; Magnuson, M.P. Impacts of Steel Mill Slag Leachate on the Water Quality of a Small Pennsylvania Stream. J. Freshw. Ecol. 2002, 17, 461–465.
