Fucoidans encompass versatile and heterogeneous sulfated biopolysaccharides of marine origin, specifically brown algae and marine invertebrates. The reported studies revealed diverse chemical skeletons in which l-fucose is the main sugar monomer. However, other sugars, i.e., galactose, mannose, etc., have been identified to be interspersed, forming several heteropolymers, including galactofucans/fucogalactans (G-fucoidans). Particularly, sulfated galactofucans are associated with rich chemistry contributing to more promising bioactivities than fucans and other marine polysaccharides. The previous reports in the last 20 years showed that G-fucoidans derived from Undaria pinnatifida were the most studied; 21 bioactivities were investigated, especially antitumor and antiviral activities, and unique biomedical applications compared to other marine polysaccharides were demonstrated.
- bioactives
- brown seaweeds
- fucoidans
- heteropolysaccharides
- structural features
- sulfated galactofucans
1. Introduction
2. Occurrence, Distribution, and Chemistry
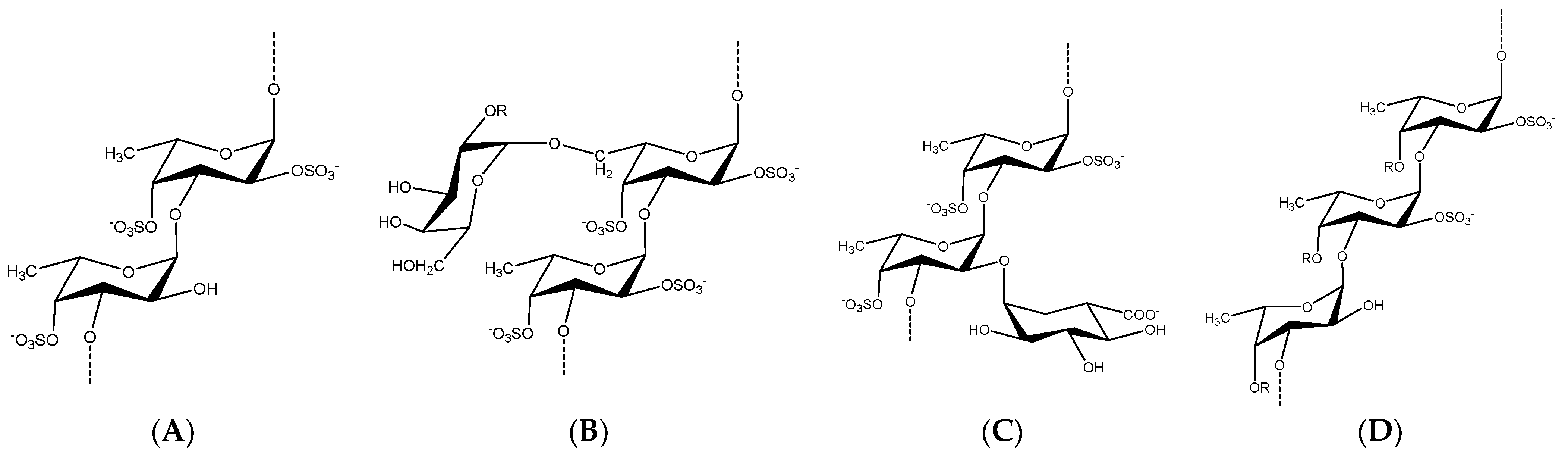
Brown seaweeds, in contrast to marine invertebrates, can synthesize more complicated, diverse, and heterogeneous fucoidan backbones, including glycosidic linkages, monomeric composition, and branching sites [53,54,55][35][36][37]. Therefore, various G-fucoidans with different fucose:galactose ratios have been reported in the different brown algae orders, including Fucales, Laminariales, and Dictyoales [56,57][38][39]. Traces of other sugars may be found, as in the case of Dictyota menstrualis [58][40] and Sargassum sp. [59][41]. Nevertheless, the presence of high percentages of glucose, i.e., fucose:galactose:glucose ratio of 1:0.3:0.25, may indicate contamination of the G-fucoidan with laminarin [60][42]. In such cases, fucoidans are partially purified by ethanol or cetyltrimethylammonium bromide (CTAB) precipitation and not purified by a specific chromatographic method, including anion exchange resin using diethylaminoethyl cellulose (DEAE-C) [61][43] or affinity chromatography [62][44]. In addition, previous studies, with the aid of advanced spectral analyses, i.e., 2D NMR (e.g., HMQC, TOCSY, and NOESY) and mass spectrometry, have attempted to reveal many structural features of G-fucoidans of various biogenic sources, including glycosidic linkages, sugar configuration, branching sites, sulfation pattern, and galactose position [6,63,64][6][45][46]. In addition, they could deduce tentative structure bioactivity relationships, as in the case of the anti-inflammatory mechanism of galactofucan isolated from Saccharina japonica [65][47]. The results of spectral analyses showed that α-l-fucopyranose (Fucp) and β-d-galactopyranose (Galp) are identified mainly, in which Fucp forms the major backbone and is linked via (1→4) and/or (1→3), while the β-d-galactopyranose molecules are found at branching sites, usually at (1→6), as in case of the G-fucoidan isolated from Hormophysa cuneiformis. In addition, the sulfation pattern is variable based on the glycosidic linkages. For instance, sulfate groups may occupy 2-O and 4-O in →3Fucp1→ or 2-O and 3-O in →4Fucp1→, in addition to 2-O in →3,4Fucp1→ [36][32]. Other models of sulfated galactofucans derived from Sargassum thunbergii were found to possess →3Fucp1→ as a main backbone with a 2-O-sulfated and 2,4-O-disulfated pattern, while the Galp residues interspersed Fucp in the main chain were linked mainly with →6Galp1→ and 4-O sulfation [46][48]. Moreover, G-fucoidan isolated from S. polycystum was built up mainly of a 4-O sulfated →3Fucp1→ backbone containing single →2Galp1→ residues sulfated similarly at the 4-O position [66][49]. Several other models are demonstrated in Figure 2 and Table 1 and in relation to their biomedical applications.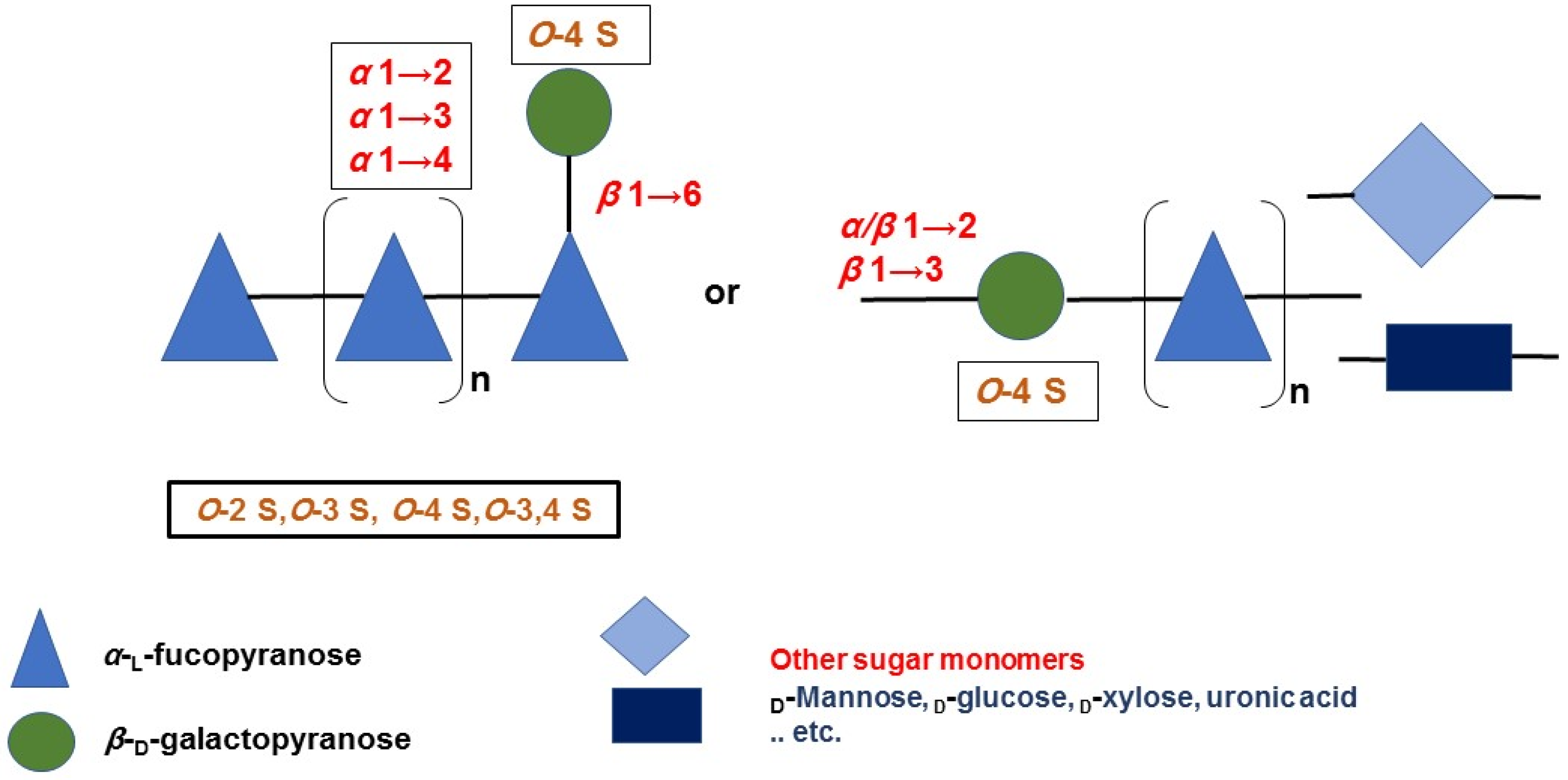
|
Brown Algae (Seaweed) Species |
Source of Seaweed Biomass |
Structural Characteristics |
References |
|||||
|---|---|---|---|---|---|---|---|---|
3.2. Antiviral Activity
Galactofucans show antiviral properties against a number of highly pathogenic viruses, including the human immunodeficiency virus (HIV-1) (Table 3). Table 3. Summarized antiviral activity of G-fucoidans with their respective sources and half-maximal effective or inhibitory concentrations (EC50/IC50). Comparisons with antiviral drugs are also shown.|
Source |
EC50/IC50 |
Compared with Antiviral Drugs? |
References |
|---|---|---|---|
|
Monosaccharide Composition |
Glycosidic Bonds of Backbone |
Molecular Weight (kDa) |
|
Source |
EC50/IC50 |
Compared with Standard/Commercial Compounds? |
References |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Fucose/Galactose Ratio | Sulfate Content (%) |
Sulfation Pattern |
||||||||||||
|
Dictyotales |
||||||||||||||
|
Adenocystis utricularis |
0.6–0.9 µg/mL (HIV-1) |
Yes. Superior to azidothymidine |
||||||||||||
|
Cystoseira compressa |
0.43 mg/mL (DPPH) | ] |
Yes. Inferior to ascorbic acid and butylated hydroxyanisole [61][ |
|||||||||||
] | [ | ] |
Canistrocarpus cervicornis |
Wild |
Gal, fuc, glcAc, xyl, |
ND |
0.3 µg/mL (HSV-1) and 0.5 µg/mL (HSV-2) |
No2 |
[ 16.5 | |||||
|
Sargassum siliquosum |
2.58 mg/mL (DPPH) | ND | ||||||||||||
No |
[10] |
Dictyota dichotoma |
Wild |
Gal, fuc, man, xyl, ara, rha, glc |
Dictyota dichotoma |
7.5 µg/mL (HSV-1), and 15.6 µg/mL (CVB3) |
Yes. Superior to ribavirin 23.6 |
1.5 |
33 |
ND |
[ |
|||
] | [ | 51] |
D. implexa |
Wild |
||||||||||
|
Saccharina japonica |
0.001–0.005 µg/mL (HIV-1) |
Gal, fuc |
ND |
1 |
18.3 |
ND |
No |
|||||||
|
Lobophora variegata |
Wild |
Gal, fuc, Glc, man, xyl, glcAc; Gal, fuc; Gal, fuc, Glc |
(1,3)- and (1,4)-α-l-fuc, and (1,3)-β-d | |||||||||||
|
S. thunbergii |
0.22 mg/mL (superoxide radical), and 0.88 mg/mL (hydroxyl radical) |
Yes. Similar (hydroxy radical) or superior (superoxide radical) to vitamin C |
[90 0.2–25 µg/mL (HSV-1)-gal |
Yes. Inferior to acyclovir and similar to heparin 35; ND; 1400 |
0.79; 0.5; 0.5 |
32.6; 0.2 *;15 |
||||||||
] | [ | , | ] | [ | ||||||||||
|
L. variegata |
ND |
Sargassum mcclurei |
0.96 µg/mL (HIV-1)Gal, fuc |
ND |
360–1600 |
0.3 |
23.3–35.5 |
ND |
[ |
Yes. Inferior to AMD3100 (plerixafor) |
||||
[ | ][69] |
Padina boryana |
Wild |
|||||||||||
|
S. patens | Gal, fuc |
1.3 µg/mL (HSV-2), 5.5 µg/mL (HSV-1), and 4.1 µg/mL (HSV-1 acyclovir-resistant strain) (1,4)-α-l-fuc, and (1,3)-β-d-gal |
No |
][71 |
1.1 |
][72] |
18.6 |
At C2 and C4 (fuc and gal) |
||||||
|
Spatoglossum schroederi |
Wild |
|||||||||||||
|
>50 µg/mL (virucidal activity against HSV-2), 1.3–1.65 µg/mL (plaque formation), 1.85–3.5 µg/mL (inhibition of virus adsorption) | Gal, fuc, xyl, glcAc; Gal, fuc, xyl; |
(1,4)-β-d-gal, (1,4)-α-l |
No-fuc, and (1,4)-β-d-xyl |
21.5; 21.5–24 |
0.5; 0.5 |
19; 2.1–2.9 * |
At C3 (gal) and C4 (fuc) |
|||||||
|
Ectocarpales |
||||||||||||||
|
1.5–5.5 mg/mL (HSV-1 replication) and 3–4 mg/mL (HSV-1 adsorption) |
Yes. Similar to acyclovir |
Adenocystis utricularis |
Wild |
Gal, fuc, rha, man; Gal, fuc, rha; Gal, fuc, man |
(1,3)-α-l-fuc |
>100 |
||||||||
|
S. polycystum | 5.53; 4.82; 5.53 | 23; 24; 23 |
At C4 (fuc and gal) |
0.34 µg/mL (HIV-1) |
Yes. Inferior to AMD3100 (plerixafor) |
|||||||||
[ | ] | [ | , | |||||||||||
|
Scytosiphon lomentaria |
Scytosiphon lomentaria Wild |
Gal, fuc, rha, xyl, man, uronic acid |
(1,3)-α- |
0.76 µg/mL (HSV-1) and 1.34 µg/mL (HSV-2) |
No l-fuc, and (1,6)-β-d-gal |
8.5 |
7.33 |
29.5 |
At C3 and C4 (fuc), and C3 (gal) |
|||||
|
Fucales |
||||||||||||||
|
Sphacelaria indica |
1.3 µg/mL (HSV-1) |
Yes. Superior to acyclovir when added to the overlay medium after penetration of the viruses into the host cell |
Cystoseira compressa |
Wild |
Gal, fuc |
(1,3)- and (1,4)-α-l-fuc |
||||||||
|
Turbinaria ornata | 100 |
0.39 µg/mL (HIV-1) |
2.32 |
14.7 |
Yes. Inferior to AMD3100 (plerixafor) At C2 and C4 (fuc) |
|||||||||
[ |
Sargassum duplicatum |
Wild |
Gal, fuc |
(1,4)-α-l-fuc and β-d-gal (alternating) |
34–191 |
1 |
31.7 |
ND |
[14] |
|||||
|
S. feldmannii |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
183–184 |
2–2.6 |
25.3–32 |
At C2, C3 and C4 (fuc), and C2, C3, C4 and C6 (gal) |
|||||||
] | [ | ] |
S. fusiforme |
Wild |
Gal, fuc, xyl, Glc, glcAc, man, uronic acid; Gal, fuc, xyl, man, rha, glcAc, Glc |
(1,3)- and (1,4)-α-l-fuc |
90; 118.3/3.9 |
2; 3.7 |
17.5; 28.5 |
At C3 (fuc) |
||||
|
S. hemiphyllum |
Wild |
Gal, fuc |
(1,6)-β-d-gal, (1,3)- and (1,4)-α-l-fuc, and (1,3)-β-d-gal |
148 |
4.5 |
32 |
At C2 and C4 (fuc) |
|||||||
|
S. mcclurei |
Wild |
Gal, fuc; Gal, fuc, man, xyl, glc |
(1,3)-α-l-fuc |
ND |
1.4; 2 |
35; 30.5 |
At C2 and C4 (fuc) |
|||||||
|
S. patens |
Wild |
Gal, fuc, man, xyl, Glc, galactosamine |
ND |
424 |
1.9 |
14.4 |
ND |
|||||||
|
S. polycystum |
Wild |
Gal, fuc, glc; Gal, fuc, man, xyl, glc |
(1,3)- | |||||||||||
|
Undaria pinnatifida |
0.77 µg/mL (HSV-1) |
Yes. Superior to acyclovir |
||||||||||||
|
32 µg/mL (HSV-1) and 0.5 µg/mL (HSV-2) |
Yes. Superior to acyclovir |
|||||||||||||
|
U. pinnatifida (sporophylls) |
2.5 µg/mL (HSV-1), 2.6 µg/mL (HSV-2), and 1.5 µg/mL (HCMV) |
No |
||||||||||||
|
U. pinnatifida |
1.1 µg/mL (HSV-1), 0.1 µg/mL (HSV-2), and 0.5 µg/mL (HCMV) |
No |
||||||||||||
|
3.1 µg/mL (HSV-1) and 1.6 µg/mL (HSV-2) |
No |
α-l-fuc, and (1,6)-β-d-gal |
39.5; ND |
5.84; 1.48 |
33.6; 23.4 |
At C2 and C4 (fuc) |
||||||||
|
S. siliquosum |
Wild |
Gal, fuc, glc, xyl, man, rha; Gal, fuc, Glc, xyl, man, rha, uronic acid |
(1,3)- and (1,4)-α-l-fuc |
107.3; ND |
1.9; 1.9 |
19.5; 20 |
At C4 and C6 (gal) |
|||||||
|
S. thunbergii |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
7.2–333.5 |
5.26–5.88 |
27.2–30.1 |
At C2 and C4 (fuc), and C4 (gal) |
|||||||
|
S. thunbergii |
Purchased from local store |
Gal, fuc |
(1,4)-α-d-gal, and (1,3)-β-l-fuc |
373 |
1.2 |
ND |
NA |
|||||||
|
S. wightii |
Wild |
Gal, fuc, Glc, man; Gal, fuc |
(1,3)-α-l-fuc |
>3.5; ND |
0.6; 3–3.5 |
379.1 †; 8.1–19.5 |
At C2 and/or C4 (fuc), or C2 and C3 (gal) |
|||||||
|
Turbinaria ornata |
Wild |
Gal, fuc; Gal, fuc, man, xyl, glc |
(1,3)-α-l-fuc |
ND |
5; 1.2 |
32; 25.6 |
At C2 and/or C4 (fuc), and/or C2, C3, C4/C6 (gal) |
|||||||
|
Laminariales |
||||||||||||||
|
Alaria angusta |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
ND |
1.1 |
24 |
At C2 (fuc), and C2 and C4 (gal) |
|||||||
|
Costaria costata |
Wild |
Gal, fuc, man, rha, xyl |
ND |
ND |
1.2 |
18.9 |
ND |
|||||||
|
Ecklonia cava |
Wild |
Gal, fuc, man, rha; Gal, fuc, rha, glc |
ND |
ND |
4.8; 3.6 |
19.1; 22.2 |
At C2 (fuc) |
|||||||
|
Laminaria hyperborea |
ND |
Gal, fuc |
(1,3)-α-l-fuc |
469 |
44.5 |
53.8 |
At C2 and C4 (fuc) |
[12] |
||||||
|
Saccharina angustata |
Wild |
Gal, fuc, xyl, uronic acid |
(1,3)-, (1,4) and (1,2)-α-l-fuc |
56 |
9.1 |
4.2 |
At C4 (fuc and gal) |
|||||||
|
S. gurjanovae |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
123 |
3.2 |
25.1 |
At C2 and C4 (fuc), and C2 and/or C3 (gal) |
|||||||
|
S. japonica |
Wild |
Gal, fuc; Gal, fuc, man, xyl; Gal, fuc, man, rham, xyl; Gal, fuc, uronic acid, man, glcAc; Gal, fuc, Glc, man, rha, xyl; Gal, fuc, xyl, Glc, glcAc, rha, uronic acid |
(1,3)-α-l-fuc |
195/13.7; 1800; ND; 106.3; 23.5; 11 |
3.6; 1.1; 1.8; 9.1; 0.5; 10 |
21; 23.3; 23; 36.9; 18; 41.3 |
At C2 and C2/C4 (fuc) |
|||||||
|
S. japonica |
Cultivated |
Gal, fuc; Gal, fuc, man, rham, xyl, Glc; Gal, fuc, man, Glc, rha, xyl, uronic acid |
(1,3)- and (1,4)-α-l-fuc |
261.7; 131.5; 8.1 |
3.8; 2.1; 5.8 |
11.4; 9.1; 41.8 |
At C4 (fuc) |
|||||||
|
S. japonica |
Provided by Fujian Yida Food Co. |
Gal, fuc, man |
ND |
527.3 |
0.9 |
26.7 |
ND |
|||||||
|
S. japonica |
ND |
Gal, fuc |
(1,3)-α-l-fuc, and (1,6)-β-d-gal |
>10 |
3.5 |
48.3 |
At C4 and/or C2/C4 (fuc), and C4 and/or C3/C4 (gal) |
|||||||
|
S. latissima |
Wild |
Gal, fuc; Gal, fuc, xyl, man, Glc |
(1,3)-α-l-fuc |
416–449; 453 |
7.8; 4.1 |
0.8 ‡; 0.6 ‡ |
ND |
|||||||
|
S. longicruris |
Wild |
Gal, fuc, xyl, man, Glc, glcAc; Gal, fuc, xyl, man, Glc, galAc, glcAc |
1529; 638 |
0.8; 0.4 |
17.6; 19.1 |
At C4 (fuc), and C3 (gal) |
||||||||
|
Undaria pinnatifida |
Wild |
Gal, fuc, man; Gal, fuc, rha; Gal, fuc, Glc, man, rha, xyl, ara |
(1,3)- or (1,4)-α-l-fuc |
ND; 290; ND |
1.1; 1.2; 1.3 |
29; 0.94 ‡; ND |
At C2, C3, C4 (fuc), or C2 and C4 (fuc and/or gal) |
|||||||
|
U. pinnatifida (sporophylls) |
Wild |
Gal, fuc, xyl, man |
(1,3)-α-l-fuc |
>150 |
1.5 |
15 |
ND |
|||||||
|
Cultivated |
Gal, fuc; Gal, fuc, man; Gal, fuc, xyl, man; Gal, fuc, man, xyl, uronic acid |
(1,3)-α-l-fuc, and (1,3)-, (1,4)-, (1,6)-β-d-gal |
ND; 1.4–3.7; 1246; 2100 |
1.4; 1.1; 1.1; 5 |
31; 8.4; 9.2; 7.4 |
At C2/C4 (fuc), and C3/C6 (gal) |
||||||||
|
From mussel farms |
Gal, fuc, xyl, Glc, man; Gal, fuc, xyl, Glc, man, uronic acid |
171; >150 |
1.5; 1.5 |
15; 15 |
ND |
|||||||||
|
U. pinnatifida |
From Marine Resources Pty Ltd. |
ND |
ND |
ND |
ND |
|||||||||
|
From Marinova Pty Ltd. |
Gal, fuc, xyl, man |
(1,3)-α-l-fuc |
51.7 |
1.3 |
21.5 |
At C2 and C4 (fuc) |
||||||||
|
ND |
ND |
ND |
ND |
ND |
||||||||||
|
U. pinnatifida (sporophylls) |
ND |
Gal, fuc; Gal, fuc, uronic acid; Gal, fuc, xyl, man |
(1,3)-α-l-fuc, and (1,3)-, (1,4)-, (1,6)-β-d-gal |
9; 9; 104.4 |
0.9; 0.9; ND |
10.4; 10.4; 21 |
At C2 (fuc), and C3 and C6 (gal) |
|||||||
|
Sphacelariales |
||||||||||||||
|
Sphacelaria indica |
Wild |
Gal, fuc, xyl, man, Glc |
(1,3)-α-l-fuc |
26 |
3.3 |
4 |
At C4 (fuc) |
|||||||
ND, not detailed; NA, not applicable; * reported as molar ratio to fucose; † reported as mg/g fucoidan; ‡ reported as degree of sulfation.
3. Potential Pharmacological Activities
3.1. Anticancer/Antitumor Activity
Several studies have reported the anticancer/antitumor activities of galactofucans in different cancer cell lines, as well as antiproliferative, antimetastasis, and antiangiogenic effects (Table 2).
Table 2. G-fucoidans showing anticancer/antitumor activity with their respective sources and half-maximal inhibitory concentrations (IC50). Comparisons with standard or commercial compounds are also shown.
|
Source |
IC50 |
Compared with Standard/Commercial Compounds? |
References |
|---|---|---|---|
|
Saccharina latissima |
0.35 µg/mL (elastase inhibition) |
Yes. Superior to commercial heparins (UFH and tinzaparin) |
|
|
Sargassum polycystum |
84.63 µg/mL (leukemia cells) and 93.62 µg/mL (breast cancer cells) |
No |
|
|
S. thunbergii |
29.7–93.5 μg/mL (inhibition of FGF1 binding) and 4.0–6.8 μg/mL (inhibition of FGF7 binding) |
No |
|
|
Undaria pinnatifida (sporophylls) |
0.10 mg/mL (breast adenocarcinoma) and 0.15 mg/mL (lung carcinoma) |
Yes. Superior to commercial fucoidan from Fucus for both cancer cell lines |
3.3. Anti-Inflammatory, Immunomodulatory, and Anticomplement Activities
Jin et al. have studied different factors that may affect the anticomplement activity of G-fucoidans. Among them were extraction methods, molecular weight, fucose:galactose molar ratio, sulfate content, uronic acid, type of glycosidic linkage, branching, and monomeric composition. The study concluded that larger molecular weights were more related to better activities [81][66]. G-fucoidans might also represent a novel and safer treatment strategy for chronic inflammation or related ailments. Six brown algal species have shown promising anti-inflammatory effects. Galactofucans from Sargassum wightii showed superior activity to aspirin, with EC90 values ranging from 0.2 to 1.22 mg/mL for inhibition of inflammatory-related enzymes [92,93][77][78]. Only the galactofucans from Saccharina japonica and Lobophora variegata have been tested in vivo with positive results [42,65,70,104][47][53][54][89]. Chen et al. showed that the investigated galactofucans from S. japonica were non-cytotoxic in the range of 3.125 to 25 μg/mL [65][47]. The anti-inflammatory was investigated in the form of fucoidan-based cream using fucoidan derived from F. vesiculosus of fucose:galactose ratio 1.0:0.05.3.4. Anticoagulant and Antithrombotic Activities
Fucoidans are well-known for their anticoagulant and antithrombotic activities. These polysaccharides have attracted extensive interest in discovering safer anticoagulants, with less hemorrhagic risk and good antithrombotic activity [135][112]. As part of this complex class of molecules, G-fucoidans also represent a source of potential antithrombotic drugs. For example, a sulfated galactofucan from Spatoglossum schroederi was 2-fold more potent than heparin in stimulating the synthesis of antithrombotic heparan sulfate by endothelial cells of rabbit aorta. In vivo experiments were key to clarifying the antithrombotic activity of this galactofucan, which initially did not show an anticoagulant effect during in vitro experiments. Such an effect was demonstrated for the fraction C at 100 µg/mL with an MW of 24 kDa [73][57]. Fucoidans can also enhance the plasma level of recombinant tissue plasminogen activator (rtPA), a protein commonly used as a non-interventional treatment to recanalize vessels occluded by acute thrombosis.3.5. Antioxidant Activity
The scavenging effect of fucoidans on harmful oxidants, such as superoxide anion, hydrogen peroxide, hydroxyl radicals, and singlet oxygen, has attracted considerable interest from the food and pharmaceutical industries [136][113]. In this regard, galactofucan from the Tunisian brown seaweed Cystoseira compressa exhibited valuable antioxidant properties when subjected to various antioxidant tests, i.e., ferrous ion chelation, ferric ion reduction, and DPPH radical scavenging assays (Table 4). For instance, the DPPH assay resulted in an IC50 value of 430 μg/mL compared to 560 μg/mL for sodium alginate isolated from the same organism [64][46].3.6. Other Biological Activities
References
- Zayed, A.; Ulber, R. Fucoidans: Downstream processes and recent applications. Mar. Drugs 2020, 18, 170.
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831.
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777.
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626.
- Zayed, A.; Haggag, Y.; Ezzat, S.M.; Salem, M.A.; Ulber, R. Fucoidans as nanoparticles: Pharmaceutical and biomedical applications. In Polysaccharide Nanoparticles; Venkatesan, J., Kim, S.-K., Anil, S., Rekha, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 413–455.
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 2020, 18, 571.
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437.
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs 2016, 14, 79.
- Zayed, A.; Hahn, T.; Finkelmeier, D.; Burger-Kentischer, A.; Rupp, S.; Krämer, R.; Ulber, R. Phenomenological investigation of the cytotoxic activity of fucoidan isolated from Fucus vesiculosus. Process Biochem. 2019, 81, 182–187.
- Wang, S.-H.; Huang, C.-Y.; Chen, C.-Y.; Chang, C.-C.; Huang, C.-Y.; Dong, C.-D.; Chang, J.-S. Structure and biological activity analysis of fucoidan isolated from Sargassum siliquosum. ACS Omega 2020, 5, 32447–32455.
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695.
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural characterization of fucoidan from Laminaria hyperborea: Assessment of coagulation and inflammatory properties and their structure–function relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892.
- Abdella, A.A.; Ulber, R.; Zayed, A. Chitosan-toluidine blue beads for purification of fucoidans. Carbohydr. Polym. 2020, 231, 115686.
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of structure and in vitro anticancer activity of native and modified fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228.
- Badrinathan, S.; Shiju, T.M.; Sharon Christa, A.S.; Arya, R.; Pragasam, V. Purification and structural characterization of sulfated polysaccharide from Sargassum myriocystum and its efficacy in scavenging free radicals. Indian J. Pharm. Sci. 2012, 74, 549–555.
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity in vitro. Carbohydr. Polym. 2017, 175, 547–556.
- Lu, J.; Shi, K.K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.N.; Merien, F.; Xie, M.; Kong, Q.; Li, J.; et al. Fucoidan extracted from the New Zealand Undaria pinnatifida-physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs 2018, 16, 461.
- Bilan, M.I.; Usov, A.I. Structural analysis of fucoidans. Nat. Prod. Commun. 2008, 3, 1934578X0800301011.
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183.
- January, G.G.; Naidoo, R.K.; Kirby-McCullough, B.; Bauer, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517.
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137.
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-assisted fucoidan extraction from brown macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296.
- Zayed, A.; Dienemann, C.; Giese, C.; Krämer, R.; Ulber, R. An immobilized perylene diimide derivative for fucoidan purification from a crude brown algae extract. Process Biochem. 2018, 65, 233–238.
- Ponce, N.M.A.; Stortz, C.A. A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312.
- Sichert, A.; Le Gall, S.; Klau, L.J.; Laillet, B.; Rogniaux, H.; Aachmann, F.L.; Hehemann, J.-H. Ion-exchange purification and structural characterization of five sulfated fucoidans from brown algae. Glycobiology 2021, 31, 352–357.
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552.
- Chang, Y.; Hu, Y.; Yu, L.; McClements, D.J.; Xu, X.; Liu, G.; Xue, C. Primary structure and chain conformation of fucoidan extracted from sea cucumber Holothuria tubulosa. Carbohydr. Polym. 2016, 136, 1091–1097.
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Li, S.; Nasri, M.; Zouari, N. Brown seaweed Cystoseira schiffneri as a promising source of sulfated fucans: Seasonal variability of structural, chemical, and antioxidant properties. Food Sci. Nutr. 2021, 9, 1551–1563.
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297.
- Leal, D.; Mansilla, A.; Matsuhiro, B.; Moncada-Basualto, M.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; De Borggraeve, W.M. Chemical structure and biological properties of sulfated fucan from the sequential extraction of subAntarctic Lessonia sp. (Phaeophyceae). Carbohydr. Polym. 2018, 199, 304–313.
- Berteau, O. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29.
- Bilan, M.I.; Ustyuzhanina, N.E.; Shashkov, A.S.; Thanh, T.T.T.; Bui, M.L.; Tran, T.T.V.; Bui, V.N.; Nifantiev, N.E.; Usov, A.I. A sulfated galactofucan from the brown alga Hormophysa cuneiformis (Fucales, Sargassaceae). Carbohydr. Res. 2018, 469, 48–54.
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130.
- Ustyuzhanina, N.E.; Bilan, M.I.; Gerbst, A.G.; Ushakova, N.A.; Tsvetkova, E.A.; Dmitrenok, A.S.; Usov, A.I.; Nifantiev, N.E. Anticoagulant and antithrombotic activities of modified xylofucan sulfate from the brown alga Punctaria plantaginea. Carbohydr. Polym. 2016, 136, 826–833.
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338.
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698.
- Yu, L.; Ge, L.; Xue, C.; Chang, Y.; Zhang, C.; Xu, X.; Wang, Y. Structural study of fucoidan from sea cucumber Acaudina molpadioides: A fucoidan containing novel tetrafucose repeating unit. Food Chem. 2014, 142, 197–200.
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160.
- Yoon, S.J.; Pyun, Y.R.; Hwang, J.K.; Mourão, P.A. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007, 342, 2326–2330.
- Albuquerque, I.R.; Queiroz, K.C.; Alves, L.G.; Santos, E.A.; Leite, E.L.; Rocha, H.A. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004, 37, 167–171.
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336.
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412.
- Je, J.-G.; Lee, H.-G.; Fernando, K.H.N.; Jeon, Y.-J.; Ryu, B. Purification and structural characterization of sulfated polysaccharides derived from brown algae, Sargassum binderi: Inhibitory mechanism of iNOS and COX-2 pathway interaction. Antioxidants 2021, 10, 822.
- Hahn, T.; Zayed, A.; Kovacheva, M.; Stadtmüller, R.; Lang, S.; Muffler, K.; Ulber, R. Dye affinity chromatography for fast and simple purification of fucoidan from marine brown algae. Eng. Life Sci. 2016, 16, 78–87.
- Jin, W.; Wu, W.; Tang, H.; Wei, B.; Wang, H.; Sun, J.; Zhang, W.; Zhong, W. Structure analysis and anti-tumor and anti-angiogenic activities of sulfated galactofucan extracted from Sargassum thunbergii. Mar. Drugs 2019, 17, 52.
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrieres, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira Compressa. Carbohydr. Polym. 2018, 198, 589–600.
- Chen, X.; Ni, L.; Fu, X.; Wang, L.; Duan, D.; Huang, L.; Xu, J.; Gao, X. Molecular mechanism of anti-inflammatory activities of a novel sulfated galactofucan from Saccharina japonica. Mar. Drugs 2021, 19, 430.
- Zhang, W.; Wu, W.; Bao, Y.; Yan, X.; Zhang, F.; Linhardt, R.J.; Jin, W.; Mao, G. Comparative study on the mechanisms of anti-lung cancer activities of three sulfated galactofucans. Food Funct. 2021, 12, 10644–10657.
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Thuy, T.T.; Van, T.T.; Ly, B.M.; Nifantiev, N.E.; Usov, A.I. Preliminary investigation of a highly sulfated galactofucan fraction isolated from the brown alga Sargassum polycystum. Carbohydr. Res. 2013, 377, 48–57.
- Camara, R.B.; Costa, L.S.; Fidelis, G.P.; Nobre, L.T.; Dantas-Santos, N.; Cordeiro, S.L.; Costa, M.S.; Alves, L.G.; Rocha, H.A. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs 2011, 9, 124–138.
- Rabanal, M.; Ponce, N.M.; Navarro, D.A.; Gomez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811.
- Shevchenko, N.M.; Usol′tseva, R.V.; Ishina, I.A.; Thinh, P.D.; Ly, B.M.; Ermakova, S.P. Structural Characteristics and in vitro Antitumor Activity of Water-Soluble Polysaccharides from Brown Algae of the Russian Far East and Vietnam. Chem. Nat. Compd. 2017, 53, 1–5.
- Medeiros, V.P.; Queiroz, K.C.; Cardoso, M.L.; Monteiro, G.R.; Oliveira, F.W.; Chavante, S.F.; Guimaraes, L.A.; Rocha, H.A.; Leite, E.L. Sulfated galactofucan from Lobophora variegata: Anticoagulant and anti-inflammatory properties. Biochemistry 2008, 73, 1018–1024.
- de Sousa Pinheiro, T.; Nascimento Santos, M.D.S.; Will Castro, L.S.E.P.; Paiva, A.A.D.O.; Alves, L.G.; Cruz, A.K.M.; Nobre, L.T.D.B.; Alves, M.G.D.C.F.; Leite, E.L. A fucan of a brown seaweed and its antitumoral property on HT-29 and immunomodulatory activity in murine RAW 264.7 macrophage cell line. J. Appl. Phycol. 2017, 29, 2061–2075.
- Queiroz, K.C.; Assis, C.F.; Medeiros, V.P.; Rocha, H.A.; Aoyama, H.; Ferreira, C.V.; Leite, E.L. Cytotoxicity effect of algal polysaccharides on HL60 cells. Biochemistry 2006, 71, 1312–1315.
- Usoltseva, R.V.; Anastyuk, S.D.; Ishina, I.A.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural characteristics and anticancer activity in vitro of fucoidan from brown alga Padina boryana. Carbohydr. Polym. 2018, 184, 260–268.
- Rocha, H.A.; Moraes, F.A.; Trindade, E.S.; Franco, C.R.; Torquato, R.J.; Veiga, S.S.; Valente, A.P.; Mourao, P.A.; Leite, E.L.; Nader, H.B.; et al. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schroederi: An ideal antithrombotic agent? J. Biol. Chem. 2005, 280, 41278–41288.
- Rocha, H.A.; Bezerra, L.C.; de Albuquerque, I.R.; Costa, L.S.; Guerra, C.M.; de Abreu, L.D.; Nader, H.B.; Leite, E.L. A xylogalactofucan from the brown seaweed Spatoglossum schroederi stimulates the synthesis of an antithrombotic heparan sulfate from endothelial cells. Planta Med. 2005, 71, 379–381.
- Nobre, L.T.; Vidal, A.A.; Almeida-Lima, J.; Oliveira, R.M.; Paredes-Gamero, E.J.; Medeiros, V.P.; Trindade, E.S.; Franco, C.R.; Nader, H.B.; Rocha, H.A. Fucan effect on CHO cell proliferation and migration. Carbohydr. Polym. 2013, 98, 224–232.
- Menezes, M.M.; Nobre, L.; Rossi, G.R.; Almeida-Lima, J.; Melo-Silveira, R.F.; Franco, C.R.C.; Trindade, E.S.; Nader, H.B.; Rocha, H.A.O. A low-molecular-weight galactofucan from the seaweed, Spatoglossum schroederi, binds fibronectin and inhibits capillary-like tube formation in vitro. Int. J. Biol. Macromol. 2018, 111, 1067–1075.
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165.
- Trinchero, J.; Ponce, N.M.; Cordoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomon, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother Res. 2009, 23, 707–712.
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Cordoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24.
- Usoltseva, R.V.; Malyarenko, O.S.; Anastyuk, S.D.; Shevchenko, N.M.; Silchenko, A.S.; Zvyagintseva, T.N.; Isakov, V.V.; Thinh, P.D.; Khanh, H.H.N.; Hang, C.T.T.; et al. The structure of fucoidan from Sargassum oligocystum and radiosensitizing activity of galactofucans from some algae of genus Sargassum. Int. J. Biol. Macromol. 2021, 183, 1427–1435.
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158.
- Jin, W.; Zhang, W.; Liang, H.; Zhang, Q. The Structure-Activity Relationship between Marine Algae Polysaccharides and Anti-Complement Activity. Mar. Drugs 2016, 14, 3.
- Jin, W.; Fang, Q.; Jiang, D.; Li, T.; Wei, B.; Sun, J.; Zhang, W.; Zhang, Z.; Zhang, F.; Linhardt, R.J.; et al. Structural characteristics and anti-complement activities of polysaccharides from Sargassum hemiphyllum. Glycoconj. J. 2020, 37, 553–563.
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476.
- Thuy, T.T.; Ly, B.M.; Van, T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128.
- Zhu, W.; Chiu, L.C.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Antiviral property and mode of action of a sulphated polysaccharide from Sargassum patens against herpes simplex virus type 2. Int. J. Antimicrob. Agents 2004, 24, 279–283.
- Zhu, W.; Chiu, L.C.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Antiviral property and mechanisms of a sulphated polysaccharide from the brown alga Sargassum patens against Herpes simplex virus type 1. Phytomedicine 2006, 13, 695–701.
- Zhu, W.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem. Cell Biol. 2003, 81, 25–33.
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.S.; Vaas, A.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; Lee, J.S.; et al. Fucoidan purified from Sargassum polycystum induces apoptosis through mitochondria-mediated pathway in HL-60 and MCF-7 cells. Mar. Drugs 2020, 18, 196.
- Wang, S.-H.; Huang, C.-Y.; Chen, C.-Y.; Chang, C.-C.; Huang, C.-Y.; Dong, C.-D.; Chang, J.-S. Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity. Biochem. Eng. J. 2021, 165, 107798.
- Luo, D.; Wang, Z.; Nie, K. Structural characterization of a novel polysaccharide from Sargassum thunbergii and its antioxidant and anti-inflammation effects. PLoS ONE 2019, 14, e0223198.
- Eluvakkal, T.; Shanthi, N.; Murugan, M.; Arunkumar, K. Extraction of antibacterial substances, galactofucoidan and alginate successively from the Gulf of Mannar brown seaweed Sargassum wightii Greville ex J. Agardh. Indian J. Nat. Prod. Resour. 2014, 5, 249–257.
- Maneesh, A.; Chakraborty, K. Pharmacological potential of sulfated polygalactopyranosyl-fucopyranan from the brown seaweed Sargassum wightii. J. Appl. Phycol. 2018, 30, 1971–1988.
- Surabhi, G.; Dhara, S.; Maneesh, A.; Chakraborty, K.; Valluru, L.; Chenchula, S.R. Polygalacto-fucopyranose from marine alga as a prospective antihypertensive lead. Int. J. Biol. Macromol. 2021, 183, 589–599.
- Ermakova, S.P.; Menshova, R.V.; Anastyuk, S.D.; Malyarenko, O.S.; Zakharenko, A.M.; Thinh, P.D.; Ly, B.M.; Zvyagintseva, T.N. Structure, chemical and enzymatic modification, and anticancer activity of polysaccharides from the brown alga Turbinaria ornata. J. Appl. Phycol. 2016, 28, 2495–2505.
- Menshova, R.V.; Anastyuk, S.D.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.I.; Zvyagintseva, T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015, 132, 118–125.
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Biotechnol. Appl. Biochem. 2011, 164, 841–850.
- Saha, S.; Navid, M.H.; Bandyopadhyay, S.S.; Schnitzler, P.; Ray, B. Sulfated polysaccharides from Laminaria angustata: Structural features and in vitro antiviral activities. Carbohydr. Polym. 2012, 87, 123–130.
- Shevchenko, N.M.; Anastyuk, S.D.; Menshova, R.V.; Vishchuk, O.S.; Isakov, V.I.; Zadorozhny, P.A.; Sikorskaya, T.V.; Zvyagintseva, T.N. Further studies on structure of fucoidan from brown alga Saccharina Gurjanovae. Carbohydr. Polym. 2015, 121, 207–216.
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776.
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014.
- Geng, L.; Hou, N.; Zhang, M.; Xu, Y.; Zhang, Q.; Wang, J.; Zhang, L.; Zhang, Q. Comparative study of the effect of different fucoidans from Sargassum maclurei and Saccharina japonica on FGFs/FGFR signaling activation in BaF3 cells. Int. J. Biol. Macromol. 2018, 107, 2429–2435.
- Geng, L.; Zhang, Q.; Wang, J.; Jin, W.; Zhao, T.; Hu, W. Glucofucogalactan, a heterogeneous low-sulfated polysaccharide from Saccharina japonica and its bioactivity. Int. J. Biol. Macromol. 2018, 113, 90–97.
- Wang, J.; Liu, H.; Jin, W.; Zhang, H.; Zhang, Q. Structure-activity relationship of sulfated hetero/galactofucan polysaccharides on dopaminergic neuron. Int. J. Biol. Macromol. 2016, 82, 878–883.
- Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated polysaccharide from Saccharina japonica suppresses LPS-induced inflammation both in a macrophage cell model via blocking MAPK/NF-κB signal pathways in vitro and a zebrafish model of embryos and larvae in vivo. Mar. Drugs 2020, 18, 593.
- Zhang, T.; Wu, S.; Ai, C.; Wen, C.; Liu, Z.; Wang, L.; Jiang, L.; Shen, P.; Zhang, G.; Song, S. Galactofucan from Laminaria japonica is not degraded by the human digestive system but inhibits pancreatic lipase and modifies the intestinal microbiota. Int. J. Biol. Macromol. 2021, 166, 611–620.
- Dhar, N.; Sarangapani, S.; Reddy, V.A.; Kumar, N.; Panicker, D.; Jin, J.; Chua, N.H.; Sarojam, R. Characterization of a sweet basil acyltransferase involved in eugenol biosynthesis. J. Exp. Bot. 2020, 71, 3638–3652.
- Schneider, T.; Ehrig, K.; Liewert, I.; Alban, S. Interference with the CXCL12/CXCR4 axis as potential antitumor strategy: Superiority of a sulfated galactofucan from the brown alga Saccharina latissima and fucoidan over heparins. Glycobiology 2015, 25, 812–824.
- Ehrig, K.; Alban, S. Sulfated galactofucan from the brown alga Saccharina latissima—Variability of yield, structural composition and bioactivity. Mar. Drugs 2014, 13, 76–101.
- Rioux, L.-E.; Moulin, V.; Beaulieu, M.; Turgeon, S.L. Human skin fibroblast response is differentially regulated by galactofucan and low molecular weight galactofucan. Bioact. Carbohydr. Diet. Fibre 2013, 1, 105–110.
- Hemmingson, J.A.; Falshaw, R.; Furneaux, R.H.; Thompson, K. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria Pinnatifida (Phaeophyta). J. Appl. Phycol. 2006, 18, 185–193.
- Wozniak, M.; Bell, T.; Denes, A.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540.
- Teng, H.; Yang, Y.; Wei, H.; Liu, Z.; Liu, Z.; Ma, Y.; Gao, Z.; Hou, L.; Zou, X. Fucoidan suppresses hypoxia-induced lymphangiogenesis and lymphatic metastasis in mouse hepatocarcinoma. Mar. Drugs 2015, 13, 3514–3530.
- Thakur, V.; Lu, J.; Roscilli, G.; Aurisicchio, L.; Cappelletti, M.; Pavoni, E.; White, W.L.; Bedogni, B. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget 2017, 8, 17887.
- Choi, Y.; Min, S.K.; Usoltseva, R.; Silchenko, A.; Zvyagintseva, T.; Ermakova, S.; Kim, J.K. Thrombolytic fucoidans inhibit the tPA-PAI1 complex, indicating activation of plasma tissue-type plasminogen activator is a mechanism of fucoidan-mediated thrombolysis in a mouse thrombosis model. Thromb. Res. 2018, 161, 22–25.
- Kim, W.-J.; Kim, S.-M.; Kim, H.G.; Oh, H.-R.; Lee, K.-B.; Lee, Y.-K.; Park, Y.-I. Purification and anticoagulant activity of a fucoidan from Korean Undaria pinnatifida sporophyll. Algae 2007, 22, 247–252.
- Lee, J.; Lee, S.; Synytsya, A.; Capek, P.; Lee, C.W.; Choi, J.W.; Cho, S.; Kim, W.J.; Park, Y.I. Low molecular weight mannogalactofucans derived from Undaria pinnatifida induce apoptotic death of human prostate cancer cells in vitro and in vivo. Mar. Biotechnol. 2018, 20, 813–828.
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-proliferation potential and content of fucoidan extracted from sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9.
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614.
- Cooper, R.; Dragar, C.; Elliot, K.; Fitton, J.; Godwin, J.; Thompson, K. GFS, a preparation of Tasmanian Undaria pinnatifida is associated with healing and inhibition of reactivation of Herpes. BMC Complementary Altern. Med. 2002, 2, 11.
- Burney, M.; Mathew, L.; Gaikwad, A.; Nugent, E.K.; Gonzalez, A.O.; Smith, J.A. Evaluation fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus in combination with anticancer drugs in human cancer orthotopic mouse models. Integr. Cancer Ther. 2018, 17, 755–761.
- Thompson, K.D.; Dragar, C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 551–555.
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094.
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza A viruses. Carbohydr. Polym. 2014, 111, 633–644.
- Wang, P.; Liu, Z.; Liu, X.; Teng, H.; Zhang, C.; Hou, L.; Zou, X. Anti-metastasis effect of fucoidan from Undaria pinnatifida sporophylls in mouse hepatocarcinoma Hca-F cells. PLoS ONE 2014, 9, e106071.
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283.
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48.
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20.
- Vo, T.-S.; Kim, S.-K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27.
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275.
