Fucoidans encompass versatile and heterogeneous sulfated biopolysaccharides of marine origin, specifically brown algae and marine invertebrates. The reported studies revealed diverse chemical skeletons in which l-fucose is the main sugar monomer. However, other sugars, i.e., galactose, mannose, etc., have been identified to be interspersed, forming several heteropolymers, including galactofucans/fucogalactans (G-fucoidans). Particularly, sulfated galactofucans are associated with rich chemistry contributing to more promising bioactivities than fucans and other marine polysaccharides. The previous reports in the last 20 years showed that G-fucoidans derived from Undaria pinnatifida were the most studied; 21 bioactivities were investigated, especially antitumor and antiviral activities, and unique biomedical applications compared to other marine polysaccharides were demonstrated.
- bioactives
- brown seaweeds
- fucoidans
- heteropolysaccharides
- structural features
- sulfated galactofucans
1. Introduction
2. Occurrence, Distribution, and Chemistry
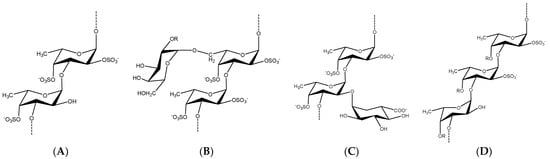
Brown seaweeds, in contrast to marine invertebrates, can synthesize more complicated, diverse, and heterogeneous fucoidan backbones, including glycosidic linkages, monomeric composition, and branching sites [35][36][37][53,54,55]. Therefore, various G-fucoidans with different fucose:galactose ratios have been reported in the different brown algae orders, including Fucales, Laminariales, and Dictyoales [38][39][56,57]. Traces of other sugars may be found, as in the case of Dictyota menstrualis [40][58] and Sargassum sp. [41][59]. Nevertheless, the presence of high percentages of glucose, i.e., fucose:galactose:glucose ratio of 1:0.3:0.25, may indicate contamination of the G-fucoidan with laminarin [42][60]. In such cases, fucoidans are partially purified by ethanol or cetyltrimethylammonium bromide (CTAB) precipitation and not purified by a specific chromatographic method, including anion exchange resin using diethylaminoethyl cellulose (DEAE-C) [43][61] or affinity chromatography [44][62]. In addition, previous studies, with the aid of advanced spectral analyses, i.e., 2D NMR (e.g., HMQC, TOCSY, and NOESY) and mass spectrometry, have attempted to reveal many structural features of G-fucoidans of various biogenic sources, including glycosidic linkages, sugar configuration, branching sites, sulfation pattern, and galactose position [6][45][46][6,63,64]. In addition, they could deduce tentative structure bioactivity relationships, as in the case of the anti-inflammatory mechanism of galactofucan isolated from Saccharina japonica [47][65]. The results of spectral analyses showed that α-l-fucopyranose (Fucp) and β-d-galactopyranose (Galp) are identified mainly, in which Fucp forms the major backbone and is linked via (1→4) and/or (1→3), while the β-d-galactopyranose molecules are found at branching sites, usually at (1→6), as in case of the G-fucoidan isolated from Hormophysa cuneiformis. In addition, the sulfation pattern is variable based on the glycosidic linkages. For instance, sulfate groups may occupy 2-O and 4-O in →3Fucp1→ or 2-O and 3-O in →4Fucp1→, in addition to 2-O in →3,4Fucp1→ [32][36]. Other models of sulfated galactofucans derived from Sargassum thunbergii were found to possess →3Fucp1→ as a main backbone with a 2-O-sulfated and 2,4-O-disulfated pattern, while the Galp residues interspersed Fucp in the main chain were linked mainly with →6Galp1→ and 4-O sulfation [48][46]. Moreover, G-fucoidan isolated from S. polycystum was built up mainly of a 4-O sulfated →3Fucp1→ backbone containing single →2Galp1→ residues sulfated similarly at the 4-O position [49][66]. Several other models are demonstrated in Figure 2 and Table 1 and in relation to their biomedical applications.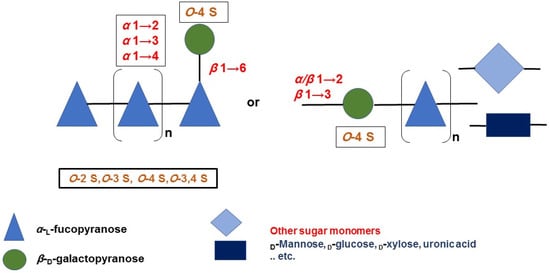
|
Brown Algae (Seaweed) Species |
Source of Seaweed Biomass |
Structural Characteristics |
References |
|||||
|---|---|---|---|---|---|---|---|---|
3.2. Antiviral Activity
Galactofucans show antiviral properties against a number of highly pathogenic viruses, including the human immunodeficiency virus (HIV-1) (Table 3). Table 3. Summarized antiviral activity of G-fucoidans with their respective sources and half-maximal effective or inhibitory concentrations (EC50/IC50). Comparisons with antiviral drugs are also shown.|
Source |
EC50/IC50 |
Compared with Antiviral Drugs? |
References |
|---|---|---|---|
|
Monosaccharide Composition |
Glycosidic Bonds of Backbone |
Molecular Weight (kDa) |
|
Source |
EC50/IC50 |
Compared with Standard/Commercial Compounds? |
References |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Fucose/Galactose Ratio | Sulfate Content (%) |
Sulfation Pattern |
|||||||||||||
|
Dictyotales |
|||||||||||||||
|
Adenocystis utricularis |
0.6–0.9 µg/mL (HIV-1) |
Yes. Superior to azidothymidine |
[61][ | ||||||||||||
|
Cystoseira compressa |
0.43 mg/mL (DPPH) |
Yes. Inferior to ascorbic acid and butylated hydroxyanisole [62], |
|||||||||||||
] | [ | ] |
Canistrocarpus cervicornis |
Wild |
Gal, fuc, glcAc, xyl, |
ND |
0.3 µg/mL (HSV-1) and 0.5 µg/mL (HSV-2) |
No2 |
[ 16.5 | ||||||
|
Sargassum siliquosum |
2.58 mg/mL (DPPH) | ND | |||||||||||||
No |
[10] |
Dictyota dichotoma |
Wild |
Gal, fuc, man, xyl, ara, rha, glc |
Dictyota dichotoma |
7.5 µg/mL (HSV-1), and 15.6 µg/mL (CVB3) |
Yes. Superior to ribavirin 23.6 |
1.5 |
33 |
ND |
[ |
||||
] | [ | 68] |
D. implexa |
Wild |
|||||||||||
|
Saccharina japonica |
0.001–0.005 µg/mL (HIV-1) |
Gal, fuc |
ND |
1 |
18.3 |
ND |
No |
||||||||
|
Lobophora variegata |
Wild |
Gal, fuc, Glc, man, xyl, glcAc; Gal, fuc; Gal, fuc, Glc |
(1,3)- and (1,4)-α-l-fuc, and (1,3)-β-d | ||||||||||||
|
S. thunbergii |
0.22 mg/mL (superoxide radical), and 0.88 mg/mL (hydroxyl radical) |
Yes. Similar (hydroxy radical) or superior (superoxide radical) to vitamin C |
[75 0.2–25 µg/mL (HSV-1)-gal |
Yes. Inferior to acyclovir and similar to heparin 35; ND; 1400 |
0.79; 0.5; 0.5 |
32.6; 0.2 *;15 |
|||||||||
] | [ | ] | [ | ] | |||||||||||
|
L. variegata |
ND |
Sargassum mcclurei |
0.96 µg/mL (HIV-1)Gal, fuc |
ND |
360–1600 |
0.3 |
23.3–35.5 |
ND |
[ |
Yes. Inferior to AMD3100 (plerixafor) |
|||||
[ | ][84] |
Padina boryana |
Wild |
||||||||||||
|
S. patens | Gal, fuc |
1.3 µg/mL (HSV-2), 5.5 µg/mL (HSV-1), and 4.1 µg/mL (HSV-1 acyclovir-resistant strain) |
No (1,4)-α-l-fuc, and (1,3)-β- |
[70]d-gal |
317.5/8.5 |
[85, |
1.1 |
18.6 |
At C2 and C4 (fuc and gal) |
||||||
|
Spatoglossum schroederi |
Wild |
||||||||||||||
|
>50 µg/mL (virucidal activity against HSV-2), 1.3–1.65 µg/mL (plaque formation), 1.85–3.5 µg/mL (inhibition of virus adsorption) | Gal, fuc, xyl, glcAc; Gal, fuc, xyl; |
(1,4)-β-d-gal, (1,4)-α-l |
No-fuc, and (1,4)-β-d-xyl |
21.5; 21.5–24 |
0.5; 0.5 |
19; 2.1–2.9 * |
At C3 (gal) and C4 (fuc) |
||||||||
|
Ectocarpales |
|||||||||||||||
|
1.5–5.5 mg/mL (HSV-1 replication) and 3–4 mg/mL (HSV-1 adsorption) |
Yes. Similar to acyclovir |
Adenocystis utricularis |
Wild |
Gal, fuc, rha, man; Gal, fuc, rha; Gal, fuc, man |
(1,3)-α-l-fuc |
>100 |
|||||||||
|
S. polycystum | 5.53; 4.82; 5.53 | 23; 24; 23 |
At C4 (fuc and gal) |
0.34 µg/mL (HIV-1) |
Yes. Inferior to AMD3100 (plerixafor) |
||||||||||
[ | ] | [ | ] | ||||||||||||
|
Scytosiphon lomentaria |
Scytosiphon lomentaria Wild |
Gal, fuc, rha, xyl, man, uronic acid |
(1,3)-α- |
0.76 µg/mL (HSV-1) and 1.34 µg/mL (HSV-2) |
No l-fuc, and (1,6)-β-d-gal |
8.5 |
7.33 |
29.5 |
At C3 and C4 (fuc), and C3 (gal) |
||||||
|
Fucales |
|||||||||||||||
|
Sphacelaria indica |
1.3 µg/mL (HSV-1) |
Yes. Superior to acyclovir when added to the overlay medium after penetration of the viruses into the host cell |
Cystoseira compressa |
Wild |
Gal, fuc |
(1,3)- and (1,4)-α-l-fuc |
|||||||||
|
Turbinaria ornata | 100 |
0.39 µg/mL (HIV-1) |
2.32 |
14.7 |
Yes. Inferior to AMD3100 (plerixafor) At C2 and C4 (fuc) |
||||||||||
[ |
Sargassum duplicatum |
Wild |
Gal, fuc |
(1,4)-α-l-fuc and β-d-gal (alternating) |
34–191 |
1 |
31.7 |
ND |
[14] |
||||||
|
S. feldmannii |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
183–184 |
2–2.6 |
25.3–32 |
At C2, C3 and C4 (fuc), and C2, C3, C4 and C6 (gal) |
||||||||
] | [ | ] |
S. fusiforme |
Wild |
Gal, fuc, xyl, Glc, glcAc, man, uronic acid; Gal, fuc, xyl, man, rha, glcAc, Glc |
(1,3)- and (1,4)-α-l-fuc |
90; 118.3/3.9 |
2; 3.7 |
17.5; 28.5 |
At C3 (fuc) |
|||||
|
S. hemiphyllum |
Wild |
Gal, fuc |
(1,6)-β-d-gal, (1,3)- and (1,4)-α-l-fuc, and (1,3)-β-d-gal |
148 |
4.5 |
32 |
At C2 and C4 (fuc) |
||||||||
|
S. mcclurei |
Wild |
Gal, fuc; Gal, fuc, man, xyl, glc |
(1,3)-α-l-fuc |
ND |
1.4; 2 |
35; 30.5 |
At C2 and C4 (fuc) |
||||||||
|
S. patens |
Wild |
Gal, fuc, man, xyl, Glc, galactosamine |
ND |
424 |
1.9 |
14.4 |
ND |
||||||||
|
S. polycystum |
Wild |
Gal, fuc, glc; Gal, fuc, man, xyl, glc |
(1,3)- | ||||||||||||
|
Undaria pinnatifida |
0.77 µg/mL (HSV-1) |
Yes. Superior to acyclovir |
|||||||||||||
|
32 µg/mL (HSV-1) and 0.5 µg/mL (HSV-2) |
Yes. Superior to acyclovir |
||||||||||||||
|
U. pinnatifida (sporophylls) |
2.5 µg/mL (HSV-1), 2.6 µg/mL (HSV-2), and 1.5 µg/mL (HCMV) |
No |
|||||||||||||
|
U. pinnatifida |
1.1 µg/mL (HSV-1), 0.1 µg/mL (HSV-2), and 0.5 µg/mL (HCMV) |
No |
|||||||||||||
|
3.1 µg/mL (HSV-1) and 1.6 µg/mL (HSV-2) |
No |
α-l-fuc, and (1,6)-β-d-gal |
39.5; ND |
5.84; 1.48 |
33.6; 23.4 |
At C2 and C4 (fuc) |
|||||||||
|
S. siliquosum |
Wild |
Gal, fuc, glc, xyl, man, rha; Gal, fuc, Glc, xyl, man, rha, uronic acid |
(1,3)- and (1,4)-α-l-fuc |
107.3; ND |
1.9; 1.9 |
19.5; 20 |
At C4 and C6 (gal) |
||||||||
|
S. thunbergii |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
7.2–333.5 |
5.26–5.88 |
27.2–30.1 |
At C2 and C4 (fuc), and C4 (gal) |
||||||||
|
S. thunbergii |
Purchased from local store |
Gal, fuc |
(1,4)-α-d-gal, and (1,3)-β-l-fuc |
373 |
1.2 |
ND |
NA |
||||||||
|
S. wightii |
Wild |
Gal, fuc, Glc, man; Gal, fuc |
(1,3)-α-l-fuc |
>3.5; ND |
0.6; 3–3.5 |
379.1 †; 8.1–19.5 |
At C2 and/or C4 (fuc), or C2 and C3 (gal) |
||||||||
|
Turbinaria ornata |
Wild |
Gal, fuc; Gal, fuc, man, xyl, glc |
(1,3)-α-l-fuc |
ND |
5; 1.2 |
32; 25.6 |
At C2 and/or C4 (fuc), and/or C2, C3, C4/C6 (gal) |
||||||||
|
Laminariales |
|||||||||||||||
|
Alaria angusta |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
ND |
1.1 |
24 |
At C2 (fuc), and C2 and C4 (gal) |
||||||||
|
Costaria costata |
Wild |
Gal, fuc, man, rha, xyl |
ND |
ND |
1.2 |
18.9 |
ND |
||||||||
|
Ecklonia cava |
Wild |
Gal, fuc, man, rha; Gal, fuc, rha, glc |
ND |
ND |
4.8; 3.6 |
19.1; 22.2 |
At C2 (fuc) |
||||||||
|
Laminaria hyperborea |
ND |
Gal, fuc |
(1,3)-α-l-fuc |
469 |
44.5 |
53.8 |
At C2 and C4 (fuc) |
[12] |
|||||||
|
Saccharina angustata |
Wild |
Gal, fuc, xyl, uronic acid |
(1,3)-, (1,4) and (1,2)-α-l-fuc |
56 |
9.1 |
4.2 |
At C4 (fuc and gal) |
||||||||
|
S. gurjanovae |
Wild |
Gal, fuc |
(1,3)-α-l-fuc |
123 |
3.2 |
25.1 |
At C2 and C4 (fuc), and C2 and/or C3 (gal) |
||||||||
|
S. japonica |
Wild |
Gal, fuc; Gal, fuc, man, xyl; Gal, fuc, man, rham, xyl; Gal, fuc, uronic acid, man, glcAc; Gal, fuc, Glc, man, rha, xyl; Gal, fuc, xyl, Glc, glcAc, rha, uronic acid |
(1,3)-α-l-fuc |
195/13.7; 1800; ND; 106.3; 23.5; 11 |
3.6; 1.1; 1.8; 9.1; 0.5; 10 |
21; 23.3; 23; 36.9; 18; 41.3 |
At C2 and C2/C4 (fuc) |
||||||||
|
S. japonica |
Cultivated |
Gal, fuc; Gal, fuc, man, rham, xyl, Glc; Gal, fuc, man, Glc, rha, xyl, uronic acid |
(1,3)- and (1,4)-α-l-fuc |
261.7; 131.5; 8.1 |
3.8; 2.1; 5.8 |
11.4; 9.1; 41.8 |
At C4 (fuc) |
||||||||
|
S. japonica |
Provided by Fujian Yida Food Co. |
Gal, fuc, man |
ND |
527.3 |
0.9 |
26.7 |
ND |
||||||||
|
S. japonica |
ND |
Gal, fuc |
(1,3)-α-l-fuc, and (1,6)-β-d-gal |
>10 |
3.5 |
48.3 |
At C4 and/or C2/C4 (fuc), and C4 and/or C3/C4 (gal) |
||||||||
|
S. latissima |
Wild |
Gal, fuc; Gal, fuc, xyl, man, Glc |
(1,3)-α-l-fuc |
416–449; 453 |
7.8; 4.1 |
0.8 ‡; 0.6 ‡ |
ND |
||||||||
|
S. longicruris |
Wild |
Gal, fuc, xyl, man, Glc, glcAc; Gal, fuc, xyl, man, Glc, galAc, glcAc |
1529; 638 |
0.8; 0.4 |
17.6; 19.1 |
At C4 (fuc), and C3 (gal) |
|||||||||
|
Undaria pinnatifida |
Wild |
Gal, fuc, man; Gal, fuc, rha; Gal, fuc, Glc, man, rha, xyl, ara |
(1,3)- or (1,4)-α-l-fuc |
ND; 290; ND |
1.1; 1.2; 1.3 |
29; 0.94 ‡; ND |
At C2, C3, C4 (fuc), or C2 and C4 (fuc and/or gal) |
||||||||
|
U. pinnatifida (sporophylls) |
Wild |
Gal, fuc, xyl, man |
(1,3)-α-l-fuc |
>150 |
1.5 |
15 |
ND |
||||||||
|
Cultivated |
Gal, fuc; Gal, fuc, man; Gal, fuc, xyl, man; Gal, fuc, man, xyl, uronic acid |
(1,3)-α-l-fuc, and (1,3)-, (1,4)-, (1,6)-β-d-gal |
ND; 1.4–3.7; 1246; 2100 |
1.4; 1.1; 1.1; 5 |
31; 8.4; 9.2; 7.4 |
At C2/C4 (fuc), and C3/C6 (gal) |
|||||||||
|
From mussel farms |
Gal, fuc, xyl, Glc, man; Gal, fuc, xyl, Glc, man, uronic acid |
171; >150 |
1.5; 1.5 |
15; 15 |
ND |
||||||||||
|
U. pinnatifida |
From Marine Resources Pty Ltd. |
ND |
ND |
ND |
ND |
||||||||||
|
From Marinova Pty Ltd. |
Gal, fuc, xyl, man |
(1,3)-α-l-fuc |
51.7 |
1.3 |
21.5 |
At C2 and C4 (fuc) |
|||||||||
|
ND |
ND |
ND |
ND |
ND |
|||||||||||
|
U. pinnatifida (sporophylls) |
ND |
Gal, fuc; Gal, fuc, uronic acid; Gal, fuc, xyl, man |
(1,3)-α-l-fuc, and (1,3)-, (1,4)-, (1,6)-β-d-gal |
9; 9; 104.4 |
0.9; 0.9; ND |
10.4; 10.4; 21 |
At C2 (fuc), and C3 and C6 (gal) |
||||||||
|
Sphacelariales |
|||||||||||||||
|
Sphacelaria indica |
Wild |
Gal, fuc, xyl, man, Glc |
(1,3)-α-l-fuc |
26 |
3.3 |
4 |
At C4 (fuc) |
||||||||
ND, not detailed; NA, not applicable; * reported as molar ratio to fucose; † reported as mg/g fucoidan; ‡ reported as degree of sulfation.
3. Potential Pharmacological Activities
3.1. Anticancer/Antitumor Activity
Several studies have reported the anticancer/antitumor activities of galactofucans in different cancer cell lines, as well as antiproliferative, antimetastasis, and antiangiogenic effects (Table 2).
Table 2. G-fucoidans showing anticancer/antitumor activity with their respective sources and half-maximal inhibitory concentrations (IC50). Comparisons with standard or commercial compounds are also shown.
|
Source |
IC50 |
Compared with Standard/Commercial Compounds? |
References |
|---|---|---|---|
|
Saccharina latissima |
0.35 µg/mL (elastase inhibition) |
Yes. Superior to commercial heparins (UFH and tinzaparin) |
|
|
Sargassum polycystum |
84.63 µg/mL (leukemia cells) and 93.62 µg/mL (breast cancer cells) |
No |
|
|
S. thunbergii |
29.7–93.5 μg/mL (inhibition of FGF1 binding) and 4.0–6.8 μg/mL (inhibition of FGF7 binding) |
No |
|
|
Undaria pinnatifida (sporophylls) |
0.10 mg/mL (breast adenocarcinoma) and 0.15 mg/mL (lung carcinoma) |
Yes. Superior to commercial fucoidan from Fucus for both cancer cell lines |
