Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Jian Yang.
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorders, which is caused by multi-factors and characterized by two histopathological hallmarks: amyloid-β (Aβ) plaques and neurofibrillary tangles of Tau proteins. Thus, researchers have been devoting tremendous efforts to developing and designing new molecules for the early diagnosis of AD and curative purposes. Curcumin and its scaffold have fluorescent and photochemical properties. Mounting evidence showed that curcumin scaffold had neuroprotective effects on AD such as anti-amyloidogenic, anti-inflammatory, anti-oxidative and metal chelating.
- Alzheimer’s disease
- amyloid-β
- tau protein
- curcumin scaffold
- AD diagnosis
1. Introduction
Alzheimer’s disease (AD) is a multi-faceted neurodegenerative disease in the elderly population with complicated pathogenesis. Generally speaking, there are two remarkable neuro-histological hallmarks in postmortem AD patient brain tissues: extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles of hyperphosphorylated tau protein [6][1]. It has been proposed that the abnormality of Aβ appears far earlier than tau abnormality, and the Aβ abnormality can induce hyperphosphorylation of tau protein and neuroinflammation [7,8,9][2][3][4]. Therefore, the amyloid cascade theory is considered to be related to the pathology of AD. Based on the research guidelines published by NIA-AA in 2018, Aβ abnormality has been considered to be an early biomarker for diagnosis in the early stages of AD.
According to amyloid cascade theory, Aβ is produced by cleavages of amyloid-β precursor protein (APP) by β- and γ-secretase and is precluded by α-secretase activity. While Aβ42 and Aβ40 are the two major Aβ species, Aβ42 is more aggregation-prone and readily forms neurotoxic oligomers and is more prevalent than Aβ40 in plaques [10,11][5][6]. Normally, the N-terminal segment has the capacity to bind metal ions, such as Fe2+, Cu2+ and Zn2+, which is simultaneously accompanied by the production of reactive oxygen species (ROS) [12][7]. During the Aβ aggregation, there are several Aβ sub-species including monomers, dimers, oligomers, and fibrils/aggregates. During the aggregation process, all the Aβ sub-species involved in the process are equilibrated [13][8]. In this equilibration process, once Aβ monomers reach a critical high concentration, over-accumulation of Aβ begins. Aβ monomers can quickly gather into large molecular weight polymeric precipitation, which turns into oligomers and eventually fibrils/aggregates [14,15,16,17,18][9][10][11][12][13] that will produce toxic effects on the surrounding neurons and synapses, and cause synaptic membrane damage, resulting in neuronal cell death [14,15,19,20,21][9][10][14][15][16]. The amyloid cascade hypothesis is shown in Figure 1.
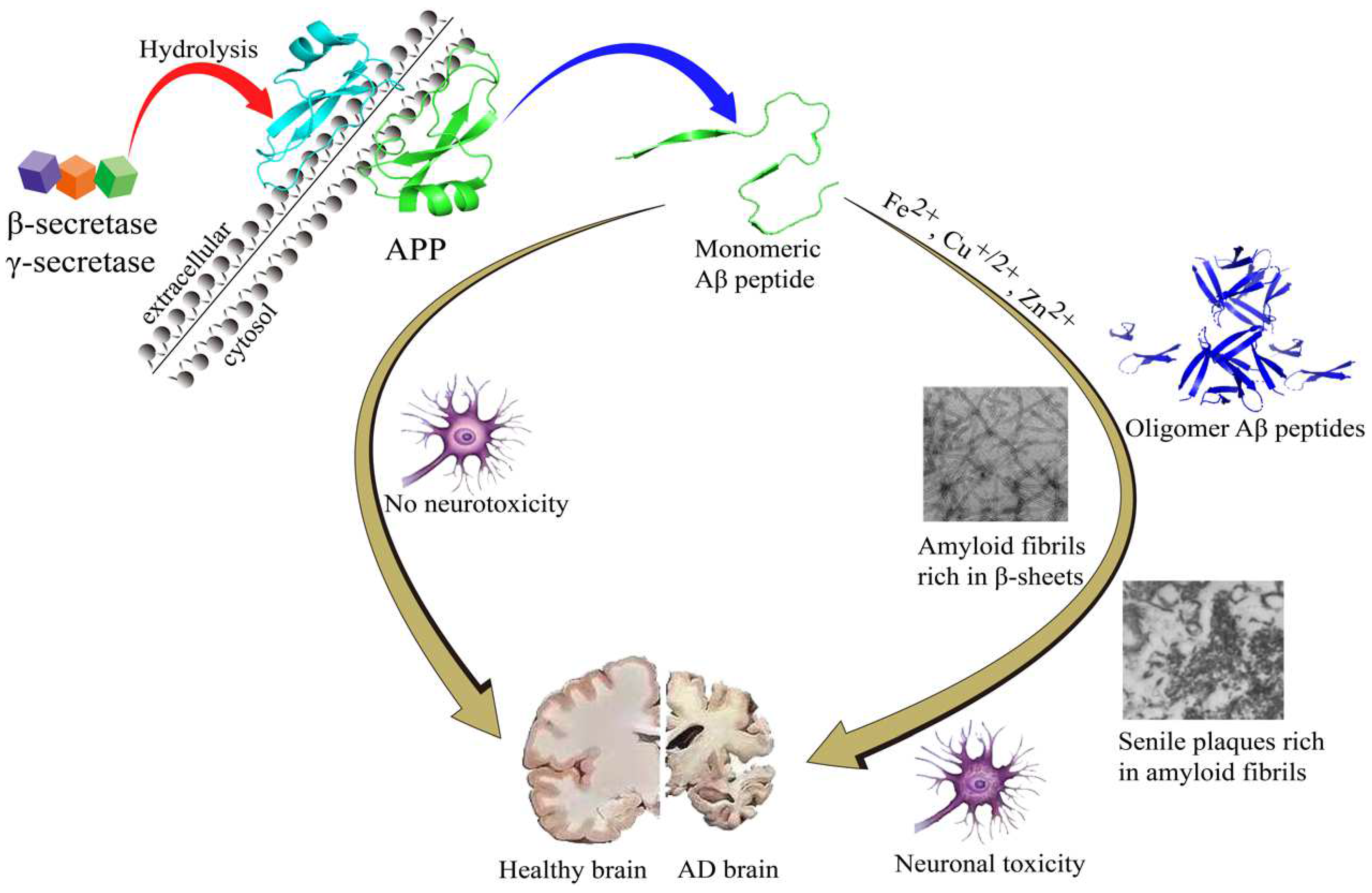
Figure 1. The amyloid cascade hypothesis.
Tau protein can stabilize the internal skeleton of nerve cells in the brain. Studies show that the total amount of tau proteins in AD brains is increased significantly, and the increased tau proteins are usually in the form of excessive abnormal phosphorylation, which means that if the tau protein is abnormally phosphorylated, it is easy to form paired helical filaments (PHF), which can lead to neurofibrillary tangles (NFTs). NFTs are highly related to the occurrence of AD [22,23,24][17][18][19]. Clearly, developing new imaging probes that target NFTs is becoming of great significance for the accurate diagnosis of AD.
In recent years, neuroinflammation is considered a highly relevant biomarker in AD, which is closely associated with oxidative stress (OS). In fact, oxidative stress always plays an important role in AD pathogenesis [25,26][20][21]. Mounting research evidence revealed that the concentration of reactive oxygen species (ROS) in AD brains is much higher than in that of healthy brains [27,28,29][22][23][24]. ROS, including superoxide radical, hydroxyl radical and hydrogen peroxide, always contribute to the pathophysiology of neurodegenerative disorders. Although ROS can modulate cell survival, once the balance of ROS was broken, it will be accompanied by oxidative damage. Multiple sources contributed to ROS production, such as mitochondria dysfunction, reactions involving peroxisomal oxidases, NAD(P)H oxidases, cytochrome P-450 enzymes, over-accumulation of metal ions and aggregation of Aβs or Tau tangles [27,30,31,32][22][25][26][27]. Recently, the relationship between OS and AD has been revealed, and the research shift is from fundamental research to clinical treatment, such as the use of antioxidants (vitamin E/C, curcumin, GSH) [33,34][28][29] and chelating agents.
It is well known that many countries have the habit of eating curry, especially in India, and curry contains abundant curcumin which has preventive and therapeutic effects on AD. That may be one reason for the low incidence of dementia in India. This phenomenon was drawing researchers’ attention in the AD field. Curcumin is a natural product of polyphenols with a diketone structure, the trans double bond and the keto-enol tautomerism make it have a long-conjugated carbon chain, which can result in several different fluorescent properties and functions. Remarkably, the neuroprotective effects of curcumin scaffold on AD have been extensively studied, such as the inhibition of Aβ accumulation, prevention of tau hyperphosphorylation and aggregation, as well as having anti-inflammatory, antioxidant, and metal ions-complexing properties [35][30].
2. Curcumin Scaffold as a Tool for Understanding AD
Aβ plaques represent a classical pathology which is found in the AD brain. Aβ peptide usually undergoes conformation changes from monomer to oligomers and fibers that are rich in β-sheets [36][31]. Martin et al. analyzed the dynamics and energetics between curcumin and 24-peptide in Aβ fibrils using molecular dynamics simulations. The results show that particular hydrophobic sites existed on the protofibril surface that can bind with curcumin, and this binding is usually associated with the complexation of curcumin with dimers, trimers, or oligomers, even at the end of the fibrillary growth axis. The hydrophobic interaction and solvation effects may contribute to the good binding between curcumin and protofibril. These interactions may be the key to reducing the toxicity of Aβ oligomers and changing the Aβ accumulation pathway to form non-toxic aggregates [37][32]. Zhao et al. demonstrated that curcumin is also a potential Aβ-toxic inhibitor because it has a high tendency to interact with certain Aβ residues which were highly likely to form hydrogen bonds with curcumin. Additionally, curcumin was observed to act as a β-sheet breaker when it was inserted into Aβ protein. The π-π stacking interactions frequently existed between the aromatic ring of curcumin and proteins such as histidine, tyrosine, and phenylalanine [38][33]. Although the interactions are temporary, they indirectly contribute to decreasing β-sheet content [39][34]. Gestwicki et al. found that hydroxyl substitution in aromatic rings on both sides of curcumin was essential for inhibiting Aβ by structure–activity relationship, and the length and flexibility of the linker between aromatic rings should be kept within an appropriate range [40][35]. Curcumin has ketone-enol tautomerism and exists in the form of enol in solution. Studies have shown that the form of enol exhibits anti-Aβ aggregation properties, not the β-dione form [41][36].
Besides inhibiting Aβ aggregation, some researches showed that curcumin has the capability to inhibit hyperphosphorylated tau protein aggregation. Molecular docking studies predicted that curcumin had a possible binding site in the tau microtubule region. A strong hydrophilic interaction between curcumin and tau D225 residues was revealed by Panda. Furthermore, the electrostatic interaction might contribute to blocking the tau-tau interaction, which was considered to be the most crucial factor in the process of tau aggregation [42][37].
Recently, oxidative stress is deemed to be another risk factor in AD [28][23]. Reactive oxygen spices (ROS) can interact with lipids, proteins, and nucleic acids, which can cause irreversible damage to neurons in the brain [43][38]. The diketone and phenolic groups in the curcumin structure can prevent the production of a variety of reactive oxygen species. In addition, the β-dione group and the hydroxyl group can complex with metal ions, such as copper ions, ferrous ions, and zinc ions [44][39]. Whereas ions are necessary for the production of hydroxyl radicals, so curcumin exerts antioxidant activity directly or indirectly.
It is well known that curcumin has fluorescence properties, which is attributed to the unique structure of the diphenylenone, and the isomerization transition between the ketone form and the enol form also gives curcumin many unique photochemical properties. Therefore, curcumin can be used as sensitive material for the detection of chemical substances. Curcumin can form complexes with Cu2+, Fe2+, Zn2+ and other cations through the structure of 1,3-diketone that can carry out keto-enol isomerization. This complexation could lead to the increase of solubility in water and the change of color [45][40]. Importantly, curcumin can cross the blood-brain barrier, so it might be used as a candidate in brain studies. In addition, curcumin can specifically bind to Aβ aggregates and abnormal tau proteins, which has been reported for staining experiments, suggesting that curcumin can bind to specific groups in proteins and produce changes in optical properties [46][41]. Therefore, curcumin has the potential to be developed as an imaging probe candidate for AD diagnosis.
Due to a variety of effects on AD, curcumin and its analogues have the potential to be a drug for AD therapy and can act as a chemical fluorescence probe for AD with great research value.
3. Curcumin Scaffold as a Tool for AD Therapy
Recent studies have revealed the close connection between curcumin scaffold and AD. Research uncovered that curcumin can prevent Aβ aggregation and cross the blood-brain barrier (BBB), reach brain cells, repair the nerve and participate in the treatment of AD, such as ameliorating cognitive decline and improving synaptic functions in animal models [75][42].3.1. Aβ Inhibition
In the last decade, curcumin has been considered an efficient inhibitor for β-amyloid (Aβ) aggregation. Mounting evidence has revealed that curcumin has a variety of anti-amyloid properties. It has been noted that curcumin and its derivatives are reported to have some potential binding sites for Aβ protein, to block its assembly. Some in vitro studies showed that curcumin has the capability of inhibiting the fibrillar Aβ formation from Aβ monomer and also destabilizing preformed fibrillar Aβ [47][43]. Meanwhile, several in vivo studies showed that curcumin could not only promote disaggregation of existing amyloid deposits but also prevent the formation of new amyloid deposits, and even reduce the size of remaining deposits [76,77][44][45]. Curcumin could also bind to Aβ oligomers and block its accumulation to lower the toxicity [78,79][46][47]. Curcumin was also found to decrease Aβ peptides levels in in vitro studies by inhibiting the expression of presenilin 1, which has a close relationship to γ secretase and leads to the generation of Aβ [80][48]. In 2020, Huang reported curcumin could inhibit BACE1 (β-amyloid precursor protein Cleaving Enzyme) gene expression in SH-SY5Y cells at transcriptional and translational levels. Furthermore, this result revealed that the signaling in nuclear factor kappa B is involved in the regulation between curcumin and BACE1 [81][49].3.2. Tau Inhibition
The formation of the neurofibrillary tangles with tau hyperphosphorylation plays an important role in the pathogenesis of AD [82,83][50][51]. The prevention of hyperphosphorylation of tau has been the most significant hotspot of AD research [84][52]. Several studies showed that curcumin can prevent tau hyperphosphorylation and decrease neurotoxicity [85][53]. Curcumin could inhibit α-synuclein accumulation and increase the protein solubility, which could lead to decreasing levels of soluble tau dimers and elevated heat shock proteins contained in the process of tau clearance in AD mice [86][54]. HSP 70 and HSP 90, two heat shock proteins, closely enhance the solubility of tau protein and promote tau-microtubule interaction [87][55]. In 2017, Panda reported that curcumin has the capability to bind the adult tau and the fetal tau. The dissociation constants were 3.3 ± 0.4 µM and 8 ± 1µM, respectively. Molecular docking studies showed that curcumin had a possible binding site in the tau microtubule-binding region, which may lead to inhibiting tau aggregation and disintegrating preformed tau oligomers. More importantly, it revealed that curcumin has the capability to break the formation of β-sheets in protein structure to inhibit aggregation [42][37]. In the same year, Sun reported that curcumin could be utilized as the lead compound to study the correlation between tau protein and Caveolin-1 (a marker protein of membranal caveolae). After being treated with curcumin, the expression of Caveolin-1, tau protein and their relationship were detected, and the potential mechanism was also explored [88][56].3.3. Metal Ion Binding
Metal ion dyshomeostasis has been recognized as a cofactor which might cause cellular death or severe dysfunction in neurodegenerative disorders such as Alzheimer’s disease (AD) [89,90][57][58]. The Aβ misfolding process, which is associated with aggregation, is related to the metal irons (i.e., copper, iron, and zinc) that could be found in both the core and rim of the AD plaques [91,92][59][60]. Studies have shown that the levels of metal ions (iron, zinc, and copper) in the brain of AD patients are 3–7 times higher than those in the normal brain [93][61]. Animal studies have confirmed that curcumin has an anti-inflammatory effect. Curcumin is a metal chelating agent, which can exhibit anti-AD effects. Reducing metal ions which can induce Aβ amyloid fibril formation is a possible mechanism [94,95][62][63]. Recent studies suggest that curcumin has the capability to bind metal irons [96,97][64][65]. The strategy of measuring the affinity between curcumin and different metal ions may verify the likelihood that curcumin could protect against AD through chelation. In 2013, Picciano et al. demonstrated that estimating Cu2+-curcumin binding affinities in the absence and presence of the Aβ peptide could provide evidence for this Cu2+ chelation role [98][66]. Moreover, a curcumin analogue named CRANAD-17 [52][67] exhibited the ability to inhibit Aβ42 cross-linking that was induced by copper. It raised the potential for CRANAD-17 to be considered a hit compound for AD therapy.3.4. ROS Scavenging and Neuroprotective Effects
Mounting evidence has revealed that oxidative stress is a key role in the onset and progression of AD [99][68]. Besides Aβ protein and tau, chronic inflammation is considered another pathological hallmark of AD, which is characterized by the increased presence of activated microglia and astrocytes, accompanied by elevated expression of acute-phase proteins and proinflammatory cytokines [100,101][69][70]. During the aggregation of Aβs, numerous ROS are generated. The generated ROS can further induce the accumulation of inflammatory cytokines such as TNFα, which can affect the microglia to release more ROS [25,30][20][25]. Many studies have shown that curcumin has potential anti-inflammatory effects such as reducing levels of IL1β and oxidized protein, microgliosis in the cortex, Aβ peptides and plaque burden [101,102,103][70][71][72]. Moreover, curcumin also has the capability to protect PC12 cells and normal human umbilical endothelial cells from oxidative stress caused by amyloid-β [104][73]. Curcumin, a PPARγ agonist, could improve mitochondrial function and reduce ROS leakage, which could indirectly reduce oxidative stress. Meanwhile, curcumin also can inhibit LPS-induced microglial activation by inhibiting NO production and reducing the release of pro-inflammatory cytokines such as IL-6, and IL-1β [105][74]. Most importantly, curcumin can attenuate microglial migration and trigger another microglial phenotype with anti-inflammatory and neuroprotective effects [106][75]. Numerous studies have revealed that curcumin exhibits good properties such as scavenging free radicals, reducing iron ions, chelating metal ions, and inhibiting lipid oxidation degradation [107][76]. Therefore, curcumin could be capable of protecting glial cells and reducing ROS.References
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192.
- Nisbet, R.M.; Polanco, J.C.; Ittner, L.M.; Gotz, J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015, 129, 207–220.
- Kumar, D.K.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra372.
- Iqbal, K.; Liu, F.; Gong, C.X. Alzheimer disease therapeutics: Focus on the disease and not just plaques and tangles. Biochem. Pharmacol. 2014, 88, 631–639.
- Sisodia, S.S.; St George-Hyslop, P.H. gamma-Secretase, Notch, Abeta and Alzheimer’s disease: Where do the presenilins fit in? Nat. Rev. Neurosci. 2002, 3, 281–290.
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18.
- Hureau, C. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 1: An overview. Coord. Chem. Rev. 2012, 256, 2164–2174.
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774.
- Feng, H.L.; Dang, H.Z.; Fan, H.; Chen, X.P.; Rao, Y.X.; Ren, Y.; Yang, J.D.; Shi, J.; Wang, P.W.; Tian, J.Z. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int. J. Immunopathol. Pharmacol. 2016, 29, 734–741.
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-beta Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 2018, 64, S567–S610.
- Kung, H.F. The beta-Amyloid Hypothesis in Alzheimer’s Disease: Seeing Is Believing. ACS Med. Chem. Lett. 2012, 3, 265–267.
- Mohamed, T.; Shakeri, A.; Rao, P.P. Amyloid cascade in Alzheimer’s disease: Recent advances in medicinal chemistry. Eur J. Med. Chem. 2016, 113, 258–272.
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-beta oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323.
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608.
- Zhang, H.; Therriault, J.; Kang, M.S.; Ng, K.P.; Pascoal, T.A.; Rosa-Neto, P.; Gauthier, S.; the Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 80.
- Elovsson, G.; Bergkvist, L.; Brorsson, A.C. Exploring Abeta Proteotoxicity and Therapeutic Candidates Using Drosophila melanogaster. Int. J. Mol. Sci. 2021, 22.
- Gray, E.G.; Paula-Barbosa, M.; Roher, A. Alzheimer’s disease: Paired helical filaments and cytomembranes. Neuropathol. Appl. Neurobiol. 1987, 13, 91–110.
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394.
- Zhang, Y.; Wu, K.M.; Yang, L.; Dong, Q.; Yu, J.T. Tauopathies: New perspectives and challenges. Mol. Neurodegener. 2022, 17, 28.
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 2015, 6, 6176.
- Fisher, L. Retraction: Chrysin attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial inflammation. RSC Adv. 2021, 11, 4173.
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121.
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619.
- Pratico, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615.
- Huang, Y.; Erdmann, N.; Peng, H.; Zhao, Y.; Zheng, J. The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell Mol. Immunol. 2005, 2, 113–122.
- Xie, H.; Hou, S.; Jiang, J.; Sekutowicz, M.; Kelly, J.; Bacskai, B.J. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 7904–7909.
- Nakao, N.; Kurokawa, T.; Nonami, T.; Tumurkhuu, G.; Koide, N.; Yokochi, T. Hydrogen peroxide induces the production of tumor necrosis factor-alpha in RAW 264.7 macrophage cells via activation of p 38 and stress-activated protein kinase. Innate Immun. 2008, 14, 190–196.
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610.
- Jahanshahi, M.; Nikmahzar, E.; Sayyahi, A. Vitamin E therapy prevents the accumulation of congophilic amyloid plaques and neurofibrillary tangles in the hippocampus in a rat model of Alzheimer’s disease. Iran. J. Basic Med. Sci. 2020, 23, 86–92.
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1003–1016.
- Walsh, D.M.; Hartley, D.M.; Kusumoto, Y.; Fezoui, Y.; Condron, M.M.; Lomakin, A.; Benedek, G.B.; Selkoe, D.J.; Teplow, D.B. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999, 274, 25945–25952.
- Martin, T.D.; Malagodi, A.J.; Chi, E.Y.; Evans, D.G. Computational Study of the Driving Forces and Dynamics of Curcumin Binding to Amyloid-beta Protofibrils. J. Phys. Chem. B 2019, 123, 551–560.
- Zhao, L.N.; Long, H.; Mu, Y.; Chew, L.Y. The toxicity of amyloid beta oligomers. Int J. Mol. Sci. 2012, 13, 7303–7327.
- Zhao, L.N.; Chiu, S.W.; Benoit, J.; Chew, L.Y.; Mu, Y.G. The Effect of Curcumin on the Stability of A beta Dimers. J. Phys. Chem. B 2012, 116, 7428–7435.
- Reinke, A.A.; Gestwicki, J.E. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215.
- Yanagisawa, D.; Kato, T.; Taguchi, H.; Shirai, N.; Hirao, K.; Sogabe, T.; Tomiyama, T.; Gamo, K.; Hirahara, Y.; Kitada, M.; et al. Keto form of curcumin derivatives strongly binds to Abeta oligomers but not fibrils. Biomaterials 2021, 270, 120686.
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers Dis. 2017, 60, 999–1014.
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19.
- Reddy, A.C.; Lokesh, B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell Biochem. 1994, 137, 1–8.
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The role of curcumin and its derivatives in sensory applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109792.
- Mutsuga, M.; Chambers, J.K.; Uchida, K.; Tei, M.; Makibuchi, T.; Mizorogi, T.; Takashima, A.; Nakayama, H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J. Vet. Med. Sci. 2012, 74, 51–57.
- Reddy, P.H.; Manczak, M.; Yin, X.L.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-beta in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 843–866.
- Yang, F.S.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901.
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104.
- Park, S.Y.; Kim, D.S. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231.
- Geng, J.; Zhao, C.; Ren, J.; Qu, X. Alzheimer’s disease amyloid beta converting left-handed Z-DNA back to right-handed B-form. Chem. Commun. 2010, 46, 7187–7189.
- Atamna, H.; Boyle, K. Amyloid-beta peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386.
- Yoshida, H.; Okumura, N.; Nishimura, Y.; Kitagishi, Y.; Matsuda, S. Turmeric and curcumin suppress presenilin 1 protein expression in Jurkat cells. Exp. Ther. Med. 2011, 2, 629–632.
- Huang, P.; Zheng, N.; Zhou, H.B.; Huang, J. Curcumin inhibits BACE1 expression through the interaction between ERbeta and NFkappaB signaling pathway in SH-SY5Y cells. Mol. Cell Biochem. 2020, 463, 161–173.
- Kruger, L.; Mandelkow, E.M. Tau neurotoxicity and rescue in animal models of human Tauopathies. Curr. Opin. Neurobiol. 2016, 36, 52–58.
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21.
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300.
- Patil, S.P.; Tran, N.; Geekiyanage, H.; Liu, L.; Chan, C. Curcumin-induced upregulation of the anti-tau cochaperone BAG2 in primary rat cortical neurons. Neurosci. Lett. 2013, 554, 121–125.
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065.
- Dou, F.; Netzer, W.J.; Tanemura, K.; Li, F.; Hartl, F.U.; Takashima, A.; Gouras, G.K.; Greengard, P.; Xu, H. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. USA 2003, 100, 721–726.
- Sun, J.; Zhang, X.; Wang, C.; Teng, Z.; Li, Y. Curcumin Decreases Hyperphosphorylation of Tau by Down-Regulating Caveolin-1/GSK-3beta in N2a/APP695swe Cells and APP/PS1 Double Transgenic Alzheimer’s Disease Mice. Am. J. Chin. Med. 2017, 45, 1667–1682.
- Kell, D.B. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch. Toxicol. 2010, 84, 825–889.
- Liu, G.; Huang, W.; Moir, R.D.; Vanderburg, C.R.; Lai, B.; Peng, Z.; Tanzi, R.E.; Rogers, J.T.; Huang, X. Metal exposure and Alzheimer’s pathogenesis. J. Struct. Biol. 2006, 155, 45–51.
- Lopes de Andrade, V.; Marreilha Dos Santos, A.P.; Aschner, M. Neurotoxicity of Metal Mixtures. Adv. Neurotoxicol. 2021, 5, 329–364.
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241.
- Zatta, P.; Drago, D.; Bolognin, S.; Sensi, S.L. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol. Sci. 2009, 30, 346–355.
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes-A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14.
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094.
- Patro, B.S.; Rele, S.; Chintalwar, G.J.; Chattopadhyay, S.; Adhikari, S.; Mukherjee, T. Protective activities of some phenolic 1,3-diketones against lipid peroxidation: Possible involvement of the 1,3-diketone moiety. Chembiochem 2002, 3, 364–370.
- Momeni, H.R.; Eskandari, N. Effect of curcumin on kidney histopathological changes, lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice. Exp. Toxicol. Pathol. 2017, 69, 93–97.
- Picciano, A.L.; Vaden, T.D. Complexation between Cu(II) and curcumin in the presence of two different segments of amyloid beta. Biophys. Chem. 2013, 184, 62–67.
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer’s disease. J. Am. Chem. Soc. 2013, 135, 16397–16409.
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522.
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357.
- Frautschy, S.A.; Hu, W.; Kim, P.; Miller, S.A.; Chu, T.; Harris-White, M.E.; Cole, G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2001, 22, 993–1005.
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386.
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208.
- Kim, D.S.; Park, S.Y.; Kim, J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA (1-42) insult. Neurosci. Lett. 2001, 303, 57–61.
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031.
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011, 8, 12.
- Chin, D.; Huebbe, P.; Pallauf, K.; Rimbach, G. Neuroprotective properties of curcumin in Alzheimer’s disease--merits and limitations. Curr. Med. Chem. 2013, 20, 3955–3985.
More
