Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Eleonora Bianchi and Version 2 by Jessie Wu.
Inorganics are generally used in tissue engineering as nanomaterials (nanoparticles, smaller than 100 nm in at least one dimension), since materials in nanoscale can efficiently support biological responses. In particular, they can interact with the biomolecules onto the cell surface and be taken up into the cytoplasm.
- nanomaterials
- metal oxides
- Cu NPs
1. Metal Oxides
Metal oxides have been studied over the years for different applications, such as catalytic, dielectric, electromechanical, and only recently research studies have been targets to explore their use in biomedical applications [1][2][53,54].
Metal oxides can exist in different shapes and sizes, which are correlated to their synthesis. When they present nano-dimensions, ranging from 1 to 100 nm, they are characterized by unique properties, particularly interesting for biomedical application. Nanoparticles having diameters less than 20 nm could more easily enter the cell membrane and the cellular organelles and could also cross the brain barrier. Moreover, they could even penetrate the bacterial cells and release toxic metal ions [3][55].
Ideally, the metal oxide nanoparticles for biomedical applications should be chemically stable, should not easily dissociate in metal ions, should not present toxicity, correlated to the size and surface properties, and lastly and importantly, they should be biocompatible [4][5][56,57]. To assure these characteristics, the use of metal oxides has recently reached internationally recognized standards. For example, zirconium dioxide must be prepared following international standard reference ISO 13356, which specifies the requirements and the related test methods for the production of biocompatible nanomaterials intended for biomedical applications [2][54]. The most important examples of metal oxide nanoparticles employed in biomedicine are represented by bioceramics and bioglasses and by magnetic nanoparticles.
1.1. Bioceramics and Bioglasses
The bioceramics are biomaterials used to treat, augment, or replace the damaged tissues, in particular the hard ones. They are characterized by properties that make them body friendly substitutes, such as biocompatibility, degradation, and high mechanical strength, suitable to improve the mechanical properties of the scaffolds.
The success of bioceramics in biomedicine is due to their biofunctionality and biocompatibility. In fact, the formation of apatite on their surface after implantation makes easier the bonding of the substrates with the body tissues [6][58].
The bioceramics are mainly classified according to the tissue response into three subclasses: bioinert, bioactive, and bioresorbable ceramics (Figure 1) [7][8][9][59,60,61].
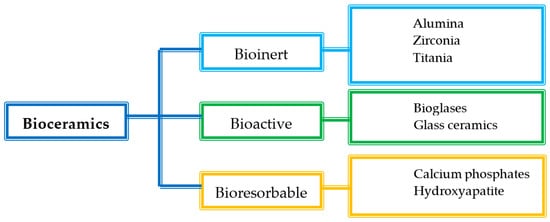
Figure 1.
Classification of bioceramics.
Bioinert ceramics are characterized by stable physicochemical properties and good biocompatibility especially with the hard tissues. They keep both their mechanical and physicochemical properties once implanted in the host, without causing an immunological rejection. The main use of bioinert materials is the production of structural supports, such as bone plates or screws, due to their ability to resist fractures [6][58]. In particular, alumina and zirconia, both alone and combined, are traditionally used for dental and orthopaedic applications, due to their improved mechanical and morphological properties and suitable biocompatibility [10][11][62,63].
Bioactive ceramics, such as bioglasses or glass ceramics, can interact with the host tissues, inducing a specific biological response that improves tissue regeneration [6][12][58,64]. Once implanted, they form a hydroxyapatite layer similar to the inorganic phase of the native bones, which can bond both the collagen fibrils of the tendons and the bone. Glass ceramics are mainly constituted by CaO and P2O5, which are also the main constituents of the bone mineral phase. For this reason, they are characterized by an optimal effectiveness. Bioglasses are widely used as implants, as they have a positive effect on living cells and tissues, due to chemically stable bonds with the skeletal system of the host. Moreover, the microstructure of bioglasses increases the bending strength and the compressive strength of the implanted material, and they also have been referred as enhancers of angiogenesis, which is a crucial step for the wound healing process [13][14][15][16][65,66,67,68].
Lastly, bioresorbable ceramics interact with the host tissues and are also able to degrade rapidly once they meet the biological fluids. Their chemical structure is broken by the tissue fluids, and they are completely absorbed by the body without producing any toxic effect. For this reason, they do not require second surgery for implant removal. The main materials of this class are hydroxyapatite (Ca10(PO4)6(OH)2) and calcium phosphates, which have been used mainly in orthopedics as bone substitutes, due to their stability, biocompatibility, and osteo-conductivity [6][16][17][58,68,69].
1.2. Magnetic Nanoparticles
The magnetic nanoparticles recognized as non-toxic in the medical field, thanks to their oxidative stability, are mainly represented by magnetite (Fe3O4) and maghemite (Fe2O3).
Magnetic nanoparticles have been extensively used for biomedical applications with different purposes, such as magnetic resonance imaging (MRI) diagnosis, drug delivery control, cell/tissue targeting, and hyperthermia in cancer treatment [16][18][19][68,70,71]. Recently, there is evidence that their inclusion into scaffolds results in unique properties to control cell signaling both in vitro and in vivo, in particular when they have small size (<100 nm) and narrow size distribution [20][72].
Interestingly, iron oxide nanoparticles are characterized by a superparamagnetic behavior, namely they show magnetism if an external magnetic field is applied. This is of interest as the nanoparticles lose magnetism after removing the field. Moreover, magnetism retention is strongly related to particles size: 10–50 nm nanoparticles can be affected by an external magnetic field [16][68].
The supposed mechanism of magnetic nanoparticles embedded in scaffolds for orthopaedic reparation is mechano-stimulation. The nano-movement induced by the magnetic field on the scaffolds seems able to cause forces in the range of pN, and cells act in response to those mechanical stimuli according to four major biochemical pathways: ion channels activation, ATP release, contraction of the cytoplasmatic actin and alteration of protein expression in particular FAK (focal adhesion kinase), which is the basis of biochemical signals, that stimulate the cells remodeling and differentiation. In this context, the magnetic scaffolds could guide the mechano-transduction signals allowing deeper tissue reparation [21][22][73,74].
1.3. Tissue Engineering Applications
Metal oxide nanoparticles has gained attention in the recent decades since they ideally could combine reparative effectiveness with antimicrobial ones [23][75].
1.3.1. Skin Applications
Bioactive glasses proved to be beneficial for wound healing in skin tissue engineering, due to their capability to stimulate hemostasis, angiogenesis, and fibroblasts proliferation both in vitro and in vivo [15][24][67,76]. Different types of formulation and particles have been considered. Wang and colleagues studied bioactive glasses nanoparticles mixed with gelatin for the production of hydrogels intended for wound dressing. The mixture was able to promote faster tissue regeneration and a more effective wound healing, due to the synergic effect of the bioactive glass and gelatin, within seven days after implantation in a murine model [25][77]. Samadian et al. developed an electrospun cellulose scaffold loaded with hydroxyapatite (HP) by means of electrospinning technique. The results showed that the concentration of HP affected the porosity, water contact angle, water uptake, water vapor transmission rate, and cells proliferation. In vivo studies showed that all dressings had higher wound closure percentage than the sterile gauze, as the control, reaching values of closure of 93.5% [26][78]. Babitha et al. investigated the stability of TiO2 nanoparticles incorporated in a zein-polydopamine nanofibrous scaffold as potential wound dressing material. The scaffold mimicked the network of the natural extracellular matrix (ECM), promoting cells adhesion and proliferation. Moreover, the in vivo evaluation of the wound healing potential proved the system as suitable for wound healing in tissue engineering applications, since complete re-epithelialization was achieved on day 15 in the group treated with the system loaded with TiO2 [27][79].
1.3.2. Orthopaedic Applications
Metal oxides have been widely considered for their capability to stimulate bone tissue repair, due to their bonding to the living tissues once implanted [16][28][68,80] and there are in vivo proofs of concept that suggest the rational for the use of metal oxides in orthopaedic tissue engineering.
In particular, Covarrubias and colleagues reported that the incorporation of bioglasses into a chitosan-gelatin matrix showed excellent cytocompatibility and enhanced the crystallization of bone-like apatite in vitro. Moreover, in vivo implantation of the scaffolds into bone defect models demonstrated that the systems significantly increased the amount of new bone production [29][81]. Li et al. produced a multifunctional poly(citrate-siloxane) (PCS) elastomer loaded with bioactive glass with intrinsic biomineralization activity and photoluminescent properties for potential bone tissue regeneration. The nanocomposite showed significantly enhanced mechanical properties, hydrophilicity, photoluminescence properties, biomineralization activity, improved osteogenic differentiation ability, osteoblasts biocompatibility, and low inflammatory response in vivo [30][82].
The efficacy of HP was also widely proven in tissue engineering application for tendon and bone regeneration. The combination of HP with various carriers, such as porous [31][83] and electrospun [32][33][84,85] scaffolds or hydrogels [34][86] showed enhancement of cellular activity. In particular, the research underlined the HP capability of increasing the mechanical properties of the scaffolds and of supporting cell adhesion and differentiation into osteo-like cells, able to produce ECM.
1.3.3. Neural Applications
One of the most promising applications of metal oxides is in nerve tissue engineering and neuroregeneration [16][68]. Various studies have been performed particularly on magnetic nanoparticles, in order to make cells magnetically sensitive and allow cell migration, proliferation and differentiation [35][36][37](Figure 2) [87,88,89].
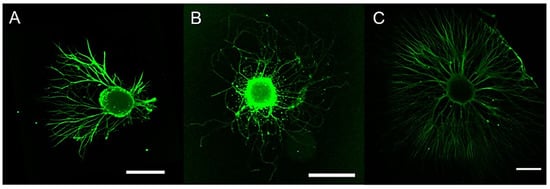
Figure 2. CLSM images (scale bar = 500 µm) showing the growth of extending neurites from dorsal root ganglia (DRG) directed by nerve growth factor (NGF) gradients generated by poly-L-lactic acid/iron oxide NPs (cNPs). DRG were subjected to (A) NGF combined with cNPs, (B) cNPs alone, and (C) NGF containing medium. (A) The combination of NGF and cNPs directed the extension of neurites from DRG. (B) Control cNPs had no effect on the directional outgrowth of neurites, and (C) NGF containing medium did not impart a growth preference on neurites. Adapted with permission from [89]. Copyright 2022 American Chemical Society.
Recently, superparamagnetic iron oxide combined with gold nanoparticles were functionalized with nerve growth factor (NGF) for neuron growth and differentiation. The functionalized systems provided higher PC12 neuronal growth and orientation under dynamic magnetic fields compared to static magnetic fields, confirming the potential of non-invasive magnetic neuron stimulation for promoting neuronal growth [38][90].
Magnetic nanoparticles have also been used by Chang et al. to control collagen fiber orientation in situ by applying an external magnetic field. In vitro, the magnetically activated neurons extended their neurites along the aligned nanofibers. This increased the cell density, and consequently also the NGF concentration, together with myelination. In a rabbit sciatic nerve model, the scaffold showed superior nerve recovery and less muscle atrophy in comparison with autograft [39][91].
In another study conducted by Vinzant et al., Fe2O3 was conjugated with a peptide antisauvagine-30 (ASV-30), since iron oxide nanoparticles are capable to efficiently cross the blood-brain barrier. The infusion of ASV-30 reduced anxiety-like behavior of rats through binding to corticotropin releasing factor type 2 receptors. In vivo results demonstrated that systemic application of iron oxide combined with ASV-30 decreased anxiety with no impact on locomotion, representing a novel approach for the peptide delivery across the blood-brain barrier [40][92].
One example of metal oxide recently recognized for its relevance in nerve reparation is represented by cerium nanoparticles [41][93]. In fact, it presents both antioxidant and anti-inflammatory effects, which could lead to cell protection and differentiation, when combined with a polymeric scaffold. Marino and coworkers developed gelatin-based nanofibers loaded with cerium oxide nanoparticles. Both topographical and antioxidant cues were confirmed. The feature of the fibers, such as their porosity, high surface area and biodegradability, permitted the ion exchange necessary for the reduction reaction of Ce3+ to Ce4+ and consequently the antioxidant effect. Meanwhile, the aligned fibers supported the axonal guidance and outgrowth of neuronal cells. They observed that the presence of metal phase at low concentration did not disturb the fiber alignment and size but increased their mechanical properties. These phenomena are fundamental for mediating the mechano-transduction pathways, such as phosphorylation of FAK, cytoskeletal rearrangements, and nuclear deformations, all outcomes observed in the differentiated SH-SY5Y nerve cells grown onto the fibers surface. Moreover, cerium nanoparticles showed beneficial effect not only on ROS control, but also on neural differentiation by releasing β3-tubulin protein [42][94].
2. Antimicrobial Properties
Metallic nanoparticles and metal oxides, such as Ag, Au, copper (Cu), titanium (Ti), and zinc (Zn), are well known to inhibit the growth of several species of bacteria, fungi, and viruses. Moreover, nanoparticles shapes and sizes have been described as key elements for the control of bacterial growth. Furthermore, microorganisms resistant to the most used antibiotics, that represent a great threat to human health, are generally sensitive to these aspecific components. For these reasons, the combination of proliferation enhancement and antimicrobial properties renders metallic and metal oxides nanoparticles very important for biomedical applications to prevent microbial infections and to promote tissue regeneration [43][118]. In particular, the nanoparticle properties could affect their antimicrobial potency. Smaller sized nanoparticles demonstrated to cause higher bacterial inhibition [44][141]. This could be attributed to the size of bacterial cell, which is in micrometer range, while their membrane pores are in nanometric dimensions and could be more easily entered by smaller nanoparticles to denature the intercellular proteins and consequently kill the bacteria [43][118]. Krishnaraj et al. have reported the anti-microbial activity against E. coli and Vibrio cholera of 20–30 nm Ag NPs [45][142]. Similarly, hydrophobic and cation-functionalized Au NPs showed a strong bactericidal activity against both Gram-negative and Gram-positive multiple drug resistant bacteria [46][143]. Cu nanoparticles (Cu NPs) also possess a great prospective to act as anti-microbial agents. The anti-microbial activity of Cu NPs was evaluated against bacteria such as Micrococcus luteus, S. aureus, E. coli, K. pneumoniae, and P. aeruginosa, and against fungi such as Aspergillus flavus, Aspergillus niger, and Candida albicans [47][144]. Table 1 summarizes the antimicrobial activities and studies caried out on various types of nanoparticles containing inorganic compounds.Table 1.
Antimicrobial activity of nanoparticles based on inorganic compounds.
| Nanoparticles | Antimicrobial Activity | References |
|---|---|---|
| Ag | Salmonella typhi, Salmonella paratyphi, V. cholera and S. aureus |
[48][145] |
| Ag | B. subtilis, Klebsiella planticola, K. pneumonia, Serratia nematodiphila, and E. coli |
[49][146] |
| Ag | B. subtilis, K. pneumonia, E. coli, P. aeruginosa and S. aureus |
[50][147] |
| Au | BCG 1 and E. coli | [51][148] |
| Au | S. aureus, E. coli, K. Pneumonia and P. aeruginosa | [52][149] |
| Cu | E. coli and C. albicans | [47][144] |
| Cu | E. coli, K. pneumonia and S. aureus | [53][150] |
| Cu | S. typhi, B. subtilus, S. aureus, K. pneumoniae and E. coli | [54][151] |
| Cu | S. aureus and P. aeruginosa | [55][152] |
| Zn | E. coli and S. aureus | [56][153] |
| TiO2 | E. coli and S. aureus | [57][154] |
| TiO2 | E. coli, S. aureus and K. pneumonia | [58][155] |
| Fe2O3 | S. aureus, E. coli, P. aeruginosa and Serratia marcescens | [58][155] |
| Iron | E. coli, Salmonella enterica, Proteus mirabilis and S. aureus |
[59][60][156,157] |
1 Bacillus Calmette-Guérin.
