Formaldehyde (FA) is the simplest aldehyde present both in the environment and in living organisms. FA is an extremely reactive compound capable of protein crosslinking and DNA damage. For a long time, FA was considered a “biochemical waste” and a by-product of normal cellular metabolism, but in recent decades the picture has changed. As a result, the need arose for novel instruments and approaches to monitor and measure not only environmental FA in water, cosmetics, and household products, but also in food, beverages and biological samples including cells and even organisms. Different methods developed for non-biological objects have been adapted for biological samples. Numerous approaches, including chemically-synthesized probes and genetically encoded FA-sensors for in cellulo and in vivo FA monitoring were developed.
- formaldehyde
- aldehyde derivatization
- fluorescent probes
- aza-Cope rearrangement
- formaldehyde sensor
1. Introduction
Formaldehyde (formula HCHO, abbreviated FA), is a simple, naturally occurring organic compound, is one of the first-row aldehydes and is the most poisonous of them [1,2]. It is a well-known fact that FA is a strong electrophile capable of reacting with various biological nucleophiles such as nucleic acids [3], and nucleophilic amino acids [4], thereby affecting the most important functions of living organisms. Nevertheless, FA is a normal component of the human organism itself, as physiological levels of FA are detected in exhaled air and blood. That fact became obvious with the development of methods by which FA could be assessed in biological samples [5]. Formaldehyde can enter the human body from outside—with polluted air or water, tobacco smoke [6,7], electronic cigarette vapour [8] and consumed food [9–139,10,11,12,13]. Moreover, there are numerous sources of endogenous FA as it is a (by-)product of different biochemical reactions in the organism performed by various enzyme systems, e.g., oxidative demethylation enzymes [14], semicarbazide-sensitive amine oxidases (SSAOs) [15–1715,16,17], serine hydroxymethyltransferase [18], dimethylglycine dehydrogenase [19], lipid oxidation enzymes [20], P450 oxidase [21] and N-methyl group demethylase [22,23] (Figure 1). One of the main sources of FA in the human organism is exogenous and endogenous methanol that is oxidized by alcohol dehydrogenases, catalase and cytochrome P450 to FA [14]. Despite the continuous incoming flow of FA in the human organism in normal conditions, it is equal to the outgoing flow—FA concentration is maintained at low levels by different mechanisms of controlling its metabolism and clearance [14].
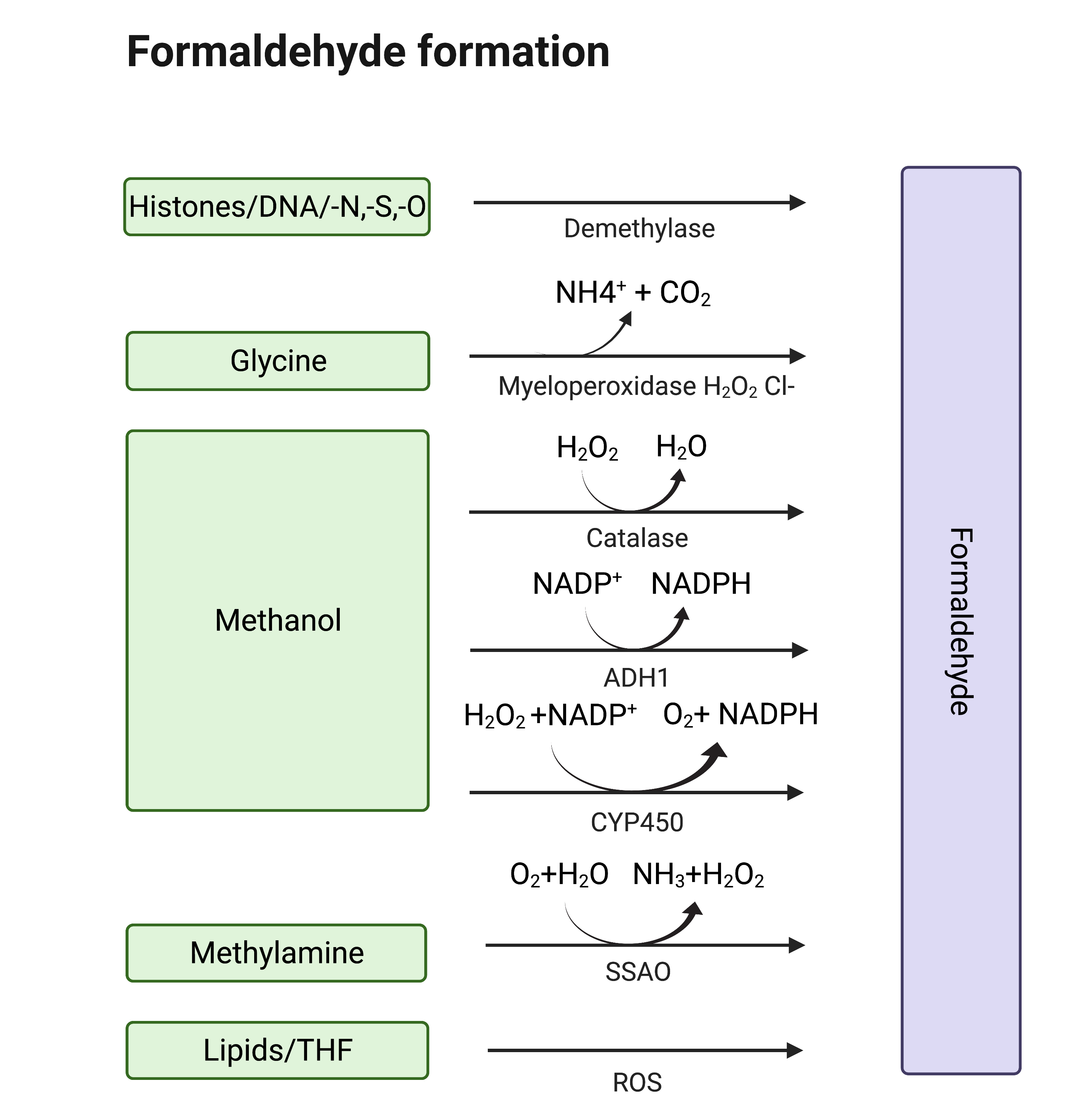
Figure 1. Sources of endogenous FA. Created Created withwith BioRender.com.
Nevertheless, in case of pathological conditions and malfunction of FA clearance systems, the increased levels of endogenous FA could lead to destructive and harmful processes. It can induce bone pain in cancer through TRPV1 activation [24] and it also plays a huge role in the origin and life of a cancer cell [25–27]. FA triggers cellular redox imbalance in human cells as it reacts with the redox-active thiol group of glutathione affecting the ratio of reduced glutathione and causing oxidative stress [28]. FA can bring genetic damage to hematopoietic stem cells, hepatocytes and nephrons [29]. Also, fluctuations of endogenous FA concentration are characteristic of the numerous pathological conditions associated with cognitive impairment [30]. FA could be assumed to be not only a by-product of endogenous biochemical processes but to also have some important functions. This assumption has risen into the hypothesis that human endogenous FA can act as a potential anticancer metabolite [27]. Moreover, endogenous FA was demonstrated to be a regulatory molecule that can help memory formation, particularly by enhancing NMDA (N-Methyl-D-aspartic acid) currents [31].
But how to measure FA concentration? The potential danger from exogenous FA was first discovered at the end of 19th–beginning of 20th century [32]. The first approach allowing FA concentration assessment was developed in the 1950s with the birth of gas chromatography. Methods for measuring FA were directed for its detection in waste water as well as in food and beverages [33], air and various types of alcoholic products [34]. For a long time, the scientific community did not consider the existence and significance of endogenous FA. But recent advances in molecular biology, biochemistry and physiology have revealed its importance indicating that FA can serve as a molecular marker for many normal and pathological biochemical processes in a living organism. It became necessary to optimize the existing FA detection methods to make them applicable for biological samples. According to that demand, numerous novel techniques were developed for FA content assessment in vitro, in cellulo and in vivo. Here researchers review a variety of approaches for FA measurement and monitoring that can be divided in to three main groups. The first group of methods includes gas and liquid chromatography and provide high sensitivity, selectivity, and reproducibility of measurements. However, these techniques are hardly applicable for FA assessment in real-time mode and in living cells and organisms. The second group comprises methods based on spectrophotometry detection of substances obtained in a reaction with FA. Some of these approaches could only be utilized for FA measurement in liquid samples (biological fluids, lysates, extracts etc.); but the others (mainly based on aza-Cope group rearrangement) could also be applied to monitor FA in cells and even living organisms. The third group includes genetically-encoded sensors that were developed for living cells and organisms. However, the variety of methods for FA assessment is not limited to those mentioned above: there are numerous other approaches for analysis of air samples using graphene- and metal oxide-based nanomaterial gas sensors [35–39], optical FA-sensors [40] and others that are out of the scope of this review.
The aim of this review is to highlight the latest developments in the field of FA detection in vitro and in vivo and their application in cellular and molecular biology, physiology and biochemistry, and to provide readers with a general overview of how FA could be assessed in biological samples. Researchers summarize data on the approaches for FA content measurement in biological fluids as well as culture medium, cell extracts and lysates, and on the suitability of different methods developed for non-biological objects. researchers discuss the potential of the numerous probes applicable for real-time FA monitoring in cell culture, tissues and living organisms. And, finally, researchers focus on the recent advances in genetically-encoded FA-sensors.
2. In Vitro FA Measurement
The first objects in which it is necessary to measure the levels of endogenous FA are various biological fluids, such as blood, saliva, and urine as well as culture medium. Cell lysates and tissue extracts could also be assigned to this group of samples. As most of these samples contain proteins, the initial step of the sample preparation for chromatography is deproteinization that is usually performed by protein precipitation using trichloroacetic acid or acetonitrile. Measurement of FA in liquid samples does not need the invention of completely new methods, but rather, requires the use of known methods that are modified to achieve the best results.
2.1. Gas Chromatography
In early 1952, Martin and James carried out a variant of gas distribution chromatography (GC). Since that time, gas chromatography became one of the fastest growing methods in analytical chemistry. GC has many advantages over other measurement methods. Due to its high separation power, GC has found wide application in the food industry [41,42] and medicine [43,44]. No other method allows analysis of water samples or multicomponent liquid systems with hundreds of components in such a short time. Comparative simplicity of equipment management and ease of use are among the most important properties of gas chromatography. Gas chromatographs are relatively affordable, reliable, and allow an automated analysis process.
High sensitivity of the chromatograph makes it possible to determine small amounts of organic compounds with great accuracy. Today, gas chromatography can reliably determine concentrations of 10-
–10-
mg/mL [45]. The obtained data may be widened when GC is combined with various instrumental methods such as mass spectrometry (MS) and Fourier-transform infrared spectroscopy.
GC–MS has become one of the most successful combined methods. By superposition of GC and MS, researchers get a tool combining the ability of GC to separate closely related molecules with the ability of MS to use data to identify and quantify separated substances.
2.1.1. FA Derivatives’ Detection with GC
How can researchers use the GC method to detect such a small metabolite as FA with a mass of only 30.026 g mol−1? It is complicated and troublesome to catch this molecule without using special protocols of sample preparation. One of the common substances used for FA derivatization is O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine (PFBHA, CAS Registry No. 57981-02-9) (Figure 2A). Thus, FA derivatives–oximes–are obtained and then analyzed by GC coupled with flame ionization detector (FID), electron-capture detector (ECD) or mass spectrometry (MS). PFBHA could be used for FA detection in water, blood [26], plasma, urine and amniotic fluid [46,47]. PFBHA reacts completely with FA within a few seconds, forming a GC-compatible oxime that is analyzed with a high-polarity capillary column and nitrogen as a carrier gas. The accuracy of FA detection using GC coupled with MS reaches the picogram (pg) level [48]. The PFBHA-based method has been recommended for FA detection by the US Environmental Protection Agency [49].
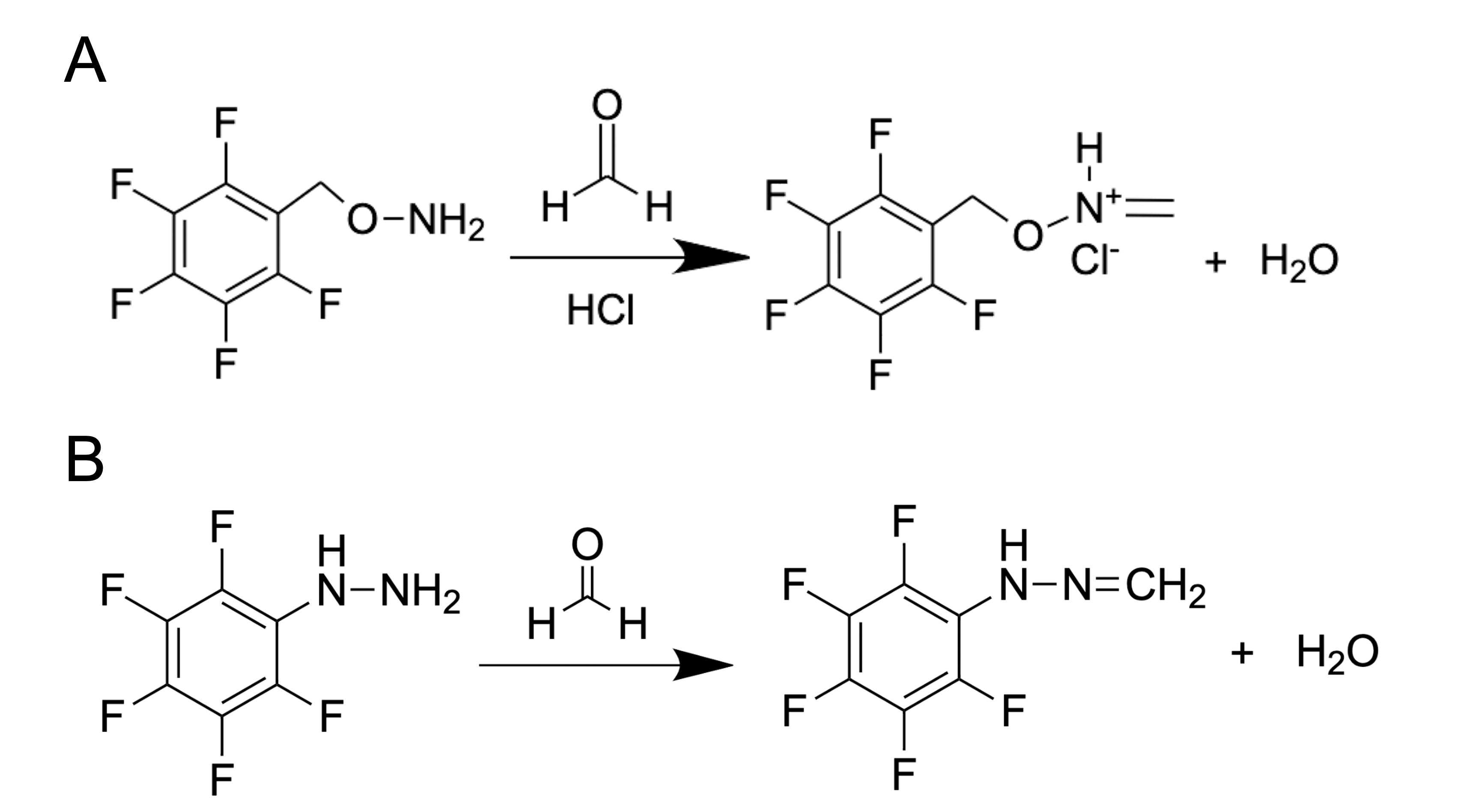 Figure 2. FA derivatization using PFBHA (O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine) (A) or PFPH (pentafluorophenylhydrazine) (B).
Figure 2. FA derivatization using PFBHA (O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine) (A) or PFPH (pentafluorophenylhydrazine) (B).
Another substance used for FA derivatization is pentafluorophenylhydrazine (PFPH) (Figure 2B). This approach was used to assess the FA content in biological samples such as homogenates obtained from different tissues [50], and the derivatization was performed directly in tissue homogenates in the presence of diluted (0.03–0.1 M) phosphoric acid (1–2h 50–55ᵒC) followed by analysis of pentafluorophenyl hydrazone by GC–MS using a capillary high polarity column and helium as a carrier gas. This method allows the detection of FA at concentrations that are characteristic of natural endogenous levels for mammals [51].
2.1.2. GC with Catalytic Hydrogenolysis Coupled with a Flame Ionization Detector (FID)
Although the GC analysis of FA derivatives detected with FID/ECD and especially MS described above provides high accuracy, it is costly and time-consuming. Jim Luong and colleagues developed a new GC technique: using a capillary column by which FA, acetaldehyde, and other components were separated from a gas-phase matrix, then the aldehydes were converted into methane and ethane, respectively, using a nickel-coated catalyst in a hydrogen atmosphere. The methane and ethane were then detected using an FID [52]. Further, the catalyst-based method was upgraded and catalytic hydrogenolysis was performed directly in a 3D-printed FID jet consisting of an FID combined with a catalyst support for hydrogenolysis. The sensitivity of this approach reaches ppm level in the range of 0.5–300 ppm [53]. The main advantages of this method are the absence of the need for preliminary preparation, derivatization, and concentration of the sample. This method can be used safely for the FA measurement in biological fluids after proper separation of the aqueous phase (precipitation of proteins, centrifugation). This promising approach has excellent prospects and the FID jet is already commercialized as Jetanizer(TM) and available.
2.2. Liquid Chromatography
Liquid chromatography (LC) is a method for separation and analysis of complex mixtures of substances in which the mobile phase is liquid [54]. The LC method is applicable for the separation of a much wider range of substances than GC, since most of the substances are not volatile and are unstable at high temperatures. LC separation is usually performed at room temperature.
High performance LC (HPLC) has long been used to work with systems of biological origin, but FA cannot be measured directly by LC due to the chemical and physical properties of FA. Moreover, the concentrations of endogenous FA in biological samples are very low. Thus, it is necessary to obtain more stable and less reactive derivatives as the products of irreversible reactions with FA. These FA derivatives could be analyzed using HPLC combined with different detectors (usually UV) or MS.
2.2.1. 2,4-Dinitrophenylhydrazine for FA measurement
The most widely used substance for binding FA is 2,4-dinitrophenylhydrazine (DNPH) [55]. As a result of this reaction, 2,4-dinitrophenylhydrazone is formed (Figure 3). This compound is separated using reverse-phase HPLC and monitored using a UV detector [56]. DNPH–HPLC can be applied to virtually any non-biological sample including water [57,58], drug substance [59], food [60], leather [61], etc. This method also allows the measurement of FA in biological samples like tissue homogenates [62], urine [63], blood and serum [64–6664, 65,66]. Despite the wide use of this approach, it still requires elaborate sample preparation and special equipment, which is not always accessible.
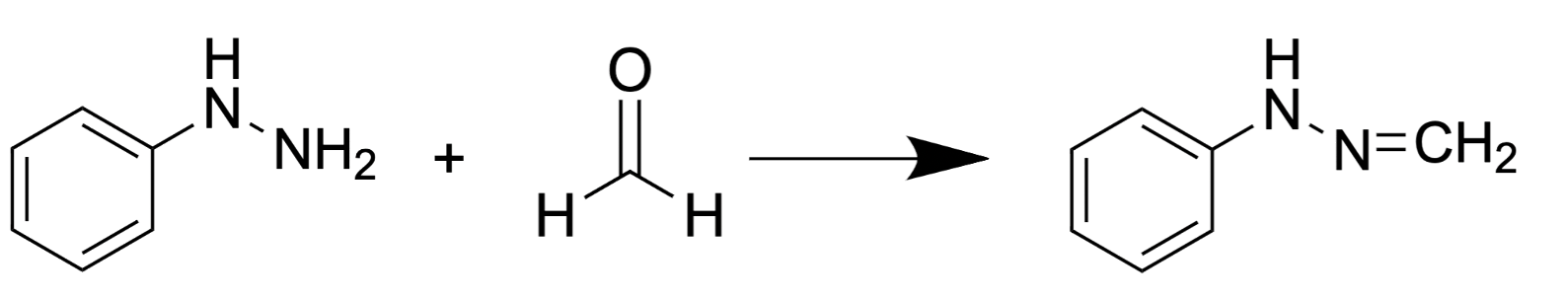 Figure 3. 2,4-dinitrophenylhydrazine reaction with formaldehyde.
Figure 3. 2,4-dinitrophenylhydrazine reaction with formaldehyde.
2.2.2. Ampicillin-Based Reaction for FA Derivatization and HPLC Analysis with Fluorescence Detection
Another substance for FA derivatization is ampicillin (D-(2)-a-aminobenzylpenicillin) that reacts with FA forming a fluorescent product (Figure 4) [67] analyzed by reverse-phase HPLC with fluorescence detection
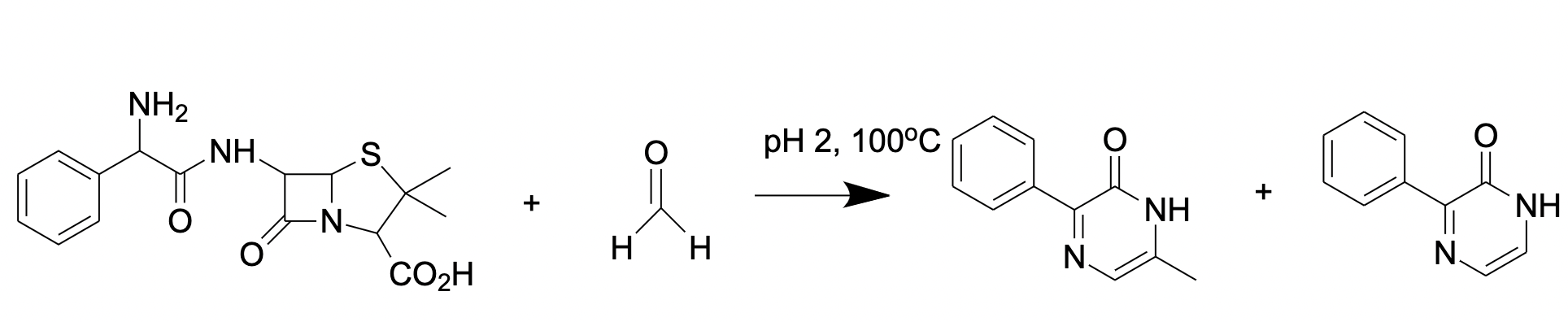
Figure 4. FA reaction with ampicillin results in formation of fluorescent pyrazine-2-one.
2.2.3. Dimedone for FA Derivatization
Tyihák and colleagues used dimedone as a derivatization agent for FA [68]. This reaction (Figure 5) is similar to the Nash reaction [69] as it is also based on the condensation reaction between FA and a reagent consisting of β-diketone and ammonium acetate. A solution of dimedone in methanol was demonstrated as a suitable reagent for the isolation of FA from different biological samples. Moreover, methanol in this protocol plays the role both of extractant and eluent [68], and no additional deproteinization stage is needed due to simultaneous extraction and derivatization. The reaction product, formaldemethone (Figure 5), could then be analyzed by reverse-phase HPLC with UV detection. Using this approach, FA assessment was performed in biological samples such as tissue (liver, muscle, tooth) lysate, urine and plant extract [68,70,71].
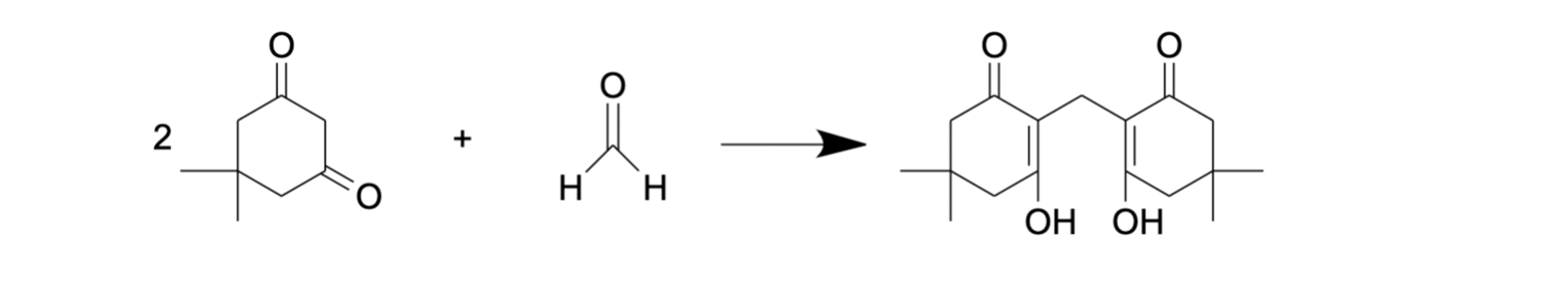
Figure 5.
FA derivatization with dimedone.
2.2.4. Detection of Reaction Products of FA and Amino Acids
It is a well-known fact that FA reacts with different amino acids [72–7472, 73, 74]. But until recently, this fact was considered only for exogenous FA, which negatively affected the organism. The elevated levels of FA/amino acid adducts are regarded as biomarkers of FA exposure [75,76]. FA applied to mammalian cell culture reacts with free cysteine and histidine residues, forming products that are stable over a wide pH range [74]. Such spontaneous reactions result in the formation of timonacic and spinacine, respectively [74]. Timonacic and spinacine concentrations could be extracted from cells or serum and analyzed by HPLC–MS [74]. The reaction product between cysteine and FA, timonacic, is more soluble than cysteine and less reactive than FA [77], which facilitates its transfer through tissues. Cysteine conversion to timonacic is reversible, which is why timonacic could be regarded as a kind of FA “storage”, which forms in some tissues and then enters the bloodstream. In the blood, it is converted back to cysteine and FA, thereby promoting the distribution of cysteine and FA throughout the body [77]. Due to all the properties described above, timonacic can be used to track relative changes in FA concentration in blood [74,77], but the absolute FA concentration cannot be assessed by this approach.
To conclude, HPLC and GC are precise, selective and well-established methods for the detection of FA in various samples, including biological. However, they are only suitable for in vitro detection. Moreover, additional steps of sample preparation, including deproteinization and derivatization, are necessary. Therefore, if all these criteria are applicable to the particular research task and the corresponding equipment as well as a qualified operator is available, then chromatography represents one of the best choices for FA assessment.
3. From In Vitro to In Cellulo
3.1. Chromogenic or Fluorogenic FA Chemosensors
There is a group of methods used for FA concentration assessment based on the measurement of absorbency or fluorescence of the products obtained in the FA reactions with particular substances [78,79]. Spectrophotometry allows measurement of chromogenic and fluorogenic substances, obtained as a result of such reactions. Most of these protocols are used for in vitro FA quantification as very convenient methods because they allow analysis of multiple samples in automatic mode (for example, microplate format); and only common devices found in molecular biology laboratories are needed including spectrophotometer, rather than specialist equipment such as HPLC or GC devices. As data on the role and function of endogenous FA in living systems accumulated, and more and more information appeared around the participation of this molecule in various processes associated with diseases, a need arose for relatively simple and widely available methods for measuring FA in biological samples in vivo in real-time conditions. The benefit afforded by the high sensitivity of the GC and HPLC methods is largely offset by the need for expensive specialist equipment and a qualified operator. Moreover, such approaches cannot be exploited for in vivo FA monitoring. By contrast, fluorescent microscopy and special FA-sensing probes allow monitoring of FA levels in cell cultures and even animals. The main criteria for the substance to be suitable for FA monitoring in living systems are low toxicity, stability, cell-penetration ability, and selectivity against other aldehydes that are present in the cell. For in vivo monitoring, a near-infrared excitation/emission profile is also preferable. Below, researchers discuss the application of FA-sensors for in vitro application, and adjustment of these molecules or development of completely new ones to make them applicable for in vivo context.
3.1.1.β-Diketone Esters and Hantzsch Reaction
This reaction was known back in 1881 thanks to Arthur Rudolf Hantzsch [80]. Subsequently, this reaction found its use for measuring FA in biological samples in 1953, when Nash modified the reaction and used acetylacetone [69]. This allowed him to measure the FA content in suspensions of living bacteria for the first time. Since then, this colorimetric reaction could be carried out under milder conditions. The acetylacetone-based protocol is now mainly used for FA detection in non-biological samples such as textiles [81], cosmetics [82], beverages [83] etc. Nevertheless, a method is suitable for biological in vitro samples: FA content was assessed in rat hepatocyte lysates [84], human erythrocyte lysates [85], hemolysate [86] and mushroom extracts [87]. Moreover, a commercial kit for different biological samples was developed (Hach Company).
Nowadays, this reaction of β-diketone ester, an aldehyde and ammonia or an alkylamine is actively used for the spectrofluorometric determination of a wide range of non-fluorescent substances which contain a primary amino group, as well as for FA detection. The most common reagents used for FA assessment based on this reaction are: acetoacetanilide (AAA) [88,89] and Fluoral-P (4-amino-3-penten-2-one) [90].
3.1.2. Fluorescent FA-Sensors Based on Formimine, Hydrazine and 2-Aza-Cope Reactions
Recently excellent reviews by Xu et al. [91], Manna et al. [79] and Pan et al. [92] summarized advances on fluorescent probes and sensors, which were developed in the last decade and can be used to monitor FA in a variety of objects both in vitro and in vivo. The main reactions which are the basis for numerous FA-sensing probes for in vivo application are: formimine, hydrazine and 2-aza-Cope reactions. In general, these numerous probes contain a fluorophore moiety (BODIPY, 1,8-naphthalimide, rhodamine, tetraphenylethene, etc) functionalized with FA-reactive groups.
2-aza-Cope chemistry requires special attention, as this approach can be used to quantitatively measure FA in living objects [93]. FA can enter into a 2-aza-Cope regrouping, a quick rearrangement with a low activation barrier [94]. The approach based on this rearrangement (Figure 6) gave a basis for multiple tools for a qualitative and quantitative measurement of FA, which are not affected by the illumination of the sample and the change in the localization of the chromophore in living objects [95]. By now, more than 30 probes have been successfully tested as FA-sensors in living systems [93]. It was shown that 2-aza-Cope rearrangement-based probes are a universal tool and can be applied to a wide variety of imaging platforms, such as multicolor and ratiometric fluorescence [96], positron emission tomography detection [97] and chemiluminescence [95]. This allows for real-time monitoring of endogenous FA levels in mammalian cells and in vivo in laboratory animals [93,97].
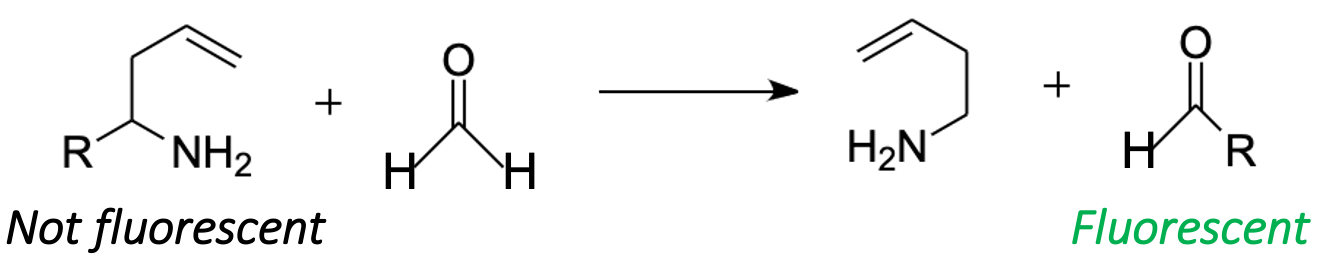
Figure 6. FA in 2-aza-Cope regrouping. R denotes probes for FA detection.
With the use of unique structures capable of entering 2-aza-Cope regrouping and the high reactivity of these analytes to FA, it is possible to work with a large number of different model organisms.
Further development of 2-aza-Cope reactions may in the future offer new approaches to the study of the role of FA in biological systems and even in humans.
4. Genetically-Encoded FA Biosensors
Recently, novel approaches based on the genetically encoded biosensors were developed. The main advantages of such sensors are: FA levels assessment in vivo; the convenient methods of detection based on the fluorescence measurement; and no dependence on expensive equipment. The invention of the first genetically-encoded FA biosensor was based on the study of pathways for FA detoxification in bacteria. To-date, only few genetically-encoded FA biosensors applicable for bacterial cells have been developed [98–10098,99,100], and until recently there was no biosensor adapted for eukaryotic cells. However, based on the properties of the Bacillus subtilis HxlR transcription factor sensitive to FA [98,101], Zhu and colleagues developed a FA biosensor (FAsor) applicable for mammalian cells [102]. The following systems are in the basis of this sensor: (1) circularly permuted fluorescence proteins (cpFPs) the ability of which to emit fluorescence is highly sensitive and strongly depends on their conformation; and (2) the ability of HxlR to undergo conformational changes induced by FA [102]. By fusing cpYFP with two HxlR subunits, they obtained a sensor HxlR-cpYFP-HxlR designated FAsor that can translate FA-induced conformational changes into a change in fluorescence signal (Figure 7).
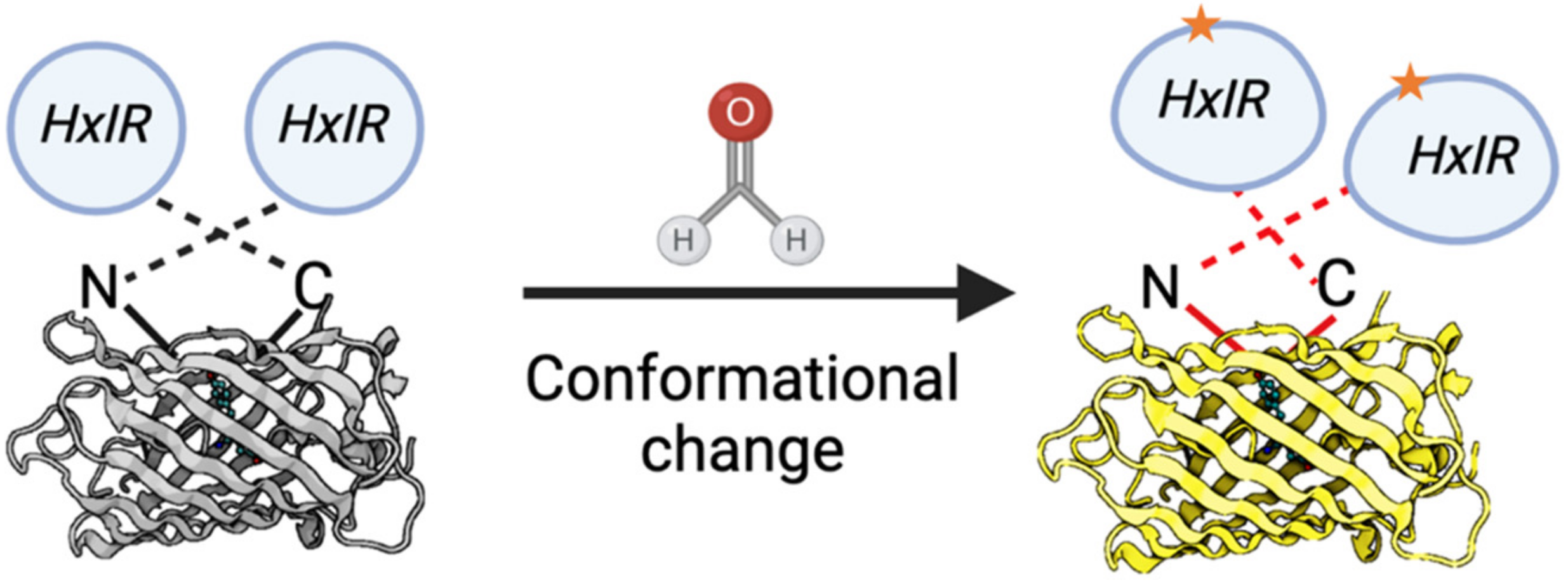
Figure 7. Schematic representation of the FA-sensor FAsor that contains cpYFP flanked with two copies of HxlR. FA induces “N-terminal helix-flipping” in each HxlR unit linking Cys11 and Lys13 residues on the HxlRα1 helix (crosslinked residues are marked with an asterisk). This event leads to a major conformational change in cpYFP module resulting in an increase in fluorescence.
5. Conclusions
FA is one of the products of cellular metabolism, and monitoring of FA levels can give the information about physiological and pathological changes in living systems. The aim of this review was to present the main categories of methods that have been optimized and applied to FA measurement in liquid biological samples, as well as to demonstrate the progress in development of novel molecules suitable for application in living systems. Finally, an additional aim was to discuss genetically encoded FA-sensors based both on existing natural FA-responsive elements, and on the combination of aza-Cope chemistry and induction of protein conformational changes. researchers presented an overview of the methods that are widely used for FA measurement in non-biological samples and are based on FA chemical reactivity; and researchers assessed challenges that occurred in their transition from non-biological to biological samples. researchers summarize the approaches discussed in the flowchart below (Figure 8).
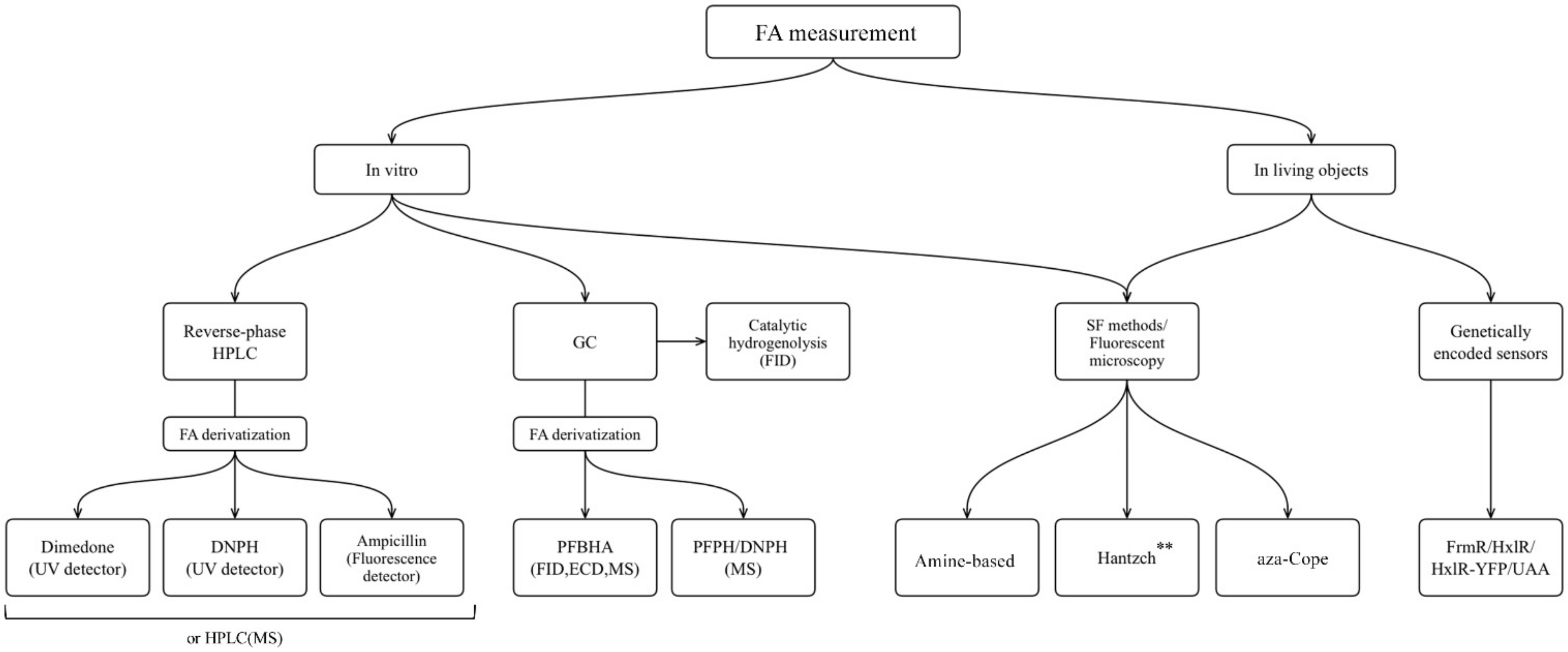
Figure 8. The approaches for FA measurement applicable for different biological samples: HPLC, high performance liquid chromatography; GC, gas chromatography; MS, mass spectrometry; FID, flame ionization detector; ECD, electron-capture detector; SF, spectrophotometry. **Only Hantzch polymers that do not show cytotoxicity could be used for living objects.
For detection of FA levels, characteristics of biological objects require the use of methods with high sensitivity, since endogenous FA concentrations and their fluctuations are in a micromolar range. In addition, these methods must meet several criteria: selectivity, reproducibility and resistance to interfering substances that may present in biological samples. Often, the amount of biological samples is limited, so this parameter should also be taken into account. The most precise and selective tools for the assessment of FA concentrations in liquid biological samples are GC–MS and HPLC–MS. However, their main disadvantages are the elaborate equipment required, and the complexity of the procedure and data interpretation. The alternative to chromatography is to use spectrophotometry-based methods. They are sensitive enough for detection of endogenous levels of FA, selective, easy to operate, suitable for microplate format and commercially available.
When it comes to measuring FA in living systems, additional requirements are added: low or negligible cytotoxicity and absence of side-effects on biological processes in cells are the essential features of such probes for monitoring FA in experimental animal organisms. Moreover, the probe emission should be close to the near-infrared range. The substance used for FA detection must be stable in the culture medium, inside the cell, and in the organism. Most probes for in vivo FA monitoring work on the basis of the aza-Cope rearrangement mechanism. Previously, the disadvantage of such substances was slow and irreversible reactions, but recently-developed novel molecules react rapidly thereby overcoming this drawback. The fundamental challenges in the innovative field of in vivo FA measurement relate to the lack of control over the concentration of the probes in a living organism due to physiological and biochemical processes. It is therefore necessary to assess the potential generalized effects of each novel probe and its “behavior” in a living system as this could complicate its usage in the research of the FA role in the organism. Currently, only short-time in vivo FA monitoring can be performed in a living organism because of the decrease of the probe concentration and its half-life.
Progress in the development of new probes for FA measurement in living systems promise to result in obtaining a new universal sensor, devoid of these drawbacks and meeting the required criteria in the near future. However, most of FA-sensing molecules developed for living systems are substances synthesized in-house and so are not available for wide use which impedes the application of this valuable tool for research.
Genetically-encoded FA-sensors have great potential as they are sensitive enough for endogenous FA level detection, could be targeted to different cellular compartments, have no cytotoxicity and do not require addition of chemical compounds to the cultured cells. However, one of the drawbacks is their sensitivity to methylglyoxal. Moreover, in vivo application of such sensors is not available yet. Thus, future efforts in upgrading of genetically-encoded sensors should include adjusted selectivity to FA and suitability for in vivo application.
A new generation of FA-sensing tools suitable for living objects is expected to be developed to meet the requirements mentioned above. The commercialization of novel probes thus making them available to the research community at large would greatly enhance research potential. researchers believe further progress in development of probes for FA monitoring in living objects could lead to breakthrough results and discoveries in the field of biochemistry of pathological conditions characterized by elevated levels of FA such as cancer and neurodegenerative disorders, providing new approaches for diagnostic and treatment.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102]
References
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A Systematic Review. Mutat Res 2011, 728, 118–138, doi:10.1016/j.mrrev.2011.07.003.Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A Systematic Review. Mutat. Res. 2011, 728, 118–138.
- Ke, Y.J.; Qin, X.D.; Zhang, Y.C.; Li, H.; Li, R.; Yuan, J.L.; Yang, X.; Ding, S.M. In Vitro Study on Cytotoxicity and Intracellular Formaldehyde Concentration Changes after Exposure to Formaldehyde and Its Derivatives. Hum Exp Toxicol 2014, 33, 822–830, doi:10.1177/0960327113510538.Ke, Y.J.; Qin, X.D.; Zhang, Y.C.; Li, H.; Li, R.; Yuan, J.L.; Yang, X.; Ding, S.M. In Vitro Study on Cytotoxicity and Intracellular Formaldehyde Concentration Changes after Exposure to Formaldehyde and Its Derivatives. Hum. Exp. Toxicol. 2014, 33, 822–830.
- French, D.; Edsall, J.T. The Reactions of Formaldehyde with Amino Acids and Proteins. Advances in Protein Chemistry 1945, 2, 277–335, doi:10.1016/S0065-3233(08)60627-0.French, D.; Edsall, J.T. The Reactions of Formaldehyde with Amino Acids and Proteins. Adv. Protein Chem. 1945, 2, 277–335.
- Fraenkel-Conrat, H.; Olcott, H.S. The Reaction of Formaldehyde with Proteins. V. Cross-Linking between Amino and Primary Amide or Guanidyl Groups. Journal of the American Chemical Society 1948, 70, 2673–2684, doi:10.1021/ja01188a018.Fraenkel-Conrat, H.; Olcott, H.S. The Reaction of Formaldehyde with Proteins. V. Cross-Linking between Amino and Primary Amide or Guanidyl Groups. J. Am. Chem. Soc. 1948, 70, 2673–2684.
- Heck, H. d’A; Casanova, M. The Implausibility of Leukemia Induction by Formaldehyde: A Critical Review of the Biological Evidence on Distant-Site Toxicity. Regul. Toxicol. Pharmacol. 2004, 40, 92–106, doi:10.1016/j.yrtph.2004.05.001.Heck, H.d.; Casanova, M. The Implausibility of Leukemia Induction by Formaldehyde: A Critical Review of the Biological Evidence on Distant-Site Toxicity. Regul. Toxicol. Pharmacol. 2004, 40, 92–106.
- Guerin, M.R.; Higgins, C.E.; Griest, W.H. The Analysis of the Particulate and Vapour Phases of Tobacco Smoke. IARC Sci. Publ. 1987, 115–139.Guerin, M.R.; Higgins, C.E.; Griest, W.H. The Analysis of the Particulate and Vapour Phases of Tobacco Smoke. IARC Sci. Publ. 1987, 81, 115–139.
- Mansfield, C.T.; Hodge, B.T.; Hege, R.B.; Hamlin, W.C. Analysis of Formaldehyde in Tobacco Smoke by High Performance Liquid Chromatography. J Chromatogr Sci 1977, 15, 301–302.Mansfield, C.T.; Hodge, B.T.; Hege, R.B.; Hamlin, W.C. Analysis of Formaldehyde in Tobacco Smoke by High Performance Liquid Chromatography. J. Chromatogr. Sci. 1977, 15, 301–302.
- Cooke, A.; Fergeson, J.; Bulkhi, A.; Casale, T.B. The Electronic Cigarette: The Good, the Bad, and the Ugly. J Allergy Clin Immunol Pract 2015, 3, 498–505, doi:10.1016/j.jaip.2015.05.022.Cooke, A.; Fergeson, J.; Bulkhi, A.; Casale, T.B. The Electronic Cigarette: The Good, the Bad, and the Ugly. J. Allergy Clin. Immunol. Pract. 2015, 3, 498–505.
- Tsuchiya, K.; Hayashi, Y.; Onodera, M.; Hasegawa, T. Toxicity of Formaldehyde in Experimental Animals--Concentrations of the Chemical in the Elution from Dishes of Formaldehyde Resin in Some Vegetables. Keio J Med 1975, 24, 19–37.Tsuchiya, K.; Hayashi, Y.; Onodera, M.; Hasegawa, T. Toxicity of Formaldehyde in Experimental Animals—Concentrations of the Chemical in the Elution from Dishes of Formaldehyde Resin in Some Vegetables. Keio J. Med. 1975, 24, 19–37.
- Hayashi, T.; Reece, C.A.; Shibamoto, T. Gas Chromatographic Determination of Formaldehyde in Coffee via Thiazolidine Derivative. J Assoc Off Anal Chem 1986, 69, 101–105.Hayashi, T.; Reece, C.A.; Shibamoto, T. Gas Chromatographic Determination of Formaldehyde in Coffee via Thiazolidine Derivative. J. Assoc. Off. Anal. Chem. 1986, 69, 101–105.
- Restani, P.; Galli, C.L. Oral Toxicity of Formaldehyde and Its Derivatives. Crit. Rev. Toxicol. 1991, 21, 315–328, doi:10.3109/10408449109019569.Restani, P.; Galli, C.L. Oral Toxicity of Formaldehyde and Its Derivatives. Crit. Rev. Toxicol. 1991, 21, 315–328.
- Kaminski, J.; Atwal, A.S.; Mahadevan, S. Determination of Formaldehyde in Fresh and Retail Milk by Liquid Column Chromatography. J AOAC Int 1993, 76, 1010–1013.Kaminski, J.; Atwal, A.S.; Mahadevan, S. Determination of Formaldehyde in Fresh and Retail Milk by Liquid Column Chromatography. J. AOAC Int. 1993, 76, 1010–1013.
- Bianchi, F.; Careri, M.; Musci, M.; Mangia, A. Fish and Food Safety: Determination of Formaldehyde in 12 Fish Species by SPME Extraction and GC–MS Analysis. Food Chemistry 2007, 100, 1049–1053, doi:10.1016/j.foodchem.2005.09.089.Bianchi, F.; Careri, M.; Musci, M.; Mangia, A. Fish and Food Safety: Determination of Formaldehyde in 12 Fish Species by SPME Extraction and GC–MS Analysis. Food Chem. 2007, 100, 1049–1053.
- Dorokhov, Y.L.; Shindyapina, A.V.; Sheshukova, E.V.; Komarova, T.V. Metabolic Methanol: Molecular Pathways and Physiological Roles. Physiol. Rev. 2015, 95, 603–644, doi:10.1152/physrev.00034.2014.Dorokhov, Y.L.; Shindyapina, A.V.; Sheshukova, E.V.; Komarova, T.V. Metabolic Methanol: Molecular Pathways and Physiological Roles. Physiol. Rev. 2015, 95, 603–644.
- Jalkanen, S.; Salmi, M. Cell Surface Monoamine Oxidases: Enzymes in Search of a Function. EMBO J. 2001, 20, 3893–3901, doi:10.1093/emboj/20.15.3893.Jalkanen, S.; Salmi, M. Cell Surface Monoamine Oxidases: Enzymes in Search of a Function. EMBO J. 2001, 20, 3893–3901.
- Strolin Benedetti, M.; Tipton, K.F.; Whomsley, R. Amine Oxidases and Monooxygenases in the in Vivo Metabolism of Xenobiotic Amines in Humans: Has the Involvement of Amine Oxidases Been Neglected? Fundam Clin Pharmacol 2007, 21, 467–480, doi:10.1111/j.1472-8206.2007.00498.x.Strolin Benedetti, M.; Tipton, K.F.; Whomsley, R. Amine Oxidases and Monooxygenases in the in Vivo Metabolism of Xenobiotic Amines in Humans: Has the Involvement of Amine Oxidases Been Neglected? Fundam. Clin. Pharmacol. 2007, 21, 467–480.
- Yu, P.H.; Wright, S.; Fan, E.H.; Lun, Z.-R.; Gubisne-Harberle, D. Physiological and Pathological Implications of Semicarbazide-Sensitive Amine Oxidase. Biochim. Biophys. Acta 2003, 1647, 193–199.Yu, P.H.; Wright, S.; Fan, E.H.; Lun, Z.-R.; Gubisne-Harberle, D. Physiological and Pathological Implications of Semicarbazide-Sensitive Amine Oxidase. Biochim. Biophys. Acta 2003, 1647, 193–199.
- Schirch, V.; Szebenyi, D.M.E. Serine Hydroxymethyltransferase Revisited. Current Opinion in Chemical Biology 2005, 9, 482–487, doi:10.1016/J.CBPA.2005.08.017.Schirch, V.; Szebenyi, D.M.E. Serine Hydroxymethyltransferase Revisited. Curr. Opin. Chem. Biol. 2005, 9, 482–487.
- Steenkamp, D.J.; Husain, M. The Effect of Tetrahydrofolate on the Reduction of Electron Transfer Flavoprotein by Sarcosine and Dimethylglycine Dehydrogenases. Biochemical Journal 1982, 203, 707–715, doi:10.1042/BJ2030707.Steenkamp, D.J.; Husain, M. The Effect of Tetrahydrofolate on the Reduction of Electron Transfer Flavoprotein by Sarcosine and Dimethylglycine Dehydrogenases. Biochem. J. 1982, 203, 707–715.
- Orrenius, S.; Dallner, G.; Ernster, L. Inhibition of the TPNH-Linked Lipid Peroxidation of Liver Microsomes by Drugs Undergoing Oxidative Demethylation. Biochemical and Biophysical Research Communications 1964, 14, 329–334, doi:10.1016/S0006-291X(64)80005-X.Orrenius, S.; Dallner, G.; Ernster, L. Inhibition of the TPNH-Linked Lipid Peroxidation of Liver Microsomes by Drugs Undergoing Oxidative Demethylation. Biochem. Biophys. Res. Commun. 1964, 14, 329–334.
- Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic Microsomal Ethanol-Oxidizing System: Solubilization, Isolation, and Characterization. Archives of Biochemistry and Biophysics 1974, 163, 404–415, doi:10.1016/0003-9861(74)90492-5.Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic Microsomal Ethanol-Oxidizing System: Solubilization, Isolation, and Characterization. Arch. Biochem. Biophys. 1974, 163, 404–415.
- Hamm, S.; Just, G.; Lacoste, N.; Moitessier, N.; Szyf, M.; Mamer, O. On the Mechanism of Demethylation of 5-Methylcytosine in DNA. Bioorganic & Medicinal Chemistry Letters 2008, 18, 1046–1049, doi:10.1016/J.BMCL.2007.12.027.Hamm, S.; Just, G.; Lacoste, N.; Moitessier, N.; Szyf, M.; Mamer, O. On the Mechanism of Demethylation of 5-Methylcytosine in DNA. Bioorgan. Med. Chem. Lett. 2008, 18, 1046–1049.
- Hopkinson, R.J.; Hamed, R.B.; Rose, N.R.; Claridge, T.D.W.; Schofield, C.J. Monitoring the Activity of 2-Oxoglutarate Dependent Histone Demethylases by NMR Spectroscopy: Direct Observation of Formaldehyde. ChemBioChem 2010, 11, 506–510, doi:10.1002/CBIC.200900713.Hopkinson, R.J.; Hamed, R.B.; Rose, N.R.; Claridge, T.D.W.; Schofield, C.J. Monitoring the Activity of 2-Oxoglutarate Dependent Histone Demethylases by NMR Spectroscopy: Direct Observation of Formaldehyde. ChemBioChem 2010, 11, 506–510.
- Wan, Y. New Mechanism of Bone Cancer Pain: Tumor Tissue-Derived Endogenous Formaldehyde Induced Bone Cancer Pain via TRPV1 Activation. In Advances in Experimental Medicine and Biology; Springer New York LLC, 2016; Vol. 904, pp. 41–58.Wan, Y. New Mechanism of Bone Cancer Pain: Tumor Tissue-Derived Endogenous Formaldehyde Induced Bone Cancer Pain via TRPV1 Activation. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2016; Volume 904, pp. 41–58.
- Kato, S.; Burke, P.J.; Koch, T.H.; Bierbaum, V.M. Formalehyde in Human Cancer Cells: Detection by Preconcentration-Chemical Ionization Mass Spectrometry. Analytical Chemistry 2001, 73, 2992–2997, doi:10.1021/ac001498q.Kato, S.; Burke, P.J.; Koch, T.H.; Bierbaum, V.M. Formalehyde in Human Cancer Cells: Detection by Preconcentration-Chemical Ionization Mass Spectrometry. Anal. Chem. 2001, 73, 2992–2997.
- Burgos-Barragan, G.; Wit, N.; Meiser, J.; Dingler, F.A.; Pietzke, M.; Mulderrig, L.; Pontel, L.B.; Rosado, I.V.; Brewer, T.F.; Cordell, R.L.; et al. Mammals Divert Endogenous Genotoxic Formaldehyde into One-Carbon Metabolism. Nature 2017, 548, 549–549, doi:10.1038/NATURE23481.Burgos-Barragan, G.; Wit, N.; Meiser, J.; Dingler, F.A.; Pietzke, M.; Mulderrig, L.; Pontel, L.B.; Rosado, I.V.; Brewer, T.F.; Cordell, R.L.; et al. Mammals Divert Endogenous Genotoxic Formaldehyde into One-Carbon Metabolism. Nature 2017, 548, 549.
- Dorokhov, Y.L.; Sheshukova, E.V.; Bialik, T.E.; Komarova, T.V. Human Endogenous Formaldehyde as an Anticancer Metabolite: Its Oxidation Downregulation May Be a Means of Improving Therapy. BioEssays 2018, 40, doi:10.1002/bies.201800136.Dorokhov, Y.L.; Sheshukova, E.V.; Bialik, T.E.; Komarova, T.V. Human Endogenous Formaldehyde as an Anticancer Metabolite: Its Oxidation Downregulation May Be a Means of Improving Therapy. BioEssays 2018, 40, 1800136.
- Umansky, C.; Morellato, A.E.; Rieckher, M.; Scheidegger, M.A.; Martinefski, M.R.; Fernández, G.A.; Pak, O.; Kolesnikova, K.; Reingruber, H.; Bollini, M.; et al. Endogenous Formaldehyde Scavenges Cellular Glutathione Resulting in Redox Disruption and Cytotoxicity. Nat Commun 2022, 13, 745, doi:10.1038/s41467-022-28242-7.Umansky, C.; Morellato, A.E.; Rieckher, M.; Scheidegger, M.A.; Martinefski, M.R.; Fernández, G.A.; Pak, O.; Kolesnikova, K.; Reingruber, H.; Bollini, M.; et al. Endogenous Formaldehyde Scavenges Cellular Glutathione Resulting in Redox Disruption and Cytotoxicity. Nat. Commun. 2022, 13, 745.
- Pontel, L.B.; Rosado, I.V.; Burgos-Barragan, G.; Garaycoechea, J.I.; Yu, R.; Arends, M.J.; Chandrasekaran, G.; Broecker, V.; Wei, W.; Liu, L.; et al. Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol. Cell 2015, 60, 177–188, doi:10.1016/j.molcel.2015.08.020.Pontel, L.B.; Rosado, I.V.; Burgos-Barragan, G.; Garaycoechea, J.I.; Yu, R.; Arends, M.J.; Chandrasekaran, G.; Broecker, V.; Wei, W.; Liu, L.; et al. Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol. Cell 2015, 60, 177–188.
- Li, T.; Wei, Y.; Qu, M.; Mou, L.; Miao, J.; Xi, M.; Liu, Y.; He, R. Formaldehyde and De/Methylation in Age-Related Cognitive Impairment. Genes 2021, 12, 913, doi:10.3390/genes12060913.Li, T.; Wei, Y.; Qu, M.; Mou, L.; Miao, J.; Xi, M.; Liu, Y.; He, R. Formaldehyde and De/Methylation in Age-Related Cognitive Impairment. Genes 2021, 12, 913.
- Fei, X.-C.; Tong, Z.-Q. [Endogenous Formaldehyde Regulates Memory]. Sheng li xue bao : [Acta physiologica Sinica] 2020, 72, 463–474.Fei, X.-C.; Tong, Z.-Q. Endogenous Formaldehyde Regulates Memory. Sheng Li Xue Bao 2020, 72, 463–474.
- Fischer, M.H. THE TOXIC EFFECTS OF FORMALDEHYDE AND FORMALIN: (A PRELIMINARY COMMUNICATION.). Journal of the Boston Society of Medical Sciences 1900, 5, 18–18.Lu, Z.; Li, C.M.; Qiao, Y.; Yan, Y.; Yang, X. Effect of Inhaled Formaldehyde on Learning and Memory of Mice. Indoor Air 2008, 18, 77–83.
- Rice, E.; Baird, R.; Eaton, A.; Clesceri, L. Standard Methods for the Examination of Water and Wastewater - TABLE OF CONTENTS. 1905.Ai, L.; Tan, T.; Tang, Y.; Yang, J.; Cui, D.; Wang, R.; Wang, A.; Fei, X.; Di, Y.; Wang, X.; et al. Endogenous Formaldehyde Is a Memory-Related Molecule in Mice and Humans. Commun. Biol. 2019, 2, 446.
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Determination of Carbonyl Compounds in Beer by Derivatisation and Headspace Solid-Phase Microextraction in Combination with Gas Chromatography and Mass Spectrometry. Journal of Chromatography A 2009, 1216, 5061–5068, doi:10.1016/J.CHROMA.2009.04.077.Fischer, M.H. The toxic effects of formaldehyde and formalin: (A Preliminary Communication.). J. Boston Soc. Med. Sci. 1900, 5, 18.
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238, doi:10.3390/s22031238.Rice, E.; Baird, R.; Eaton, A.; Clesceri, L. Standard Methods for the Examination of Water and Wastewater—Table of Contents; American Public Health Association (APHA): Washington, DC, USA, 1905.
- Huang, Q.; Zeng, D.; Li, H.; Xie, C. Room Temperature Formaldehyde Sensors with Enhanced Performance, Fast Response and Recovery Based on Zinc Oxide Quantum Dots/Graphene Nanocomposites. Nanoscale 2012, 4, 5651–5658, doi:10.1039/C2NR31131C.Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Determination of Carbonyl Compounds in Beer by Derivatisation and Headspace Solid-Phase Microextraction in Combination with Gas Chromatography and Mass Spectrometry. J. Chromatogr. A 2009, 1216, 5061–5068.
- Dirksen, J.A.; Duval, K.; Ring, T.A. NiO Thin-Film Formaldehyde Gas Sensor. Sensors and Actuators B: Chemical 2001, 80, 106–115, doi:10.1016/S0925-4005(01)00898-X.Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238.
- Liu, J.; Chen, Y.; Zhang, H. Study of Highly Sensitive Formaldehyde Sensors Based on ZnO/CuO Heterostructure via the Sol-Gel Method. Sensors 2021, 21, 4685, doi:10.3390/s21144685.Huang, Q.; Zeng, D.; Li, H.; Xie, C. Room Temperature Formaldehyde Sensors with Enhanced Performance, Fast Response and Recovery Based on Zinc Oxide Quantum Dots/Graphene Nanocomposites. Nanoscale 2012, 4, 5651–5658.
- Vajhadin, F.; Mazloum-Ardakani, M.; Amini, A. Metal Oxide-Based Gas Sensors for Detection of Exhaled Breath Markers. Med Devices Sens 2020, e10161, doi:10.1002/mds3.10161.Dirksen, J.A.; Duval, K.; Ring, T.A. NiO Thin-Film Formaldehyde Gas Sensor. Sens. Actuators B Chem. 2001, 80, 106–115.
- Winkowski, M.; Stacewicz, T. Optical Detection of Formaldehyde in Air in the 3.6 µm Range. Biomed. Opt. Express, BOE 2020, 11, 7019–7031, doi:10.1364/BOE.405384.Liu, J.; Chen, Y.; Zhang, H. Study of Highly Sensitive Formaldehyde Sensors Based on ZnO/CuO Heterostructure via the Sol-Gel Method. Sensors 2021, 21, 4685.
- Jeong, H.; Chung, H.; Song, S.; Kim, C.; Lee, J.; Kim, Y. Validation and Determination of the Contents of Acetaldehyde and Formaldehyde in Foods. Toxicological research 2015, 31, 273–278, doi:10.5487/TR.2015.31.3.273.Vajhadin, F.; Mazloum-Ardakani, M.; Amini, A. Metal Oxide-Based Gas Sensors for Detection of Exhaled Breath Markers. Med. Dev. Sens. 2020, 4, e10161.
- Lobo, F.; Santos, T.; Vieira, K.; Osório, V.; Taylor, J. Determination of Formaldehyde in Hair Creams by Gas Chromatography-Mass Spectrometry. Drug testing and analysis 2015, 7, 848–852, doi:10.1002/DTA.1808.Winkowski, M.; Stacewicz, T. Optical Detection of Formaldehyde in Air in the 3.6 µm Range. Biomed. Opt. Express 2020, 11, 7019–7031.
- Takeuchi, A.; Takigawa, T.; Abe, M.; Kawai, T.; Endo, Y.; Yasugi, T.; Endo, G.; Ogino, K. Determination of Formaldehyde in Urine by Headspace Gas Chromatography. Bulletin of environmental contamination and toxicology 2007, 79, 1–4, doi:10.1007/S00128-007-9172-0.Jeong, H.; Chung, H.; Song, S.; Kim, C.; Lee, J.; Kim, Y. Validation and Determination of the Contents of Acetaldehyde and Formaldehyde in Foods. Toxicol. Res. 2015, 31, 273–278.
- Lv, G.; Hu, D.; Zhao, J.; Li, S. Quality Control of Sweet Medicines Based on Gas Chromatography-Mass Spectrometry. Drug discoveries & therapeutics 2015, 9, 94–106, doi:10.5582/DDT.2015.01020.Lobo, F.; Santos, T.; Vieira, K.; Osório, V.; Taylor, J. Determination of Formaldehyde in Hair Creams by Gas Chromatography-Mass Spectrometry. Drug Test. Anal. 2015, 7, 848–852.
- Dorman, F.; Whiting, J.; Cochran, J.; Gardea-Torresdey, J. Gas Chromatography. Analytical chemistry 2010, 82, 4775–4785, doi:10.1021/AC101156H.Takeuchi, A.; Takigawa, T.; Abe, M.; Kawai, T.; Endo, Y.; Yasugi, T.; Endo, G.; Ogino, K. Determination of Formaldehyde in Urine by Headspace Gas Chromatography. Bull. Environ. Contam. Toxicol. 2007, 79, 1–4.
- Cancilla, D.A.; Que Hee, S.S. O-(2,3,4,5,6-Pentafluorophenyl)Methylhydroxylamine Hydrochloride: A Versatile Reagent for the Determination of Carbonyl-Containing Compounds. J Chromatogr 1992, 627, 1–16, doi:10.1016/0021-9673(92)87181-7.Lv, G.; Hu, D.; Zhao, J.; Li, S. Quality Control of Sweet Medicines Based on Gas Chromatography-Mass Spectrometry. Drug Discov. Ther. 2015, 9, 94–106.
- Hoffmann, G.F.; Sweetman, L. O-(2,3,4,5,6-Pentafluorobenzyl)Oxime-Trimethylsilyl Ester Derivatives for Sensitive Identification and Quantitation of Aldehydes, Ketones, and Oxoacids in Biological Fluids. Clin Chim Acta 1991, 199, 237–242, doi:10.1016/0009-8981(91)90117-u.Dorman, F.; Whiting, J.; Cochran, J.; Gardea-Torresdey, J. Gas Chromatography. Anal. Chem. 2010, 82, 4775–4785.
- Tsai, S.W.; Chang, C.M. Analysis of Aldehydes in Water by Solid-Phase Microextraction with on-Fiber Derivatization. Journal of Chromatography A 2003, 1015, 143–150, doi:10.1016/S0021-9673(03)01241-X.Baba, K.; Ishikawa, S.; Hanada, Y.; Uchimura, Y.; Subta, S.; Kido, K. Determination of Saturated and Alpha-Unsaturated Aliphatic Aldehydes in Environmental Water Samples by GC. Bunseki Kagaku Anal. Chem. 1988, 37, 519–523.
- Office of Research and Development, U.E., Method 556, 1998. US Environmental Protection Agency, Determination of carbonyl compounds in drinking water by pentafluorobenzylhydroxylamine derivatization and capillary gas chromatography with electron capture detection, US Environmental Protection Agency, Determination of Carbonyl Compounds in Drinking Water by Pentafluorobenzylhydroxylamine Derivatization and Capillary Gas Chromatography with Electron Capture Detection. Office of Research and Development, US EPA 1998, Method 556.Bao, M.L.; Pantani, F.; Griffini, O.; Burrini, D.; Santianni, D.; Barbieri, K. Determination of Carbonyl Compounds in Water by Derivatization-Solid-Phase Microextraction and Gas Chromatographic Analysis. J. Chromatogr. A 1998, 809, 75–87.
- Heck, H. d’A; White, E.L.; Casanova-Schmitz, M. Determination of Formaldehyde in Biological Tissues by Gas Chromatography/Mass Spectrometry. Biomedical Mass Spectrometry 1982, 9, 347–353, doi:10.1002/bms.1200090808.Cancilla, D.A.; Que Hee, S.S. O-(2,3,4,5,6-Pentafluorophenyl)Methylhydroxylamine Hydrochloride: A Versatile Reagent for the Determination of Carbonyl-Containing Compounds. J. Chromatogr. 1992, 627, 1–16.
- Heck, H.D.; Casanova-Schmitz, M.; Dodd, P.B.; Schachter, E.N.; Witek, T.J.; Tosun, T. Formaldehyde (CH2O) Concentrations in the Blood of Humans and Fischer-344 Rats Exposed to CH2O under Controlled Conditions. Am Ind Hyg Assoc J 1985, 46, 1–3, doi:10.1080/15298668591394275.Hoffmann, G.F.; Sweetman, L. O-(2,3,4,5,6-Pentafluorobenzyl)Oxime-Trimethylsilyl Ester Derivatives for Sensitive Identification and Quantitation of Aldehydes, Ketones, and Oxoacids in Biological Fluids. Clin. Chim. Acta 1991, 199, 237–242.
- Luong, J.; Sieben, L.; Fairhurst, M.; De Zeeuw, J. Determination of Low Levels of Formaldehyde and Acetaldehyde by Gas Chromatography/Flame Ionization Detection with a Nickel Catalyst. Journal of High Resolution Chromatography 1996, 19, 591–594, doi:10.1002/jhrc.1240191013.Tsai, S.W.; Chang, C.M. Analysis of Aldehydes in Water by Solid-Phase Microextraction with on-Fiber Derivatization. J. Chromatogr. A 2003, 1015, 143–150.
- Luong, J.; Yang, X.; Hua, Y.; Yang, P.; Gras, R. Gas Chromatography with In Situ Catalytic Hydrogenolysis and Flame Ionization Detection for the Direct Measurement of Formaldehyde and Acetaldehyde in Challenging Matrices. Analytical Chemistry 2018, 90, 13855–13859, doi:10.1021/ACS.ANALCHEM.8B04563.Office of Research and Development, U.E.; US Environmental Protection Agency. Determination of carbonyl compounds in drinking water by pentafluorobenzylhydroxylamine derivatization and capillary gas chromatography with electron capture detection, US Environmental Protection Agency, Determination of Carbonyl Compounds in Drinking Water by Pentafluorobenzylhydroxylamine Derivatization and Capillary Gas Chromatography with Electron Capture Detection. Off. Res. Dev. US EPA 1998, 556.
- Nguyen, D.T.-T.; Guillarme, D.; Rudaz, S.; Veuthey, J.-L. Fast Analysis in Liquid Chromatography Using Small Particle Size and High Pressure. Journal of Separation Science 2006, 29, 1836–1848, doi:10.1002/JSSC.200600189.Heck, H.d.; White, E.L.; Casanova-Schmitz, M. Determination of Formaldehyde in Biological Tissues by Gas Chromatography/Mass Spectrometry. Biomed. Mass Spectrom. 1982, 9, 347–353.
- Luo, W.; Li, H.; Zhang, Y.; Ang, C.Y. Determination of Formaldehyde in Blood Plasma by High-Performance Liquid Chromatography with Fluorescence Detection. J Chromatogr B Biomed Sci Appl 2001, 753, 253–257, doi:10.1016/s0378-4347(00)00552-1.Heck, H.D.; Casanova-Schmitz, M.; Dodd, P.B.; Schachter, E.N.; Witek, T.J.; Tosun, T. Formaldehyde (CH2O) Concentrations in the Blood of Humans and Fischer-344 Rats Exposed to CH2O under Controlled Conditions. Am. Ind. Hyg. Assoc. J. 1985, 46, 1–3.
- Koivusalmi, E.; Haatainen, E.; Root, A. Quantitative RP-HPLC Determination of Some Aldehydes and Hydroxyaldehydes as Their 2,4-Dinitrophenylhydrazone Derivatives. Anal. Chem. 1999, 71, 86–91, doi:10.1021/ac980699f.Gabrio, T.; Bertsch, A. Determination of Carbonyl Compounds in Pool Water with O-(2,3,4,5,6-Pentafluorobenzyl)Hydroxyamine Hydrochloride and Gas Chromatographic-Tandem Mass Spectrometric Analysis. J. Chromatogr. A 2004, 1046, 293–296.
- Van Hoof, F.; Wittocx, A.; Van Buggenhout, E.; Janssens, J. Determination of Aliphatic Aldehydes in Waters by High-Performance Liquid Chromatography. Analytica Chimica Acta 1985, 169, 419–424, doi:10.1016/S0003-2670(00)86251-0.Lo, K.M.; Yen, L. Integration of Headspace Solid Phase Micro-Extraction with Gas Chromatography for Quantitative Analysis of Formaldehyde. Bull. Korean Chem. Soc. 2013, 34, 139–142.
- Peters, R.; Hellenbrand, J.; Mengerink, Y.; Wal, S.V. der On-Line Determination of Carboxylic Acids, Aldehydes and Ketones by High-Performance Liquid Chromatography-Diode Array Detection-Atmospheric Pressure Chemical Ionisation Mass Spectrometry after Derivatization with 2-Nitrophenylhydrazine. Journal of chromatography. A 2004, 1031, 35–50, doi:10.1016/J.CHROMA.2003.10.100.Pragst, F. Application of Solid-Phase Microextraction in Analytical Toxicology. Anal. Bioanal. Chem. 2007, 388, 1393–1414.
- Soman, A.; Qiu, Y.; Li, Q.C. HPLC-UV Method Development and Validation for the Determination of Low Level Formaldehyde in a Drug Substance. Journal of chromatographic science 2008, 46, 461–465, doi:10.1093/CHROMSCI/46.6.461.Beránek, J.; Kubátová, A. Evaluation of Solid-Phase Microextraction Methods for Determination of Trace Concentration Aldehydes in Aqueous Solution. J. Chromatogr. A 2008, 1209, 44–54.
- Sebaei, A.; Gomaa, A.; El-Zwahry, A.; Emara, E. Determination of Formaldehyde by HPLC with Stable Precolumn Derivatization in Egyptian Dairy Products. International journal of analytical chemistry 2018, 2018, doi:10.1155/2018/2757941.Martos, P.A.; Pawliszyn, J. Sampling and Determination of Formaldehyde Using Solid-Phase Microextraction with On-Fiber Derivatization. Anal. Chem. 1998, 70, 2311–2320.
- Bourgeois, C.; Blanc, N.; Cannot, J.; Demesmay, C. Towards a Non-Biased Formaldehyde Quantification in Leather: New Derivatization Conditions before HPLC Analysis of 2,4-Dinitrophenylhydrazine Derivatives. Molecules (Basel, Switzerland) 2020, 25, doi:10.3390/MOLECULES25235765.Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of Aldehydes in Beer Using Solid-Phase Microextraction with on-Fiber Derivatization and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944.
- Storey, J.; Andersen, W.; Heise, A.; Turnipseed, S.; Lohne, J.; Thomas, T.; Madson, M. A Rapid Liquid Chromatography Determination of Free Formaldehyde in Cod. Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment 2015, 32, 657–664, doi:10.1080/19440049.2015.1020530.Luong, J.; Sieben, L.; Fairhurst, M.; De Zeeuw, J. Determination of Low Levels of Formaldehyde and Acetaldehyde by Gas Chromatography/Flame Ionization Detection with a Nickel Catalyst. J. High Resolut. Chromatogr. 1996, 19, 591–594.
- Deng, Y.; Yu, P. Simultaneous Determination of Formaldehyde and Methylglyoxal in Urine: Involvement of Semicarbazide-Sensitive Amine Oxidase-Mediated Deamination in Diabetic Complications. Journal of chromatographic science 1999, 37, 317–322, doi:10.1093/CHROMSCI/37.9.317.Luong, J.; Yang, X.; Hua, Y.; Yang, P.; Gras, R. Gas Chromatography with In Situ Catalytic Hydrogenolysis and Flame Ionization Detection for the Direct Measurement of Formaldehyde and Acetaldehyde in Challenging Matrices. Anal. Chem. 2018, 90, 13855–13859.
- Cordis, G.A.; Maulik, N.; Bagchi, D.; Engelman, R.M.; Das, D.K. Estimation of the Extent of Lipid Peroxidation in the Ischemic and Reperfused Heart by Monitoring Lipid Metabolic Products with the Aid of High-Performance Liquid Chromatography. J Chromatogr 1993, 632.Nguyen, D.T.-T.; Guillarme, D.; Rudaz, S.; Veuthey, J.-L. Fast Analysis in Liquid Chromatography Using Small Particle Size and High Pressure. J. Sep. Sci. 2006, 29, 1836–1848.
- Komarova, T.V.; Petrunia, I.V.; Shindyapina, A.V.; Silachev, D.N.; Sheshukova, E.V.; Kiryanov, G.I.; Dorokhov, Y.L. Endogenous Methanol Regulates Mammalian Gene Activity. PLoS ONE 2014, 9, e90239, doi:10.1371/journal.pone.0090239.Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; ISBN 978-1-118-59201-4.
- Shindyapina, A.V.; Komarova, T.V.; Sheshukova, E.V.; Ershova, N.M.; Tashlitsky, V.N.; Kurkin, A.V.; Yusupov, I.R.; Mkrtchyan, G.V.; Shagidulin, M.Y.; Dorokhov, Y.L. The Antioxidant Cofactor Alpha-Lipoic Acid May Control Endogenous Formaldehyde Metabolism in Mammals. Frontiers in Neuroscience 2017, 0, 651–651, doi:10.3389/FNINS.2017.00651.Perrett, D.; Herbert, K.; Morris, G.; Simmonds, H. Optimised Conditions for the Routine HPLC Separation of Nucleotides in Cell Extracts. Adv. Exp. Med. Biol. 1989, 253B, 463–468.
- Luo, W.; Ang, C.Y.; Thompson, H.C. Rapid Method for the Determination of Ampicillin Residues in Animal Muscle Tissues by High-Performance Liquid Chromatography with Fluorescence Detection. J Chromatogr B Biomed Sci Appl 1997, 694, 401–407, doi:10.1016/s0378-4347(97)00171-0.Luo, W.; Li, H.; Zhang, Y.; Ang, C.Y. Determination of Formaldehyde in Blood Plasma by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 253–257.
- Sárdi, E.; Tyihák, E. Simple Determination of Formaldehyde in Dimedone Adduct Form in Biological Samples by High Performance Liquid Chromatography. Biomed Chromatogr 1994, 8, 313–314, doi:10.1002/bmc.1130080614.Koivusalmi, E.; Haatainen, E.; Root, A. Quantitative RP-HPLC Determination of Some Aldehydes and Hydroxyaldehydes as Their 2,4-Dinitrophenylhydrazone Derivatives. Anal. Chem. 1999, 71, 86–91.
- Nash, T. The Colorimetric Estimation of Formaldehyde by Means of the Hantzsch Reaction. Biochemical Journal 1953, 55, 416–416, doi:10.1042/BJ0550416.Deng, Y.; Yu, P. Simultaneous Determination of Formaldehyde and Methylglyoxal in Urine: Involvement of Semicarbazide-Sensitive Amine Oxidase-Mediated Deamination in Diabetic Complications. J. Chromatogr. Sci. 1999, 37, 317–322.
- Różyło, T.K.; Siembida, R.; Tyihák, E. Measurement of Formaldehyde as Dimedone Adduct and Potential Formaldehyde Precursors in Hard Tissues of Human Teeth by Overpressured Layer Chromatography. Biomedical Chromatography 1999, 13, 513–515, doi:10.1002/(SICI)1099-0801(199912)13:8<513::AID-BMC917>3.0.CO;2-5.Van den Bergh, H.; Coeckelberghs, H.; Vankerckhoven, F.; Compernolle, C. Vinckier Study of the Carbonyl Products of Terpene/OH Radical Reactions: Detection of the 2,4-DNPH Derivatives by HPLC-MS. Anal. Bioanal. Chem. 2004, 379, 484–494.
- Sárdi, E.; Tyihák, E. Relationship between Dimedone Concentration and Formaldehyde Captured in Plant Tissues. Acta Biol Hung 1998, 49, 291–301.Van Hoof, F.; Wittocx, A.; Van Buggenhout, E.; Janssens, J. Determination of Aliphatic Aldehydes in Waters by High-Performance Liquid Chromatography. Anal. Chim. Acta 1985, 169, 419–424.
- Wadsworth, A.; Pangborn, M.C. THE REACTION OF FORMALDEHYDE WITH AMINO ACIDS. Journal of Biological Chemistry 1936, 116, 423–436, doi:10.1016/S0021-9258(18)74695-4.Peters, R.; Hellenbrand, J.; Mengerink, Y.; Van der Wal, S. On-Line Determination of Carboxylic Acids, Aldehydes and Ketones by High-Performance Liquid Chromatography-Diode Array Detection-Atmospheric Pressure Chemical Ionisation Mass Spectrometry after Derivatization with 2-Nitrophenylhydrazine. J. Chromatogr. A 2004, 1031, 35–50.
- Kitamoto, Y.; Maeda, H. Reevaluation of the Reaction of Formaldehyde at Low Concentration with Amino Acids. The Journal of Biochemistry 1980, 87, 1519–1530, doi:10.1093/OXFORDJOURNALS.JBCHEM.A132893.Soman, A.; Qiu, Y.; Li, Q.C. HPLC-UV Method Development and Validation for the Determination of Low Level Formaldehyde in a Drug Substance. J. Chromatogr. Sci. 2008, 46, 461–465.
- Kamps, J.J.A.G.; Hopkinson, R.J.; Schofield, C.J.; Claridge, T.D.W. How Formaldehyde Reacts with Amino Acids. Communications Chemistry 2019 2:1 2019, 2, 1–14, doi:10.1038/s42004-019-0224-2.Sebaei, A.; Gomaa, A.; El-Zwahry, A.; Emara, E. Determination of Formaldehyde by HPLC with Stable Precolumn Derivatization in Egyptian Dairy Products. Int. J. Anal. Chem. 2018, 2018, 2757941.
- Chiarella, P.; Tranfo, G.; Pigini, D.; Carbonari, D. Is It Possible to Use Biomonitoring for the Quantitative Assessment of Formaldehyde Occupational Exposure? Biomarkers in Medicine 2016, 10, 1287–1303, doi:10.2217/bmm-2016-0146.Bourgeois, C.; Blanc, N.; Cannot, J.; Demesmay, C. Towards a Non-Biased Formaldehyde Quantification in Leather: New Derivatization Conditions before HPLC Analysis of 2,4-Dinitrophenylhydrazine Derivatives. Molecules 2020, 25, 5765.
- Edrissi, B.; Taghizadeh, K.; Moeller, B.C.; Yu, R.; Kracko, D.; Doyle-Eisele, M.; Swenberg, J.A.; Dedon, P.C. N6-Formyllysine as a Biomarker of Formaldehyde Exposure: Formation and Loss of N6-Formyllysine in Nasal Epithelium in Long-Term, Low-Dose Inhalation Studies in Rats. Chem Res Toxicol 2017, 30, 1572–1576, doi:10.1021/acs.chemrestox.7b00075.Storey, J.; Andersen, W.; Heise, A.; Turnipseed, S.; Lohne, J.; Thomas, T.; Madson, M. A Rapid Liquid Chromatography Determination of Free Formaldehyde in Cod. Food Addit. Contam. Part A 2015, 32, 657–664.
- Pietzke, M.; Burgos-Barragan, G.; Wit, N.; Tait-Mulder, J.; Sumpton, D.; Mackay, G.M.; Patel, K.J.; Vazquez, A. Amino Acid Dependent Formaldehyde Metabolism in Mammals. Communications Chemistry 2020 3:1 2020, 3, 1–10, doi:10.1038/s42004-020-0324-z.Cordis, G.A.; Maulik, N.; Bagchi, D.; Engelman, R.M.; Das, D.K. Estimation of the Extent of Lipid Peroxidation in the Ischemic and Reperfused Heart by Monitoring Lipid Metabolic Products with the Aid of High-Performance Liquid Chromatography. J. Chromatogr. 1993, 632, 97–103.
- Hladová, M.; Martinka, J.; Rantuch, P.; Nečas, A. Review of Spectrophotometric Methods for Determination of Formaldehyde. Research Papers Faculty of Materials Science and Technology Slovak University of Technology 2019, 27, 105–120, doi:10.2478/rput-2019-0012.Komarova, T.V.; Petrunia, I.V.; Shindyapina, A.V.; Silachev, D.N.; Sheshukova, E.V.; Kiryanov, G.I.; Dorokhov, Y.L. Endogenous Methanol Regulates Mammalian Gene Activity. PLoS ONE 2014, 9, e90239.
- Manna, S.K.; Achar, T.K.; Mondal, S. Recent Advances in Selective Formaldehyde Detection in Biological and Environmental Samples by Fluorometric and Colorimetric Chemodosimeters. Anal. Methods 2021, 13, 1084–1105, doi:10.1039/D0AY02252G.Shindyapina, A.V.; Komarova, T.V.; Sheshukova, E.V.; Ershova, N.M.; Tashlitsky, V.N.; Kurkin, A.V.; Yusupov, I.R.; Mkrtchyan, G.V.; Shagidulin, M.Y.; Dorokhov, Y.L. The Antioxidant Cofactor Alpha-Lipoic Acid May Control Endogenous Formaldehyde Metabolism in Mammals. Front. Neurosci. 2017, 11, 651.
- Hantzsch, A. Condensationsprodukte Aus Aldehydammoniak Und Ketonartigen Verbindungen. Berichte der deutschen chemischen Gesellschaft 1881, 14, 1637–1638, doi:10.1002/CBER.18810140214.Luo, W.; Ang, C.Y.; Thompson, H.C. Rapid Method for the Determination of Ampicillin Residues in Animal Muscle Tissues by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. B Biomed. Sci. Appl. 1997, 694, 401–407.
- Kawakami, Y.; Maruo, Y.Y.; Nakagawa, T.; Saito, H. A Screening Method for Detecting Formaldehyde Emitted from Textile Products. Measurement 2015, 62, 41–46, doi:10.1016/j.measurement.2014.11.005.Reinbold, R.; John, T.; Spingardi, P.; Kawamura, A.; Thompson, A.L.; Schofield, C.J.; Hopkinson, R.J. Formaldehyde Quantification Using Ampicillin Is Not Selective. Sci. Rep. 2019, 9, 18289.
- Gryllaki-Berger, M.; Mugny, C.; Perrenoud, D.; Pannatier, A.; Frenk, E. A Comparative Study of Formaldehyde Detection Using Chromotropic Acid, Acetylacetone and HPLC in Cosmetics and Household Cleaning Products. Contact Dermatitis 1992, 26, 149–154, doi:10.1111/j.1600-0536.1992.tb00284.x.Sárdi, E.; Tyihák, E. Simple Determination of Formaldehyde in Dimedone Adduct Form in Biological Samples by High Performance Liquid Chromatography. Biomed. Chromatogr. 1994, 8, 313–314.
- Na, C.; ManLing, Z.; YanLing, T. Determination of the formaldehyde content in beer by acetyl acetone method. Journal of Food Safety and Quality 2016, 7, 906–910.Nash, T. The Colorimetric Estimation of Formaldehyde by Means of the Hantzsch Reaction. Biochem. J. 1953, 55, 416.
- MacAllister, S.L.; Choi, J.; Dedina, L.; O’Brien, P.J. Metabolic Mechanisms of Methanol/Formaldehyde in Isolated Rat Hepatocytes: Carbonyl-Metabolizing Enzymes versus Oxidative Stress. Chemico-Biological Interactions 2011, 191, 308–314, doi:10.1016/j.cbi.2011.01.017.Różyło, T.K.; Siembida, R.; Tyihák, E. Measurement of Formaldehyde as Dimedone Adduct and Potential Formaldehyde Precursors in Hard Tissues of Human Teeth by Overpressured Layer Chromatography. Biomed. Chromatogr. 1999, 13, 513–515.
- Hallier, E.; Schröder, K.R.; Asmuth, K.; Dommermuth, A.; Aust, B.; Goergens, H.W. Metabolism of Dichloromethane (Methylene Chloride) to Formaldehyde in Human Erythrocytes: Influence of Polymorphism of Glutathione Transferase Theta (GST T1-1). Arch Toxicol 1994, 68, 423–427, doi:10.1007/s002040050092.Sárdi, E.; Tyihák, E. Relationship between Dimedone Concentration and Formaldehyde Captured in Plant Tissues. Acta Biol. Hung. 1998, 49, 291–301.
- Bruhn, C.; Brockmöller, J.; Kerb, R.; Roots, I.; Borchert, H.-H. Concordance between Enzyme Activity and Genotype of Glutathione S-Transferase Theta (GSTT1). Biochemical Pharmacology 1998, 56, 1189–1193, doi:10.1016/S0006-2952(98)00191-9.Wadsworth, A.; Pangborn, M.C. The reaction of formaldehyde with amino acids. J. Biol. Chem. 1936, 116, 423–436.
- Pinto, G.F.; Rocha, D.L.; Richter, E.M.; Muñoz, R.A.A.; Silva, S.G. da A Multicommuted Flow System for Spectrophotometric Determination of Formaldehyde in Mushroom. J. Braz. Chem. Soc. 2018, 29, 1400–1405.Kitamoto, Y.; Maeda, H. Reevaluation of the Reaction of Formaldehyde at Low Concentration with Amino Acids. J. Biochem. 1980, 87, 1519–1530.
- Li, Q.; Sritharathikhun; Motomizu Development of Novel Reagent for Hantzsch Reaction for the Determination of Formaldehyde by Spectrophotometry and Fluorometry. Analytical sciences : the international journal of the Japan Society for Analytical Chemistry 2007, 23, 413–417, doi:10.2116/ANALSCI.23.413.Kamps, J.J.A.G.; Hopkinson, R.J.; Schofield, C.J.; Claridge, T.D.W. How Formaldehyde Reacts with Amino Acids. Commun. Chem. 2019, 21, 1–14.
- Li, Q.; Oshima, M.; Motomizu, S. Flow-Injection Spectrofluorometric Determination of Trace Amounts of Formaldehyde in Water after Derivatization with Acetoacetanilide. Talanta 2007, 72, 1675–1680, doi:10.1016/j.talanta.2007.01.054.Chiarella, P.; Tranfo, G.; Pigini, D.; Carbonari, D. Is It Possible to Use Biomonitoring for the Quantitative Assessment of Formaldehyde Occupational Exposure? Biomark. Med. 2016, 10, 1287–1303.
- Compton, B.J.; Purdy, W.C. The Mechanism of the Reaction of the Nash and the Sawicki Aldehyde Reagent. Can. J. Chem. 1980, 58, 2207–2211, doi:10.1139/v80-355.Edrissi, B.; Taghizadeh, K.; Moeller, B.C.; Yu, R.; Kracko, D.; Doyle-Eisele, M.; Swenberg, J.A.; Dedon, P.C. N6-Formyllysine as a Biomarker of Formaldehyde Exposure: Formation and Loss of N6-Formyllysine in Nasal Epithelium in Long-Term, Low-Dose Inhalation Studies in Rats. Chem. Res. Toxicol. 2017, 30, 1572–1576.
- Xu, Z.; Chen, J.; Hu, L.L.; Tan, Y.; Liu, S.H.; Yin, J. Recent Advances in Formaldehyde-Responsive Fluorescent Probes. Chinese Chemical Letters 2017, 28, 1935–1942, doi:10.1016/J.CCLET.2017.07.018.Regazzoni, L.G.; Grigoryan, H.; Ji, Z.; Chen, X.; Daniels, S.I.; Huang, D.; Sanchez, S.; Tang, N.; Sillé, F.C.M.; Iavarone, A.T.; et al. Using Lysine Adducts of Human Serum Albumin to Investigate the Disposition of Exogenous Formaldehyde in Human Blood. Toxicol. Lett. 2017, 268, 26–35.
- Pan, S.; Roy, S.; Choudhury, N.; Behera, P.P.; Sivaprakasam, K.; Ramakrishnan, L.; De, P. From Small Molecules to Polymeric Probes: Recent Advancements of Formaldehyde Sensors. null 2022, 23, 49–63, doi:10.1080/14686996.2021.2018920.Yang, M.; Ospina, M.; Tse, C.; Toth, S.; Caudill, S.P.; Vesper, H.W. Ultraperformance Liquid Chromatography Tandem Mass Spectrometry Method to Determine Formaldehyde Hemoglobin Adducts in Humans as Biomarker for Formaldehyde Exposure. Chem. Res. Toxicol. 2017, 30, 1592–1598.
- Du, Y.; Zhang, Y.; Huang, M.; Wang, S.; Wang, J.; Liao, K.; Wu, X.; Zhou, Q.; Zhang, X.; Wu, Y.-D.; et al. Systematic Investigation of the Aza-Cope Reaction for Fluorescence Imaging of Formaldehyde in Vitro and in Vivo. Chem Sci 2021, 12, 13857–13869, doi:10.1039/d1sc04387k.Pietzke, M.; Burgos-Barragan, G.; Wit, N.; Tait-Mulder, J.; Sumpton, D.; Mackay, G.M.; Patel, K.J.; Vazquez, A. Amino Acid Dependent Formaldehyde Metabolism in Mammals. Commun. Chem. 2020, 31, 1–10.
- Horowitz, R.M.; Geissman, T.A. A Cleavage Reaction of α-Allylbenzylamines. Journal of the American Chemical Society 2002, 72, 1518–1522, doi:10.1021/JA01160A025.Hladová, M.; Martinka, J.; Rantuch, P.; Nečas, A. Review of Spectrophotometric Methods for Determination of Formaldehyde. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2019, 27, 105–120.
- Bruemmer, K.J.; Green, O.; Su, T.A.; Shabat, D.; Chang, C.J. Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice. Angewandte Chemie International Edition 2018, 57, 7508–7512, doi:10.1002/ANIE.201802143.Manna, S.K.; Achar, T.K.; Mondal, S. Recent Advances in Selective Formaldehyde Detection in Biological and Environmental Samples by Fluorometric and Colorimetric Chemodosimeters. Anal. Methods 2021, 13, 1084–1105.
- Brewer, T.F.; Burgos-Barragan, G.; Wit, N.; Patel, K.J.; Chang, C.J. A 2-Aza-Cope Reactivity-Based Platform for Ratiometric Fluorescence Imaging of Formaldehyde in Living Cells. Chemical Science 2017, 8, 4073–4081, doi:10.1039/C7SC00748E.Hantzsch, A. Condensationsprodukte Aus Aldehydammoniak Und Ketonartigen Verbindungen. Berichte Dtsch. Chem. Ges. 1881, 14, 1637–1638.
- Liu, W.; Truillet, C.; Flavell, R.R.; Brewer, T.F.; Evans, M.J.; Wilson, D.M.; Chang, C.J. A Reactivity-Based [18F]FDG Probe for in Vivo Formaldehyde Imaging Using Positron Emission Tomography. Chemical Science 2016, 7, 5503–5507, doi:10.1039/C6SC01503D.Kawakami, Y.; Maruo, Y.Y.; Nakagawa, T.; Saito, H. A Screening Method for Detecting Formaldehyde Emitted from Textile Products. Measurement 2015, 62, 41–46.
- Yurimoto, H.; Hirai, R.; Matsuno, N.; Yasueda, H.; Kato, N.; Sakai, Y. HxlR, a Member of the DUF24 Protein Family, Is a DNA-Binding Protein That Acts as a Positive Regulator of the Formaldehyde-Inducible HxlAB Operon in Bacillus Subtilis. Mol Microbiol 2005, 57, 511–519, doi:10.1111/j.1365-2958.2005.04702.x.Gryllaki-Berger, M.; Mugny, C.; Perrenoud, D.; Pannatier, A.; Frenk, E. A Comparative Study of Formaldehyde Detection Using Chromotropic Acid, Acetylacetone and HPLC in Cosmetics and Household Cleaning Products. Contact Dermat. 1992, 26, 149–154.
- Tralau, T.; Lafite, P.; Levy, C.; Combe, J.P.; Scrutton, N.S.; Leys, D. An Internal Reaction Chamber in Dimethylglycine Oxidase Provides Efficient Protection from Exposure to Toxic Formaldehyde. J Biol Chem 2009, 284, 17826–17834, doi:10.1074/jbc.M109.006262.Na, C.; ManLing, Z.; YanLing, T. Determination of the formaldehyde content in beer by acetyl acetone method. J. Food Saf. Qual. 2016, 7, 906–910.
- Woolston, B.M.; King, J.R.; Reiter, M.; Hove, B.V.; Stephanopoulos, G. Improving Formaldehyde Consumption Drives Methanol Assimilation in Engineered E. Coli. Nature Communications 2018, 9, doi:10.1038/S41467-018-04795-4.MacAllister, S.L.; Choi, J.; Dedina, L.; O’Brien, P.J. Metabolic Mechanisms of Methanol/Formaldehyde in Isolated Rat Hepatocytes: Carbonyl-Metabolizing Enzymes versus Oxidative Stress. Chem. Biol. Interact. 2011, 191, 308–314.
- Law, J.R. Molecular Basis of Bacterial Formaldehyde Sensing; The University of Manchester (United Kingdom): Manchester, UK, 2012; ISBN 1-07-392387-8.Hallier, E.; Schröder, K.R.; Asmuth, K.; Dommermuth, A.; Aust, B.; Goergens, H.W. Metabolism of Dichloromethane (Methylene Chloride) to Formaldehyde in Human Erythrocytes: Influence of Polymorphism of Glutathione Transferase Theta (GST T1-1). Arch. Toxicol. 1994, 68, 423–427.
- Zhu, R.; Zhang, G.; Jing, M.; Han, Y.; Li, J.; Zhao, J.; Li, Y.; Chen, P.R. Genetically Encoded Formaldehyde Sensors Inspired by a Protein Intra-Helical Crosslinking Reaction. Nat Commun 2021, 12, 581, doi:10.1038/s41467-020-20754-4.Bruhn, C.; Brockmöller, J.; Kerb, R.; Roots, I.; Borchert, H.-H. Concordance between Enzyme Activity and Genotype of Glutathione S-Transferase Theta (GSTT1). Biochem. Pharmacol. 1998, 56, 1189–1193.
