The aim of this study was to investigate whether oxygen is a rate limiting factor for any of the main cognitive domains in healthy young individuals. Subjects were randomly assigned to either increased oxygen supply using hyperbaric oxygen (two atmospheres of 100% oxygen) or to a “sham” treatment (simulation of increased pressure in the chamber breathing normal air). While in the chamber, participants went through a battery of tests evaluating the major cognitive domains including information processing speed, episodic memory, working memory, cognitive flexibility, and attention. The results demonstrated that from all evaluated cognitive domains, a statistically significant improvement was found in the episodic memory of the hyper-oxygenized group. The hyper-oxygenized group demonstrated a better learning curve and a higher resilience to interference. The results of this study indicate that memory function is a continuum that does not reach its maximal ceiling effect at the normal sea level environment even in healthy young individuals. Understanding the biological limitation of our cognitive functions is important for future development of interventional tools that can be used in the daily clinical practice.
The aim of this entry was to investigate whether oxygen is a rate limiting factor for any of the main cognitive domains in healthy young individuals. Subjects were randomly assigned to either increased oxygen supply using hyperbaric oxygen (two atmospheres of 100% oxygen) or to a “sham” treatment (simulation of increased pressure in the chamber breathing normal air). While in the chamber, participants went through a battery of tests evaluating the major cognitive domains including information processing speed, episodic memory, working memory, cognitive flexibility, and attention. The results demonstrated that from all evaluated cognitive domains, a statistically significant improvement was found in the episodic memory of the hyper-oxygenized group. The hyper-oxygenized group demonstrated a better learning curve and a higher resilience to interference. The results of this study indicate that memory function is a continuum that does not reach its maximal ceiling effect at the normal sea level environment even in healthy young individuals. Understanding the biological limitation of our cognitive functions is important for future development of interventional tools that can be used in the daily clinical practice.
- Episodic memory
- hyperoxia
- hyperbaric oxygen
- cognitive enhancement
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Cognition, “the mental action or process of acquiring knowledge and understanding through thought, experience, and the senses,” [1] is crucial for human functionality. Cognition is the sum of different aspects of intellectual domains such as attention, memory and working memory, processing speed, cognitive flexibility, and executive functions. Similar to any other physical capability, cognition is both enabled and limited by tissue biology, in this case, brain biology. Most research on the biology of cognition relates to pathophysiological conditions and how they cause cognitive decline, and less is known about the biological rate-limiting factors that prevent us from enhancing cognitive functions. In this study, we challenged the different cognitive domains to evaluate whether, in normal healthy brains, oxygen delivery is a rate-limiting factor preventing enhanced cognitive performance.
The brain has unique thermodynamic characteristics. It comprises about 2% of the body’s total weight, yet it utilizes about 20% of the total oxygen supply and consumes about 30% of the body’s total energy. At normal oxygen (normoxic) conditions, oxygen is continuously consumed by the brain tissue, and brain tissue oxygenation (PbTO2) values range from a maximal intra-capillary 90 mmHg to less than 30 mmHg [2]. At any given moment, the brain utilizes all oxygen delivered, and the perfusion differentially changes based on neuronal activity, as demonstrated and utilized by functional MRI. When neurons become active, local blood flow to those brain regions increases at the expense of other less active brain regions [3]. Apparently, since oxygen is a limited resource, many neurological functions are regularly activated at suboptimal levels. Therefore, it is safe to assume that cognition is another such affected function.
Data relating to the dependency of different cognitive functions on brain oxygenation have been mostly gathered from pathologic conditions [4,5][4][5]. Previous research on hypoxia has demonstrated a decline in cognitive function when oxygen’s partial pressure goes below 50 mmHg [6]. In such cases, there is a decline in memory performance [7[7][8],8], attention skills [9], working memory [10], and executive functions [11].
Very few studies have investigated data on the immediate effects of increasing oxygen delivery to the brain (hyperoxia) on cognitive function. Scholey et al. demonstrated that a short period of hyperoxia, induced up to five minutes prior to learning a set of words, can enhance later word recall [12]. Chung et al. have demonstrated that doubling the breathing oxygen concentration to 40% oxygen administration leads to increases in the N-back task performance [13]. In addition, in a previous study, we demonstrated that oxygen is indeed a rate-limiting factor for performing a multitask paradigm (motor + cognitive tasks) [14].
2. Results
Sixty-two subjects signed the informed consent form and were randomized to either study group. Out of the 62, 5 did not perform an in-chamber test and were excluded: 1 had intercurrent disease that prevented him from going into the chamber and 4 lost interest after the training session. Another subject did not complete the tests in the chamber. Accordingly, 56 individuals were included in the final analyses, 27 subjects in the HBOT group and 29 in the sham group (see Figure 4Figure 1).
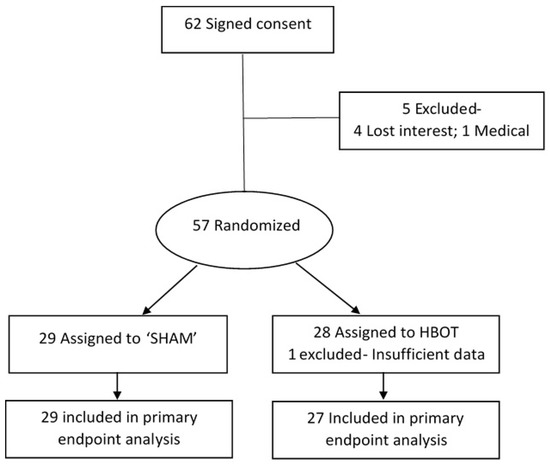
Figure 41.
Subject inclusion flow diagram.
The baseline characteristics are presented in Table 2Table 1.
Table 21.
Baseline characteristics.
| HBOT (N = 27) | SHAM (N = 29) | p | Value |
|---|
| Mean Age (Stdev) | 27.92 ± 4.77 | 26.96 ± 3.95 | 0.41 |
| Years of Education (Stdev) | 15.36 ± 2.11 | 14.64 ± 1.7 | 0.178 |
| Gender (% Females) | 59% | 45% | 0.28 |
The mean age of the participants was 27.42 ± 4.35; 51.7% were female and the average number of years of education among them was 14.98 ± 1.92. There was no significant difference in any of the participants’ characteristics between the two groups (see Table Table 21). The cognitive test results are summarized in Table 3Table 2. The learning curve, as demonstrated by the total number of words recalled from a list of words read repetitively, significantly improved while breathing hyperbaric oxygen in comparison to subjects from the sham group. Subjects from the HBOT group recalled significantly more words in total compared to the sham group (t = 4.76, p < 0.03), although this result did not withstand the FDR correction. The most notable difference between the groups was in memory decay. Participants in the HBOT group were less vulnerable to memory decay, as indicated by their preferable retrieval rates after distraction (i.e., a trial in which they had to memorize and recall a new set of words) (t = 15.1, p < 0.001). Cohen’s D revealed a strong effect size (0.85) indicating that the significant difference between the experimental groups was also clinically valuable. There was no other significant difference in any of the other evaluated cognitive parameters (see Table Table 32).
Table 32.
Cognitive domains results.
| Cognitive Domain | HBOT (N = 27) | SHAM (N = 29) | t | -Test ( | p | ) | FDR ( | p | ) | Effect Size (Cohen’s D) |
|---|
| Digit span (forward: highest correct) | 7.78 (1.5) | 7.46 (0.85) | 0.37 | 0.55 | 0.26 | |
| Digit span (forward: overall correct) | 41.3 (11.98) | 38.27 (6.55) | 0.25 | 0.75 | 0.31 | |
| Digit span (backward: highest correct) | 7.34 (1.52) | 6.85 (1.81) | 0.28 | 0.67 | 0.29 | |
| Digit span (backward: overall correct) | 40.85 (10.62) | 42 (9.04) | 0.66 | 0.79 | 0.12 | |
| Stroop (% correct) | 59.03 (15.39) | 62.96 (15.99) | 0.35 | 0.6 | 0.25 | |
| Multi-tasking (% total accuracy) | 66.6 (7.97) | 64.24 (8.47) | 0.29 | 0.58 | 0.29 | |
| Symbol search (no. correct) | 31.66 (6.3) | 33.03 (8.47) | 0.49 | 0.65 | 0.18 | |
| N-back (% correct) | 91.19 (9.57) | 90.42 (10.05) | 0.76 | 0.83 | 0.08 | |
| Series simple (no. correct) | 15.3 (5.9) | 15.27 (5.48) | 0.98 | 0.98 | 0.00 | |
| Series complex (no. correct) | 12 (4.51) | 13.41 (4.35) | 0.23 | 0.92 | 0.32 | |
| CVLT (total correct) | 22.4 (2.96) | 20.51 (3.75) | 0.04 | 0.24 | 0.54 | |
| CVLT (no. of words—post interference) | 11.33 (2.3) | 9.03 (2.93) | 0.001 | 0.012 | * | 0.85 |
References
- Oxford University Press. Lexico. Available online: https://www.lexico.com (accessed on 17 August 2020).
- Zauner, A.; Daugherty, W.P.; Bullock, M.R.; Warner, D.S. Brain oxygenation and energy metabolism: Part I-biological function and pathophysiology. Neurosurgery 2002, 51, 289–301.
- Bandettini, A.P. Neuronal or Hemodynamic? Grappling with the Functional MRI Signal. Brain Connect. 2014, 4, 487–498.
- Huppert, F. Memory impairment associated with chronic hypoxia. Thorax 1982, 37, 858–860.
- Stuss, D.T.; Peterkin, I.; Guzman, D.A.; Guzman, C.; Troyer, A.K. Chronic obstructive pulmonary disease: Effects of hypoxia on neurological and neuropsychological measures. J. Clin. Exp. Neuropsychol. 1997, 19, 515–524.
- Areza-Fegyveres, R.; Kairalla, R.A.; Carvalho, C.R.; Nitrini, R. Cognition and chronic hypoxia in pulmonary diseases. Dement. Neuropsychol. 2010, 4, 14–22.
- Kida, M.; Imai, A. Cognitive performance and event-related brain potentials under simulated high altitudes. J. Appl. Physiol. 1993, 74, 1735–1741.
- Shukitt-Hale, B.; Stillman, M.J.; Welch, D.I.; Levy, A.; Devine, J.A.; Lieberman, H.R. Hypobaric hypoxia impairs spatial memory in an elevation-dependent fashion. Behav. Neural Boil. 1994, 62, 244–252.
- Zani, A.; Tumminelli, C.; Proverbio, A.M. Electroencephalogram (EEG) Alpha Power as a Marker of Visuospatial Attention Orienting and Suppression in Normoxia and Hypoxia. An Exploratory Study. Brain Sci. 2020, 10, 140.
- Malle, C.; Quinette, P.; Laisney, M.; Bourrilhon, C.; Boissin, J.; Desgranges, B.; Eustache, F.; Piérard, C. Working memory impairment in pilots exposed to acute hypobaric hypoxia. Aviat. Space Environ. Med. 2013, 84, 773–779.
- Ochi, G.; Kanazawa, Y.; Hyodo, K.; Suwabe, K.; Shimizu, T.; Fukuie, T.; Byun, K.; Soya, H. Hypoxia-induced lowered executive function depends on arterial oxygen desaturation. J. Physiol. Sci. 2018, 68, 847–853.
- Scholey, A.; Moss, M.; Wesnes, K. Oxygen and cognitive performance: The temporal relationship between hyperoxia and enhanced memory. Psychopharmacology 1998, 140, 123–126.
- Chung, S.-C.; Kwon, J.-H.; Lee, H.-W.; Tack, G.-R.; Lee, B.; Yi, J.-H.; Lee, S.-Y. Effects of high concentration oxygen administration onn-back task performance and physiological signals. Physiol. Meas. 2007, 28, 389–396.
- Vadas, D.; Kalichman, L.; Hadanny, A.; Efrati, S. Hyperbaric Oxygen Environment Can Enhance Brain Activity and Multitasking Performance. Front. Integr. Neurosci. 2017, 11, 25.
References
- Oxford University Press. Lexico. Available online: https://www.lexico.com (accessed on 17 August 2020).
- Zauner, A.; Daugherty, W.P.; Bullock, M.R.; Warner, D.S. Brain oxygenation and energy metabolism: Part I-biological function and pathophysiology. Neurosurgery 2002, 51, 289–301.
- Bandettini, A.P. Neuronal or Hemodynamic? Grappling with the Functional MRI Signal. Brain Connect. 2014, 4, 487–498.
- Huppert, F. Memory impairment associated with chronic hypoxia. Thorax 1982, 37, 858–860.
- Stuss, D.T.; Peterkin, I.; Guzman, D.A.; Guzman, C.; Troyer, A.K. Chronic obstructive pulmonary disease: Effects of hypoxia on neurological and neuropsychological measures. J. Clin. Exp. Neuropsychol. 1997, 19, 515–524.
- Areza-Fegyveres, R.; Kairalla, R.A.; Carvalho, C.R.; Nitrini, R. Cognition and chronic hypoxia in pulmonary diseases. Dement. Neuropsychol. 2010, 4, 14–22.
- Kida, M.; Imai, A. Cognitive performance and event-related brain potentials under simulated high altitudes. J. Appl. Physiol. 1993, 74, 1735–1741.
- Shukitt-Hale, B.; Stillman, M.J.; Welch, D.I.; Levy, A.; Devine, J.A.; Lieberman, H.R. Hypobaric hypoxia impairs spatial memory in an elevation-dependent fashion. Behav. Neural Boil. 1994, 62, 244–252.
- Zani, A.; Tumminelli, C.; Proverbio, A.M. Electroencephalogram (EEG) Alpha Power as a Marker of Visuospatial Attention Orienting and Suppression in Normoxia and Hypoxia. An Exploratory Study. Brain Sci. 2020, 10, 140.
- Malle, C.; Quinette, P.; Laisney, M.; Bourrilhon, C.; Boissin, J.; Desgranges, B.; Eustache, F.; Piérard, C. Working memory impairment in pilots exposed to acute hypobaric hypoxia. Aviat. Space Environ. Med. 2013, 84, 773–779.
- Ochi, G.; Kanazawa, Y.; Hyodo, K.; Suwabe, K.; Shimizu, T.; Fukuie, T.; Byun, K.; Soya, H. Hypoxia-induced lowered executive function depends on arterial oxygen desaturation. J. Physiol. Sci. 2018, 68, 847–853.
- Scholey, A.; Moss, M.; Wesnes, K. Oxygen and cognitive performance: The temporal relationship between hyperoxia and enhanced memory. Psychopharmacology 1998, 140, 123–126.
- Chung, S.-C.; Kwon, J.-H.; Lee, H.-W.; Tack, G.-R.; Lee, B.; Yi, J.-H.; Lee, S.-Y. Effects of high concentration oxygen administration onn-back task performance and physiological signals. Physiol. Meas. 2007, 28, 389–396.
- Vadas, D.; Kalichman, L.; Hadanny, A.; Efrati, S. Hyperbaric Oxygen Environment Can Enhance Brain Activity and Multitasking Performance. Front. Integr. Neurosci. 2017, 11, 25.
