Leucaena leucocephala (Lam.) de Wit is native to southern Mexico and Central America and is now naturalized in more than 130 countries. The spread of L. leucocephala is probably due to its multipurpose use such as fodder, timber, paper pulp, shade trees, and soil amendment. However, the species is listed in the world’s 100 worst invasive alien species, and an aggressive colonizer. It forms dense monospecific stands and threatens native plant communities, especially in oceanic islands. Phytotoxic chemical interactions such as allelopathy have been reported to play an important role in the invasion of several invasive plant species.
- invasive plant
- allelochemical
- mimosine
- phytotoxicity
- rhizosphere soil
1. Introduction

2. Allelopathy
Allelopathy is chemical interaction among plants and caused by allelochemicals [27], which are produced in plants and released into the vicinity of the plants including rhizosphere soil either by root exudation, decomposition of plant litter and residues, and rainfall leachates and volatilization from the plant parts [28][29][30].2.1. Plant Extract
Allelopathic activity of the extracts of leaves, seeds, bark, and aerial parts of L. leucocephala on crops and weeds were determined since allelochemicals are synthesized and accumulated in certain plant parts [27][28][29][30]. Aqueous extracts of the leaves of L. leucocephala suppressed the radicle growth of Lactuca sativa L. and Oryza sativa L. seedlings [31], and the seedling growth of Ischaemum rugosum Saisb and Vigna radiata (L.) R. Wilczek [32]. Its aqueous leaf and seed extracts showed the inhibitory activity on the germination and seedling growth of three weed species, Ageratum conyzoides L. Tridax procumbens L., and Emilia sonchifolia (L.) DC. Ex Wight [33]. Aqueous leaf, seed, and bark extracts of L. leucocephala inhibited the germination, growth, and crop yield of Zea mays L. under pot culture conditions [34].2.2. Leachate
For the simulation of rainfall conditions, plant tissues were soaked in water, and its supernatant was used as leaches from the tissues by rainfall [35][36][37][38][39]. The senescent leaves of L. leucocephala was soaked in water for 48 h, and its supernatant showed inhibitory activity on the germination and growth of Raphanus sativus L. [37]. The soaking water of L. leucocephala leaves also enhanced electrolyte leakage from the leaf cells of Eichhornia crassipes (Martius) Solms. and increased the activities of catalase and ascorbate peroxidase in the leaves [38].2.3. Plant Litter and Residue
Leaf litter of L. leucocephala was mixed with soil, and the seeds of a woody plant, Albiza procera (Roxb.) Benth., and crop plants, Vigna unguiculata (L.) Walp., Cicer arietinum L. and Cajanus cajan (L.) Millsp. were sown into the mixture. The treatments resulted in the suppression of the germination and growth of these test plant species [39]. Soil mixture with decomposing leaves of L. leucocephala increased the mortality of five tree species, Alnus formosana (Burkill) Makino, Acacia confusa Marr., Liquidambar formosana Hance, Casuarina glauca Sieber, and Mimosa pudica L. [31]. Aqueous extracts of L. leucocephala litter, which accumulated on the forest floors, showed the suppression of the germination and radicle growth of Lolium multiflorum Lam. [31], and Ageratum conyzoides L. Tridax procumbens L., and Emilia sonchifolia (L.) DC. ex Wight [40]. Leaf mulch of L. leucocephala covered on soil surface or mixed with soil inhibited the germination and growth of Vigna unguiculata (L.) Walp., and the root nodulation of V. unguiculate [41]. Those observations indicate that leaf litter and residues of L. leucocephala may contain certain allelochemicals, and some of them may be liberated into the soil during their decomposition processes.2.4. Rhizosphere Soil and Root Exudate
The seeds of Ageratum conyzoides L., Tridax procumbens L., and Emilia sonchifolia (L.) DC. ex Wight were sown into the soil collected from L. leucocephala infested areas. The treatments resulted in the suppression of the germination and growth of those plant species [40]. Rhizosphere soil of L. leucocephala also inhibited the germination and growth of Vigna radiata (L.) R.Wilczek and Glycine max L. [42]. Aqueous extracts of the soil of the forest floors under L. leucocephala trees showed the inhibition of the radicle growth of Lactuca sativa L. [31]. In addition, root exudates from L. leucocephala showed the suppression of the germination and growth of Ageratum conyzoides L., Tridax procumbens L., and Emilia sonchifolia (L.) DC. ex Wight [40]. Those observations suggest that rhizosphere soil of L. leucocephala may contain certain allelochemicals, which may be supplied through root exudation, decomposition of plant litter and residues, and rainfall leachates.3. Allelochemical
Phenolic acids, flavonoids, and mimosine were isolated and identified from L. leucocephala as its allelopathic agents (Figure 2).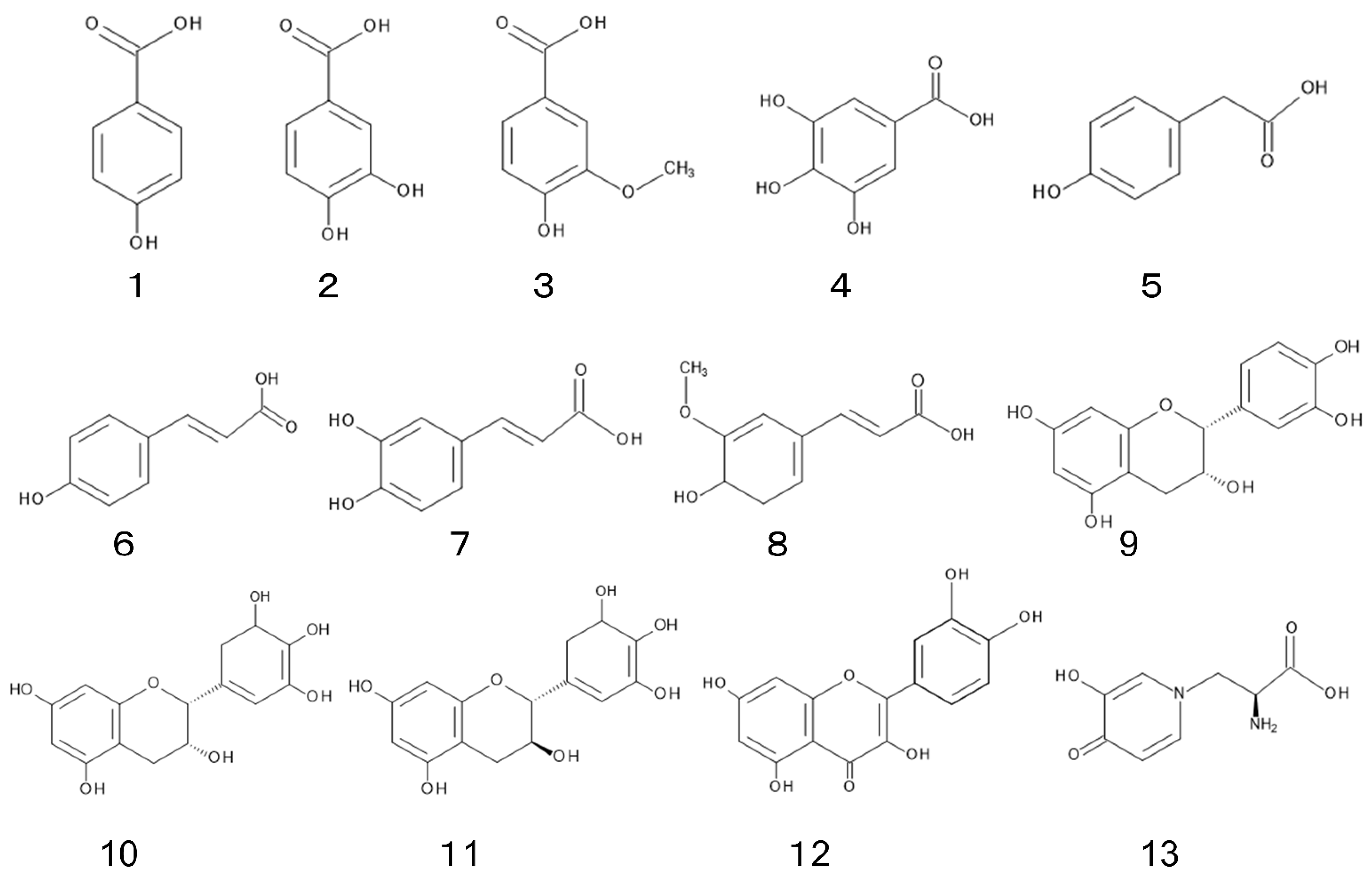
3.1. Phenolic Acid
Phenolic acids such as p-hydroxybenzoic acid (1), protocatechuic acid (2), vanillic acid (3), gallic acid (4), p-hydroxyphenylacetic acid (5), p-hydroxycinnamic acid (6), caffeic acid (7), and ferulic acid (8) were identified in the leaves of L. leucocephala. Total concentrations of those phenolic acids in young leaves were 2-fold greater than those in mature leaves [31]. The concentration of total phenolic compounds in L. leucocephala plants was estimated to be 1.3 to 2.8 mg g−1 of dry weight of the plants [43]. Phenolic acids have been found in a wide range of plants, plant residues, and soils, and their involvement has been often mentioned in the allelopathy of those plant species [44][45].
3.2. Flavonoid
Flavonoids such as epicatechin (9), epigallocatechin (10), and gallocatechin (11) were identified in L. leucocephala roots. Those compounds inhibited the nitrification process, which is an important step in the nitrogen cycle in soil [46]. Quercetin (12) and other 16 flavonoids were identified in L. leucocephala leaves, and some of them showed antioxidant activity [47]. L. leucocephala was reported to contain condensed tannins, which may contribute towards the plant resistance to pathogens and insects. [48][49][50].3.3. Mimosine
Mimosine (13); L-mimosine, synonym; leucenol) is a non-protein amino acid. It was first isolated from Mimosa pudica L [51], and found in some other species of the genus, Mimosa and Leucaena, including L. leucocephala [52][53][54]. Mimosine possesses a wide range of pharmacological and biological properties, such as anti-tumor, apoptotic, anti-inflammation, anti-viral, and cell cycle blocking effects [55]. It also possesses the inhibitory activity on the germination and growth of several plant species [31][56][57]. Therefore, mimosine is possibly involved in the allelopathy of L. leucocephala.4. Conclusions
Although the economic value of L. leucocephala is widely recognized, the species is listed in the world’s 100 worst invasive alien species. It is an aggressive colonizer and forms dense monospecific stands. It interferes the regeneration and replacement of native plant species in its dominant forests. The species richness in L. leucocephala invaded forests was lower than that in its uninvaded forests, and seedling establishment of native plant species under L. leucocephala invaded areas was also low. Sunlight intensity and other conditions of the forest floors between L. leucocephala forests and native forests were not apparent. In addition, plant extracts, leachates, root exudates, plant litter and residues, and rhizosphere soil of L. leucocephala showed the enhancement of the mortality and suppression of the germination and growth of several plant species including weeds and woody plants. Those observations suggest that L. leucocephala is allelopathic and contains allelochemicals which affect the plant mortality, germination, and growth, and some of the allelochemicals may be released into the vicinity of L. leucocephala, including its rhizosphere soil. L. leucocephala produces a large amount of mimosine and accumulates it in almost all parts of the plants. Mimosine showed growth inhibitory activity against several plant species including some shrubs and another invasive plant species. Mimosine blocked cell division of protoplasts from Petunia hybrida between G1 and S phases, and disturbed some enzyme activities such as peroxidase, catalase, and IAA oxidase. In addition, several phenolic acids and flavonoids were identified in L. leucocephala. However, the concentrations of mimosine, phenolic acids, and flavonoids in the rhizosphere soil and vicinity of L. leucocephala have not yet been reported. The information is essential to evaluate the contribution of mimosine, phenolic acids, and flavonoids to the allelopathy of L. leucocephala.References
- Invasive Species Compendium. Leucaena leucocephala (Leucaena). Available online: https://www.cabi.org/ISC/abstract/20127801953 (accessed on 8 April 2022).
- Global Invasive Species Database. Species Profile: Leucaena leucocephala. Available online: http://www.iucngisd.org/gisd/speciesname/Leucaena+leucocephala (accessed on 8 April 2022).
- National Research Council. Leucaena: Promising Forage and Tree Crop for the Tropics; The National Academies Press: Washington, DC, USA, 1984; pp. 1–129.
- Van den Beldt, R.J.; Brewbaker, J.L. Leucaena Wood Production and Use; Nitrogen Fixing Tree Association: Waimanalo, HI, USA, 1985; pp. 1–50.
- Verma, S. A review study on Leucaena leucocephala: A multipurpose tree. Int. J. Sci. Res. Sci. Eng. Technol. 2016, 2, 103–105.
- Abair, A.; Hughes, C.E.; Bailey, C.D. The evolutionary history of Leucaena: Recent research, new genomic resources and future directions. Trop. Grassl. Forrajes Trop. 2019, 7, 65–73.
- Sohtome, Y.; Tokunaga, T.; Ueda, K.; Yamamura, S.; Ueda, M. Leaf-closing substance in Leucaena leucocephala. Biosci. Biotechnol. Biochem. 2002, 66, 51–56.
- Walton, C. Leucaena (Leucaena leucocephala) in Queensland; Department of Natural Resources and Mines: Brisbane, QLD, Australia, 2003; pp. 1–51.
- Olckers, T. Biological control of Leucaena leucocephala (Lam.) de Wit (Fabaceae) in South Africa: A tale of opportunism, seed feeders and unanswered questions. Afr. Entomol. 2011, 19, 356–365.
- Brewbaker, J.L.; Hegde, N.; Hutton, E.M.; Jones, R.J.; Lowry, J.B.; Moog, F.; van den Beldt, R. Leucaena—Forage Production and Use; Nitrogen Fixing Tree Association: Waimanalo, HI, USA, 1985; pp. 1–39.
- Campbell, S.; Vogler, W.; Brazier, D.; Vitelli, J.; Brooks, S. Weed Leucaena and its significance, implications and control. Trop. Grassl. Forrajes Trop. 2019, 7, 280–289.
- Garcia, G.W.; Ferguson, T.U.; Neckels, F.A.; Archibald, K.A.E. The nutritive value and forage productivity of Leucaena leucocephala. Anim. Feed Sci. Technol. 1996, 60, 29–41.
- Wanapat, M.; Kang, S.; Polyorach, S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J. Anim. Sci. Biotechnol. 2013, 4, 32.
- De Angelis, A.; Gasco, L.; Parisi, G.; Danieli, P.P. A multipurpose Leguminous plant for the mediterranean countries: Leucaena leucocephala as an alternative protein source: A review. Animals 2021, 11, 2230.
- Durango, S.G.; Barahona, R.; Bolívar, D.; Chirinda, N.; Arango, J. Feeding strategies to increase nitrogen retention and improve rumen fermentation and rumen imcrobial population in beef steers fed with tropical forages. Sustainability 2021, 13, 10312.
- Anupam, K.; Swaroop, V.; Deepika, L.P.S.; Bist, V. Turning Leucaena leucocephala bark to biochar for soil application via statistical modelling and optimization technique. Ecol. Eng. 2015, 82, 26–39.
- Jha, P.; Neenu, S.; Rashmi, I.; Meena, B.P.; Jatav, R.C.; Lakaria, B.L.; Biswas, A.L.; Singh, M.; Patra, A.K. Ameliorating effects of Leucaena biochar on soil acidity and exchangeable Ions. Commun. Soil Sci. Plant Anal. 2016, 47, 1252–1262.
- Dalzell, S.A. Leucaena cultivars—Current releases and future opportunities. Trop. Grass. 2019, 7, 56–64.
- Khanna, N.K.; Shukla, O.P.; Gogate, M.G.; Narkhede, S.L. Leucaena for paper industry in Gujarat, India: Case study. Trop. Grassl. Forrajes Trop. 2019, 7, 200–209.
- Mac Dicken, K.G. Nitrogen Fixing Trees for Wastelands; FAO Regional Office for Asia and the Pacific—Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 1988; pp. 1–104.
- Hansted, A.L.S.; Nakashima, G.T.; Martins, M.P.; Yamamoto, H.; Yamaji, F.M. Comparative analyses of fast growing species in different moisture content for high quality solid fuel production. Fuel 2016, 184, 180–184.
- Nguyen, M.P.; Vaast, P.; Pagella, T.; Sinclair, F. Local knowledge about ecosystem services provided by trees in coffee agroforestry practices in northwest Vietnam. Land 2020, 9, 486.
- Yu, G.; Huang, H.Q.; Wang, Z.; Brierley, G.; Zhang, K. Rehabilitation of a debris-flow prone mountain stream in southwestern China—Strategies, effects and implications. J. Hydrol. 2012, 414–415, 231–243.
- Adhikary, P.P.; Hombegowda, H.C.; Barman, D.; Jakhar, P.; Madhu, M. Soil erosion control and carbon sequestration in shifting cultivated degraded highlands of eastern India: Performance of two contour hedgerow systems. Agrof. Syst. 2017, 91, 757–771.
- Ishihara, K.L.; Honda, M.D.H.; Bageel, A.; Borthakur, D. Leucaena leucocephala: A leguminous tree suitable for eroded habitats of Hawaiian Islands. In Ravine Lands: Greening for Livelihood and Environmental Security; Dagar, J., Singh, A., Eds.; Springer: Singapore, 2018; pp. 413–431.
- Standley, P.C. Trees and Shrubs of Mexico, Contributions from the United State National Herbarium; Government Printing Office: Washington, DC, USA, 1922; pp. 1–1721.
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578.
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326.
- Chou, C.H.; Kuo, Y.L. Allelopathic research of subtropical vegetation in Taiwan. J. Chem. Ecol. 1986, 12, 1431–1448.
- Boaprem, P. Allelopathic effects of leucaena leaves extract (Leucaena leucocephala (Lam.) de Wit) on the growth of rice (Oryza sativa L.), wrinkle duck-beak (Ischaemum rugosum Salisb), and mung bean (Vigna radiata (L.) R. Wilczek). Songklanakarin. J. Sci. Technol. 2019, 41, 619–623.
- Ishak, M.S.; Sahid, I. Allelopathic effects of the aqueous extract of the leaf and seed of Leucaena leucocephala on three selected weed species. AIP Conf. Proc. 2016, 1744, 020029.
- Sahoo, U.K.; Upadhyaya, K.; Meitei, C.B. Allelopathic effects of Leucaena leucocephala and Tectona grandis on germination and growth of maize. Allelopath. J. 2007, 20, 135–144.
- John, J.; Sreekumar, K.M.; Rekha, P. Allelopathic effects of leaf leachates of multipurpose trees on vegetables. Allelopath. J. 2007, 19, 507–516.
- Maiti, P.P.; Bhakat, R.K.; Bhattacharjee, A. Allelopathic effects of Lantana camara on physio-biochemical parameters of Mimosa pudica seeds. Allelopath. J. 2008, 22, 59–67.
- Kalpana, P.; Navin, M.K. Assessment of allelopathic potential of Leucaena leucocephala (Lam) De Vit on Raphanus sativus L. Int. J. Sci. Res. Publ. 2015, 5, 1–3.
- Chai, T.T.; Ooh, K.F.; Ooi, P.W.; Chue, P.S.; Wong, F.C. Leucaena leucocephala leachate compromised membrane integrity, respiration and antioxidative defense of water hyacinth leaf tissues. Bot. Stud. 2013, 54, 8.
- Ahmed, T.; Hoque, A.T.M.R.; Hossain, M.K. Allelopathic effects of Leucaena leucocephala leaf litter on some forest and agricultural crops grown in nursery. J. For. Res. 2008, 19, 298–302.
- Ishak, M.S.; Ismail, B.S.; Yusoff, N. Allelopathic potential of Leucaena leucocephala (Lam.) de Wit on the germination and seedling growth of Ageratum conyzoides L., Tridax procumbens L. and Emilia sonchifolia (L.) DC. Allelopath. J. 2016, 37, 109–122.
- Kamara, A.Y.; Akobundu, I.O.; Sanginga, N.; Jutzi, S.C. Effects of mulch from 14 multipurpose tree species (MPTs) on early growth and nodulation of cowpea (Vigna unguiculata L.). J. Agron. Crop Sci. 1999, 182, 127–133.
- Parvin, R.; Shapla, T.L.; Amin, M.H.A. Effect of leafs and Leucaena leucocephala different three depth soil on the allelopathy of agricultural crops. J. Innov. Dev. Strategy 2011, 5, 61–69.
- Sharma, P.; Chaurasia, S. Evaluation of total phenolic, flavonoid contents and antioxidant activity of Acokanthera oppositifolia and Leucaena leucocephala. Int. J. Pharmacogn. Phytochem. Res. 2014, 15, 175–180.
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202.
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principals and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74.
- Erickson, A.J.; Ramsewak, R.S.; Smucker, A.J.; Nair, M.G. Nitrification Inhibitors from the Roots of Leucaena leucocephala. J. Agric. Food Chem. 2000, 48, 6174–6177.
- Xu, Y.; Tao, Z.; Jin, Y.; Yuan, Y.; Dong, T.T.X.; Tsim, K.W.K.; Zhou, Z. Flavonoids, a potential new insight of Leucaena leucocephala foliage in ruminant health. J. Agric. Food Chem. 2018, 66, 7616–7626.
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565.
- Zhou, M.; Wei, L.; Sun, Z.; Gao, L.; Meng, Y.; Tang, Y.; Wu, Y. Production and transcriptional regulation of proanthocyanidin biosynthesis in forage legumes. Appl. Microbiol Biotechnol. 2015, 99, 3797–3806.
- Paengkoum, S.; Petlum, A.; Purba, R.A.P.; Paengkoum, P. Protein binding affinity of various condensed tannin molecular weights from tropical leaf peel. J. Appl. Pharm. Sci. 2021, 11, 114–120.
- Renz, J. Mimosine. Physiol. Chem. 1936, 244, 153–158.
- Brewbaker, J.L.; Hylin, J.W. Variations in mimosine content among Leucaena species and related mimosaceae. Crop Sci. 1965, 5, 348–349.
- Smith, I.K.; Fowden, L.A. Study of mimosine toxicity in plants. J. Exp. Bot. 1996, 17, 750–761.
- Soedarjo, M.; Borthakur, D. Mimosine produced by the tree-legume Leucaena provides growth advantages to some Rhizobium strains that utilize it as a source of carbon and nitrogen. Plant Soil 1996, 186, 87–92.
- Nguyen, B.C.; Tawata, S. The chemistry and biological activities of mimosine: A review. Phytother. Res. 2016, 30, 1230–1242.
- John, J.; Narwal, S.S. Allelopathic plants. 9. Leucaena Leucocephala (Lam.) De Wit. Allelopath. J. 2003, 12, 13–36.
- Hussain, M.I.; Gonzalez-Rodriguez, L.; Roger, M.R. Germination and growth response of four plant species to different allelochemicals and herbicides. Allelopath. J. 2008, 22, 101–110.
