You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Carla Fernandes.
New psychoactive substances represent a public health threat since they are not controlled by international conventions, are easily accessible online and are sold as a legal alternative to illicit drugs. Among them, synthetic cathinones are widely abused due to their stimulant and hallucinogenic effects. To circumvent the law, new derivatives are clandestinely synthesized and, therefore, synthetic cathinones keep emerging on the drug market, with their chemical and toxicological properties still unknown.
- synthetic cathinones
- chirality
- enantioselectivity
- enantiomeric resolution
1. Introduction
The use of new psychoactive substances (NPS) has been growing since 2000 [1]. These substances started to replace illicit drugs as legal alternatives being known as “legal highs”, “smart drugs” or “research chemicals” [2,3][2][3]. They can be sold, as bath salts, plant fertilizers or air fresheners. Although these products are frequently labeled as “not for human consumption”, they are mostly purchased with that purpose. Therefore, NPS are defined as new narcotics or psychotropic substances, in pure form or in mixture preparations, that are not controlled by international conventions but can represent a public health concern [3,4][3][4].
Between 1997 and 2020, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) was monitoring more than 820 NPS. Despite this number, it is possible to observe that, since 2015, the number of NPS notified for the first time has been decreasing (Figure 1). In 2019, 53 NPS were reported for the first time and in 2020, until October, the number was 38 [5].
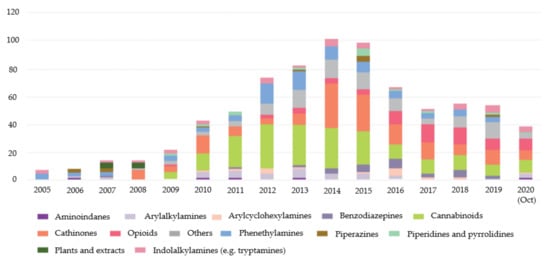
Figure 1.
New psychoactive substances reported for the first time from 2005 to 2020 (October) divided by categories [5].
The popularity of these drugs had a sharp increase due to their easy accessibility online. Human and animal studies involving these NPS are very limited and non-existent for some of them. Therefore, available information about the pharmacological and toxicological properties of these substances is still very limited. The actual composition of NPS sold online can be very different from the package label and, therefore, consumers might purchase and use them mistakenly. All these factors explain why the world of new psychoactive substances represents a huge danger for public health [3,4][3][4].
The two groups of NPS reported on a larger scale are synthetic cannabinoids and synthetic cathinones, representing more than two-thirds of all available compounds since 2005 [5,6][5][6]. The present paper will focus on synthetic cathinones, which comprise a vast group of compounds derived from cathinone (1), an alkaloid found in khat (Catha edulis) leaves, structurally identical and similar in action to amphetamine (2) (Figure 2) [7].
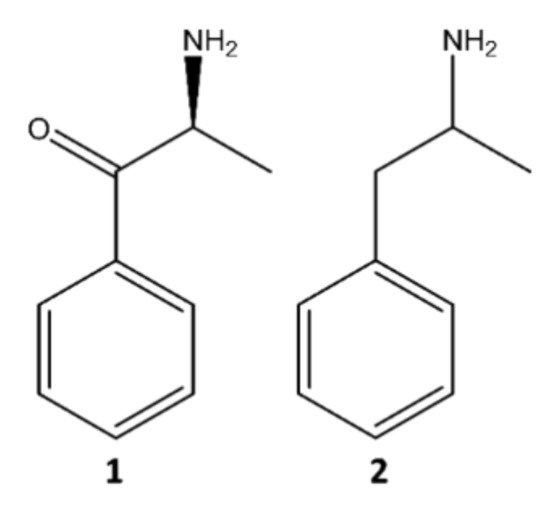
Figure 2.
Structures of cathinone (
1
) and amphetamine (
2
).
Moreover, chewing of fresh khat leaves has been a tradition for centuries in some cultures. The leaves contain more than 40 components such as alkaloids, flavonoids, amino acids, glycosides, sterols, vitamins and minerals (Figure 3) [8]. In 1930, cathine or (+)-norpseudoephedrine (3) was identified as the active principle of khat. However, the activity of cathine (3) was insufficient to be responsible for all the pharmacological effects observed. Later on, in 1975, cathinone was isolated and found to be seven to ten times more bioactive than cathine (3) [6].
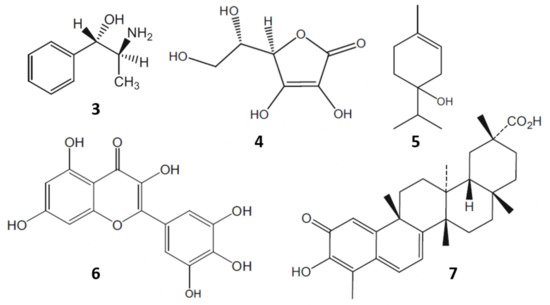
Figure 3.
Some bioactive components of khat: cathine (
3
); ascorbic acid (
4
); α-terpineol (
5
); myricetin (
6
); and celastrol (
7
).
Although both cathine and cathinone are internationally controlled, the World Health Organization considered that the evidence was insufficient to justify the international control of khat. Nonetheless, they advised the development of campaigns to educate the public about the potential adverse effects of the excessive use of khat [9]. Khat has been used as starting material to synthesize derivatives resulting in the first synthetic cathinones that, in the 2000s, emerged in the drug market [6]. Synthetic cathinones gradually became available in smartshops, internet and other drug paraphernalia stores [10]. They were commonly found as “bath salts” under names like Bloom, Ivory Wave, Vanilla Sky, Blue Silk, or Purple Wave [7]. By 2013, approximately 600 “dark web” sites were identified in Europe that allowed the purchase of these compounds anonymously using untraceable digital currencies [11].
Synthetic cathinones are widely abused due to their stimulant and hallucinogenic effects, replacing 3,4-methylenedioxymethamphetamine (MDMA), cocaine and amphetamines, which are much more expensive. However, synthetic cathinones can be much more potent than the drugs that they are intended to mimic, increasing the risk of overdose and death [12].
2. Classification of Synthetic Cathinones
Synthetic cathinones are β-keto phenethylamine derivatives presenting the same core structure. Moreover, they are structurally similar to amphetamine, with the difference being the presence of a keto group [14,16][13][14]. Cathinone derivatives can be synthesized by the addition of several substituents at different sites of the cathinone scaffold as represented in Figure 4 [17][15].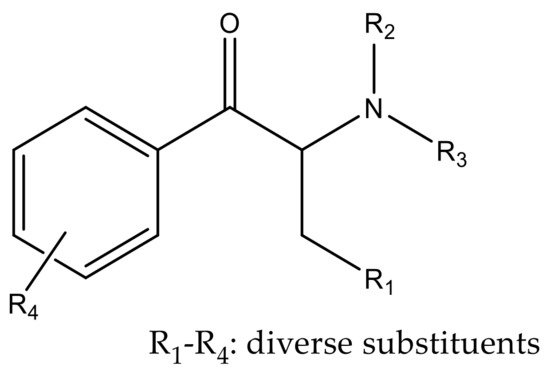
Figure 4.
Core structure of cathinone derivatives.
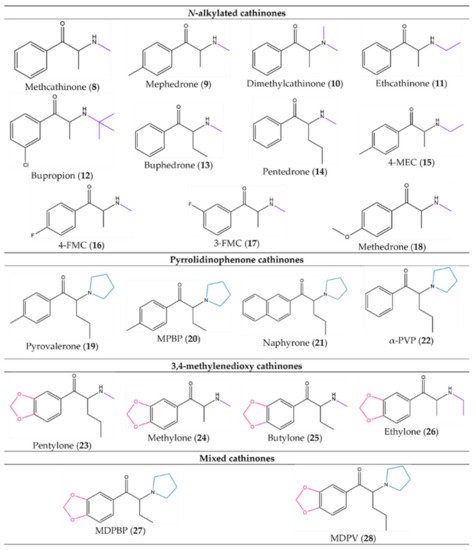
Figure 5. Examples of synthetic cathinones from each group based on the substitution pattern: (8)–(18) from the N-alkylated cathinones, (19)–(22) from the pyrrolidinophenone cathinones, (23)–(26) from the 3,4-methylenedioxy cathinones and (27) and (28) from the mixed cathinones.
3. Chronological Evolution and Recent Developments of Cathinone Derivatives
In Figure 6, some of the most important landmarks of the history of synthetic cathinones are summarized.
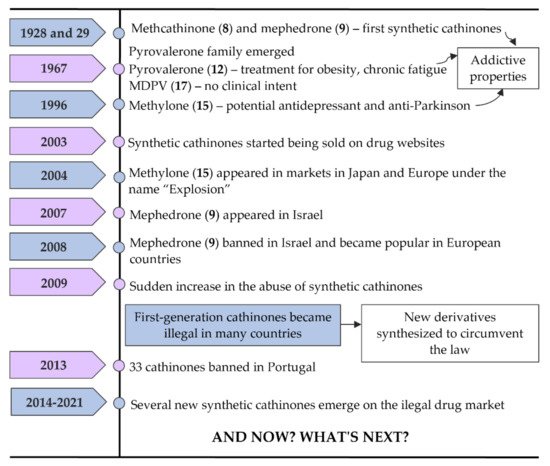

Figure 6.
Timeline of events related to the history of synthetic cathinones.
Methcathinone (α-methylamino-propiophenone or ephedrone (EPH), (8)) and mephedrone (4-methylmethcathinone or MEPH, (9)) (Figure 5) were the first synthetic cathinones, arising in 1928 and 1929, respectively [2]. Methcathinone (8) was meant to reach the market as an antidepressant, but latter it was found to have powerful addictive properties [6,18][6][16]. As a consequence, this synthetic derivative was responsible for several intoxications in the Soviet Union in the 70s and in the USA in the 90s being known in the streets as “Cat”, “Jeff” and “Mulka” [13][17].
The pyrrolidinophenone family comprises a range of compounds that began to be reported at the end of the 60s. Pyrovalerone (19, Figure 5) is a member of this family and was firstly synthesized as a treatment for obesity, chronic fatigue and lethargy but, due to its addictive potential, the clinical use was stopped after these reports of abuse [6]. However, other derivatives of this family, such as 3,4-methylenedioxypyrovalerone (MDPV (28), Figure 5) in 1967, were synthetized with no clinical intent [2,6][2][6].
In 1996, methylone (3,4-methylenedioxy-N-methylcathinone or ßk-MDMA, (24) appeared as a potential antidepressant and anti-Parkinson agent but this compound never reached the market due to their psychostimulant properties identical to MDMA [6,19][6][18].
From the few cathinones synthetized with a medicinal intent, only bupropion (12) succeeded for that purpose, being currently used as an antidepressant and a support to smoking cessation [13,20][17][19].
Synthetic cathinones had barely any attention until 2003 when they were first reported online on drug websites as a legal replacement to MDMA [7].
Around 2004, methylone (24) started to appear in markets in Japan and Europe under the name “Explosion”, also being the first of these substances to be sold via smartshops and online [13][17]. In 2007, mephedrone (9) made its appearance on the market, first in Israel, although it was banned in this country in 2008. After this, mephedrone (9), also known in the markets as “Meph”, “TopCat”, “Mcat”, “Meow Meow”, among other names, became more popular in European countries [6,13][6][17]. Many drug users began to replace cocaine and ecstasy with mephedrone (9) due to a decrease in the purity and availability of the first two drugs. Additionally, mephedrone (9) was less expensive and more potent. This explains why, in 2009, there was a sudden increase in the abuse of synthetic cathinones, especially mephedrone (9) [13][17].
Later, this first-generation of cathinones became illegal in many countries. To overcome this, clandestine chemists started to modify their structures to obtain new derivatives that could circumvent the law. Thus, several new cathinones were synthetized such as buphedrone (13), butylone (25), ethylone (26), pentedrone (14) and its constitutional isomer 4-methyl-N-ethylcathinone (4-MEC, (15)). Additionally, flephedrone (4-fluoromethcathinone or 4-FMC (16)) and 3-fluoromethcathinone (3-FMC (17)), two derivatives of mephedrone (9) were also reported [14][13].
Naphyrone (naphthylpyrovalerone (21)), which appeared after mephedrone (9) was marketed in the UK under the name “Energy-1” (NRG-1) as a legal alternative [13][17]. Simultaneously with pentedrone (14), a derivative from the same group, α-pyrrolidinopentiophenone (α-PVP, (22)) also emerged [14][13]. The popularity of this compound increased greatly in Europe and the USA between 2011 and 2015, being found in the markets as “Flakka” or “Gravel”. After several fatal or almost-fatal cases of abuse, α-PVP (22) began to be controlled internationally [13][17]. Moreover, in Portugal, in April of 2013, because of new legislative control measures, the commercialization and use of 33 cathinone derivatives were prohibited and all smartshops were closed [6,21][6][20].
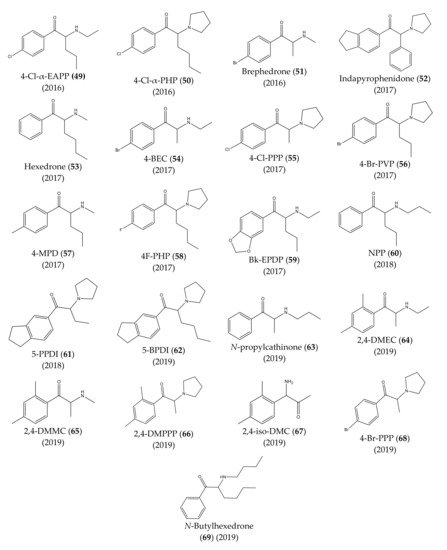
The chemical structures of the first cathinones are being constantly modified. Each year several new derivatives emerge on the illegal drug market. The chemical structures of synthetic cathinones (29–69) clandestinely synthetized and reported since 2014 are represented in Figure 7 and Figure 8.
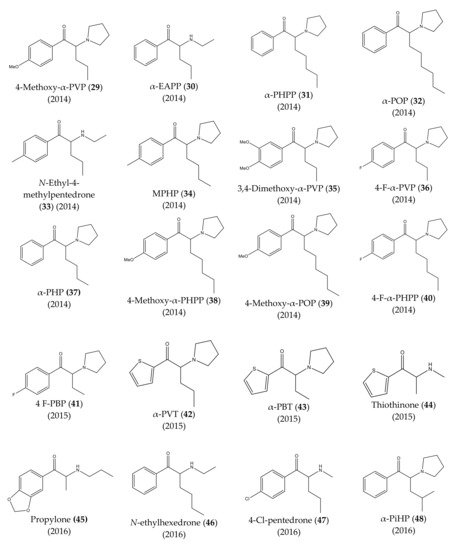

Figure 7.
Structures of the most recent cathinone derivatives (
29
)–(
48
).

Figure 8.
Structures of the most recent cathinone derivatives (
49
)–(
69
).
In 2014, several new synthetic cathinones were found in the Japanese market, being sold as “aroma liquids” and “fragrance powders”. They included 4-methoxy-α-PVP (29), α-EAPP (30), α-PHPP or PV8 (31), α-POP or PV9 (32), N-ethyl-4-methylpentedrone (33), MPHP (34), 3,4-dimethoxy-α-PVP (35), 4-F-α-PVP (36). Almost half a year later, α-PHP (37), 4-methoxy-α-PHPP (38), 4-methoxy-α-POP (39), and 4-F-α-PHPP (40) were also discovered [13,14,22,23,24][13][17][21][22][23].
Moreover, in Portugal, 4-F-PBP (41) was reported for the first time in 2015 [13,25][17][24]. In the same year, the first thienyl cathinone derivatives, α-PVT (42), α-PBT (43) and bromothienyl analogs were identified [14,26][13][25]. The thiothinone (44), another thienyl cathinone derivative was also discovered, at the same time [14,27][13][26].
In 2016, propylone (45), N-ethylhexedrone (46), 4-chloro-pentedrone (47), α-PiHP (48), 4-Cl-α-EAPP (49) and 4-Cl-α-PHP (50) were identified for the first time [13,28][17][27]. In the same year, brephedrone (51) was identified in seized samples from Brazilian streets. However, this synthetic cathinone had already been reported in other countries [29][28].
In 2017, an unknown compound found in seized drugs in the UK was identified and characterized as indapyrophenidone (52), a novel cathinone derivative [30][29]. Additionally, hexedrone (53), 4-BEC (54), 4-Cl-PPP (55) and 4-Br-PVP (56) were first reported in Poland [13,31][17][30]. Furthermore, in the same year, three emerging cathinone derivatives: 4-MPD (57), 4F-PHP (58) and bk-EPDP (59) were detected, identified, and fully characterized [32][31].
In 2018, 5-PPDI (61), a novel synthetic cathinone, was identified and characterized in an unknown white powder [33][32].
One year later, seven other new synthetic cathinones were reported in Poland: 5-BPDI (62), N-propylcathinone (63), 2,4-DMEC (64), 2,4-DMMC (65), 2,4-DMPPP (66), 2,4-iso-DMC (67) and 4-Br-PPP (68) [34][33]. Additionally, in the same year, N-butylhexedrone (69) was identified in seized material [35][34], which was, later on, characterized by spectroscopic and crystallographic analysis [36][35].
Besides that, some studies have described novel synthetic cathinones (70–80) that were synthesized in controlled laboratories (instead of found in the drug market) with the purpose of studying potential effects and to develop analytical techniques for the identification and characterization of future cathinones (Figure 9).
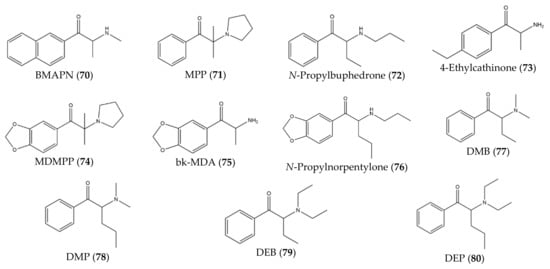

Figure 9.
Structures of cathinone derivatives synthesized in controlled laboratories (
70
)–(
80
).
For instance, Botanas et al. [37][36] (2017) synthesized a novel synthetic cathinone, BMAPN (70), with the purpose of studying its rewarding and reinforcing properties. Since this compound presents a naphthalene substituent on the aromatic ring, this study can be helpful to predict the abuse potential of future cathinones with aromatic ring substitutions [37][36]. Moreover, Carlsson et al. [38][37] (2018) synthesized six novel synthetic cathinones: MPP, 71), N-propylbuphedrone (72), 4-ethylcathinone (73), MDMPP (74), bk-MDA (75), N-propylnorpentylone (76). This study described the synthesis of these analogs and provided spectroscopic data [38][37]. With the same purpose, Gaspar et al. [39][38] synthesized four novel synthetic cathinones: DMB (77), DMP (78), DEB (79) and DEP (80).
Some of the new cathinone derivatives have been described in cases of abuse. For instance, Hasegawa et al. [40][39] (2014) reported a fatal poisoning case of a woman after oral ingestion of an ‘‘aroma liquid’’-type drug bought in a drug shop. This study identified and quantified PV9 (32) in the ‘‘aroma liquid’’ product as well as in antemortem and postmortem samples [40][39].
Majchrzak et al. [41][40] (2018) reported the first case of fatal poisoning with N-PP (60), a novel synthetic cathinone. This compound was identified in a white powder found at the scene and high concentrations were found in postmortem specimens collected from the autopsy [41][40]. Moreover, Pieprzyca et al. [42][41] (2018) reported two fatal poisoning cases in which PV8 (31) was detected and quantified in biological samples and found to be the cause of the deaths.
Adamowicz et al. [43][42] (2020) reported a fatal intoxication with α-PiHP (48). This substance was detected and quantified in all postmortem samples except in hair and was reported as the main cause of death, although 4-CMC, N-ethylhexedrone, benzoylecgonine and MDMA were also detected in some analyzed materials [43][42].
Currently, hundreds of synthetic cathinones have been identified and up to 250 new cathinone-related chemical entities are estimated to emerge every year [44][43]. The identification of these compounds and the implementation of a drug library with their structures and physicochemical and pharmacological properties are of great importance for chemists and toxicologists [14][13].
References
- UNODC. World Drugs Report. 2014. Available online: https://www.unodc.org/documents/data-and-analysis/WDR2014/World_Drug_Report_2014_web.pdf (accessed on 18 March 2021).
- Zanda, M.T.; Fattore, L. Chapter 29—Novel Psychoactive Substances: A New Behavioral and Mental Health Threat. In Addictive Substances and Neurological Disease; Watson, R.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 341–353.
- Coppola, M.; Mondola, R.; Oliva, F.; Picci, R.L.; Ascheri, D.; Trivelli, F. Chapter 63—Treating the Phenomenon of New Psychoactive Substances: Synthetic Cannabinoids and Synthetic Cathinones. In Neuropathology of Drug Addictions and Substance Misuse; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 679–686.
- Zawilska, J.B. Chapter Thirteen—“Legal Highs”—An Emerging Epidemic of Novel Psychoactive Substances. In International Review of Neurobiology; Taba, P., Lees, A., Sikk, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 273–300.
- EMCDDA. New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic-An Update from the EU Early Warning System. 2020. Available online: https://www.emcdda.europa.eu/publications/rapid-communication/new-psychoactive-substances-global-markets-glocal-threats-and-covid-19-pandemic_en (accessed on 18 March 2021).
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45.
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. Life Sci. 2014, 97, 2–8.
- Getasetegn, M. Chemical composition of Catha edulis (khat): A review. Phytochem. Rev. 2016, 15, 907–920.
- World Health Organization. WHO Expert Committee on Drug Dependence: Thirty-Fourth Report; Technical Report Series No. 942; World Health Organization: Geneva, Switzerland, 2006.
- Bretteville-Jensen, A.L.; Tuv, S.S.; Bilgrei, O.R.; Fjeld, B.; Bachs, L. Synthetic cannabinoids and cathinones: Prevalence and markets. Forensic Sci. Rev. 2013, 25, 7–26.
- EMCDDA. European Drug Report 2015: Trends and Developments; Publications Office of the European Union: Luxembourg, 2015; p. 86.
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Schindler, C.W. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacol 2013, 38, 552–562.
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 36, 33–50.
- Zuba, D.; Byrska, B. Prevalence and co-existence of active components of ‘legal highs’. Drug Test. Anal. 2013, 5, 420–429.
- Paillet-Loilier, M.; Cesbron, A.; Boisselier, R.; Bourgine, J.; Debruyne, D. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abus. Rehabil. 2014, 5, 37–52.
- Young, R.; Glennon, R.A. Cocaine-stimulus generalization to two new designer drugs: Methcathinone and 4-methylaminorex. Pharmacol. Biochem. Behav. 1993, 45, 229–231.
- Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. Synthetic cathinones: An evolving class of new psychoactive substances. Crit. Rev. Toxicol. 2019, 49, 549–566.
- Dal Cason, T.A.; Young, R.; Glennon, R.A. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 1997, 58, 1109–1116.
- Dasgupta, A. 3-Designer drugs including bath salts and spices. In Alcohol, Drugs, Genes and the Clinical Laboratory; Dasgupta, A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 53–73.
- Government Decreto-Lei n.º 54/2013 Diário da República nº75/2013. 2013, pp. 2250–2254. Available online: https://dre.pt/dre/detalhe/decreto-lei/54-2013-260418 (accessed on 18 March 2021).
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Identification of two new-type designer drugs, piperazine derivative MT-45 (I-C6) and synthetic peptide Noopept (GVS-111), with synthetic cannabinoid A-834735, cathinone derivative 4-methoxy-α-PVP, and phenethylamine derivative 4-methylbuphedrine from illegal products. Forensic Toxicol. 2014, 32, 9–18.
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Shimokawa, Y.; Kikura-Hanajiri, R.; Aritake, K.; Goda, Y. Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci. Int. 2014, 243, 1–13.
- Uchiyama, N.; Shimokawa, Y.; Kawamura, M.; Kikura-Hanajiri, R.; Hakamatsuka, T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014, 32, 266–281.
- Gaspar, H.; Bronze, S.; Ciríaco, S.; Queirós, C.R.; Matias, A.; Rodrigues, J.; Santos, S. 4F-PBP (4′-fluoro-α-pyrrolidinobutyrophenone), a new substance of abuse: Structural characterization and purity NMR profiling. Forensic Sci. Int. 2015, 252, 168–176.
- Doi, T.; Asada, A.; Takeda, A.; Tagami, T.; Katagi, M.; Matsuta, S.; Kamata, H.; Obana, H. Identification and characterization of α-PVT, α-PBT, and their bromothienyl analogs found in illicit drug products. Forensic Toxicol. 2016, 34, 76–93.
- Gambaro, V.; Casagni, E.; Dell’Acqua, L.; Roda, G.; Tamborini, L.; Visconti, G.L.; Demartin, F. Identification and characterization of a new designer drug thiothinone in seized products. Forensic Toxicol. 2016, 34, 174–178.
- Liu, C.; Jia, W.; Li, T.; Hua, Z.; Qian, Z. Identification and analytical characterization of nine synthetic cathinone derivatives N-ethylhexedrone, 4-Cl-pentedrone, 4-Cl-α-EAPP, propylone, N-ethylnorpentylone, 6-MeO-bk-MDMA, α-PiHP, 4-Cl-α-PHP, and 4-F-α-PHP. Drug Test. Anal. 2017, 9, 1162–1171.
- Machado, Y.; Coelho Neto, J.; Barbosa, P.E.N.; Lordeiro, R.A.; Alves, R.B. Brephedrone: A new psychoactive substance seized in Brazil. Forensic Sci. Int. 2017, 275, 302–307.
- Bijlsma, L.; Miserez, B.; Ibáñez, M.; Vicent, C.; Guillamón, E.; Hernán, F. Identification and characterization of a novel cathinone derivative 1-(2,3-dihydro-1H-inden-5-yl)-2-phenyl-2-(pyrrolidin-1-yl)-ethanone seized by customs in Jersey. Forensic Toxicol. 2016, 34, 144–150.
- Błażewicz, A.; Bednarek, E.; Sitkowski, J.; Popławska, M.; Stypułkowska, K.; Bocian, W.; Kozerski, L. Identification and structural characterization of four novel synthetic cathinones: α-methylaminohexanophenone (hexedrone, HEX), 4-bromoethcathinone (4-BEC), 4-chloro-α-pyrrolidinopropiophenone (4-Cl-PPP), and 4-bromo-α-pyrrolidinopentiophenone (4-Br-PVP) after their seizures. Forensic Toxicol. 2017, 35, 317–332.
- Apirakkan, O.; Frinculescu, A.; Shine, T.; Parkin, M.C.; Cilibrizzi, A.; Frascione, N.; Abbate, V. Analytical characterization of three cathinone derivatives, 4-MPD, 4F-PHP and bk-EPDP, purchased as bulk powder from online vendors. Drug Test. Anal. 2018, 10, 372–378.
- Fabregat-Safont, D.; Carbón, X.; Gil, C.; Ventura, M.; Sancho, J.V.; Hernández, F.; Ibáñez, M. Reporting the novel synthetic cathinone 5-PPDI through its analytical characterization by mass spectrometry and nuclear magnetic resonance. Forensic Toxicol. 2018, 36, 447–457.
- Blazewicz, A.; Bednarek, E.; Poplawska, M.; Olech, N.; Sitkowski, J.; Kozerski, L. Identification and structural characterization of synthetic cathinones: N-propylcathinone, 2,4-dimethylmethcathinone, 2,4-dimethylethcathinone, 2,4-dimethyl-α-pyrrolidinopropiophenone, 4-bromo-α-pyrrolidinopropiophenone, 1-(2,3-dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)hexan-1-one and 2,4-dimethylisocathinone. Forensic Toxicol. 2019, 37, 288–307.
- Shevyrin, V.; Eltsov, O.; Shafran, Y. Identification and analytical characterization of the synthetic cathinone N-butylhexedrone. Drug Test. Anal. 2020, 12, 159–163.
- Rojkiewicz, M.; Kuś, P.; Kusz, J.; Książek, M.; Sochanik, A. Spectroscopic and crystallographic characterization of a new cathinone derivative: 1-phenyl-2-(butylamino)hexan-1-one hydrochloride (N-butylhexedrone). Forensic Toxicol. 2020, 38, 481–489.
- Botanas, C.J.; Yoon, S.S.; de la Peña, J.B.; de la Peña, I.J.; Kim, M.; Woo, T.; Cheong, J.H. A novel synthetic cathinone, 2-(methylamino)-1-(naphthalen-2-yl) propan-1-one (BMAPN), produced rewarding effects and altered striatal dopamine-related gene expression in mice. Behav. Brain Res. 2017, 317, 494–501.
- Carlsson, A.; Sandgren, V.; Svensson, S.; Konradsson, P.; Dunne, S.; Josefsson, M.; Dahlén, J. Prediction of designer drugs: Synthesis and spectroscopic analysis of synthetic cathinone analogs that may appear on the Swedish drug market. Drug Test. Anal. 2018, 10, 1076–1098.
- Gaspar, H.; Bronze, S.; Oliveira, C.; Victor, B.; Machuqueiro, M.; Pacheco, R.; Caldeira, M.J.; Santos, S. Proactive response to tackle the threat of emerging drugs: Synthesis and toxicity evaluation of new cathinones. Forensic Sci. Int. 2018, 290, 146–156.
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I. Identification and quantitation of a new cathinone designer drug PV9 in an “aroma liquid” product, antemortem whole blood and urine specimens, and a postmortem whole blood specimen in a fatal poisoning case. Forensic Toxicol. 2014, 32, 243–250.
- Majchrzak, M.; Celiński, R.; Kowalska, T.; Sajewicz, M. Fatal case of poisoning with a new cathinone derivative: α-propylaminopentiophenone (N-PP). Forensic Toxicol. 2018, 36, 525–533.
- Pieprzyca, E.; Skowronek, R.; Korczyńska, M.; Kulikowska, J.; Chowaniec, M. A two fatal cases of poisoning involving new cathinone derivative PV8. Leg. Med. 2018, 33, 42–47.
- Adamowicz, P.; Jurczyk, A.; Gil, D.; Szustowski, S. A case of intoxication with a new cathinone derivative α-PiHP–A presentation of concentrations in biological specimens. Leg. Med. 2020, 42, 101626.
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156.
More
