You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Lord Abbey.
microalgae is an aquatic microorganism with a plethora of diverse bioactive compounds including phenolics, carotenoids, vitamin B12 and peptides. Microalgal bioactive compounds have been shown to possess positive health effects such as antihypertensive, anti-obesity, antioxidative, anticancer and cardiovascular protection.
- microalgae
- bioactive compounds
- functional food
1. Introduction
Functional role of foods has shifted from only the provision of energy and basic nutrients to include the supply of non-nutritive bioactive compounds capable of offering protection against the development of chronic diseases. Plant food groups (e.g., grains, legumes, nuts, fruits and vegetables, etc.) with these duo characteristics are defined as functional foods, which can be consumed as fresh foods, minimally processed or as ingredients in formulated foods [1]. However, with the increasing world population and consumer knowledge of the relationship between food intake and development of diseases, there is the need to exploit other food sources that can (i) sustain the functional food industry and (ii) meet the demanding health needs of the growing populace. In pursuit of this, an underexploited aquatic microorganism with potential functional food supply is microalgae.
Compared to terrestrial plants, microalgae are sustainable due to their rapid growth, ease of cultivation and non-competition for arable land. Microalgae are unicellular aquatic microorganisms with over 50,000 classified species. Some notable examples include Nostoc commune, Arthrospira platensis, Aphanizomenon flosaquae, Chlorella vulgaris and Chlorella pyrenoidosa [2,3][2][3]. Besides their appreciable levels of primary metabolites (e.g., protein, carbohydrates, polyunsaturated fatty acids and vitamins), the health benefits of microalgae are mainly correlated to the presence of high value secondary metabolites. Secondary metabolites are non-nutritive compounds produced in plants as defense agents against environmental stress [4]. Microalgae research has shown substantial concentrations of diverse secondary metabolites such as pigments (e.g., phenolics, carotenoids, etc.), phytosterols and mycosporine-like amino acids [5] (Figure 1).
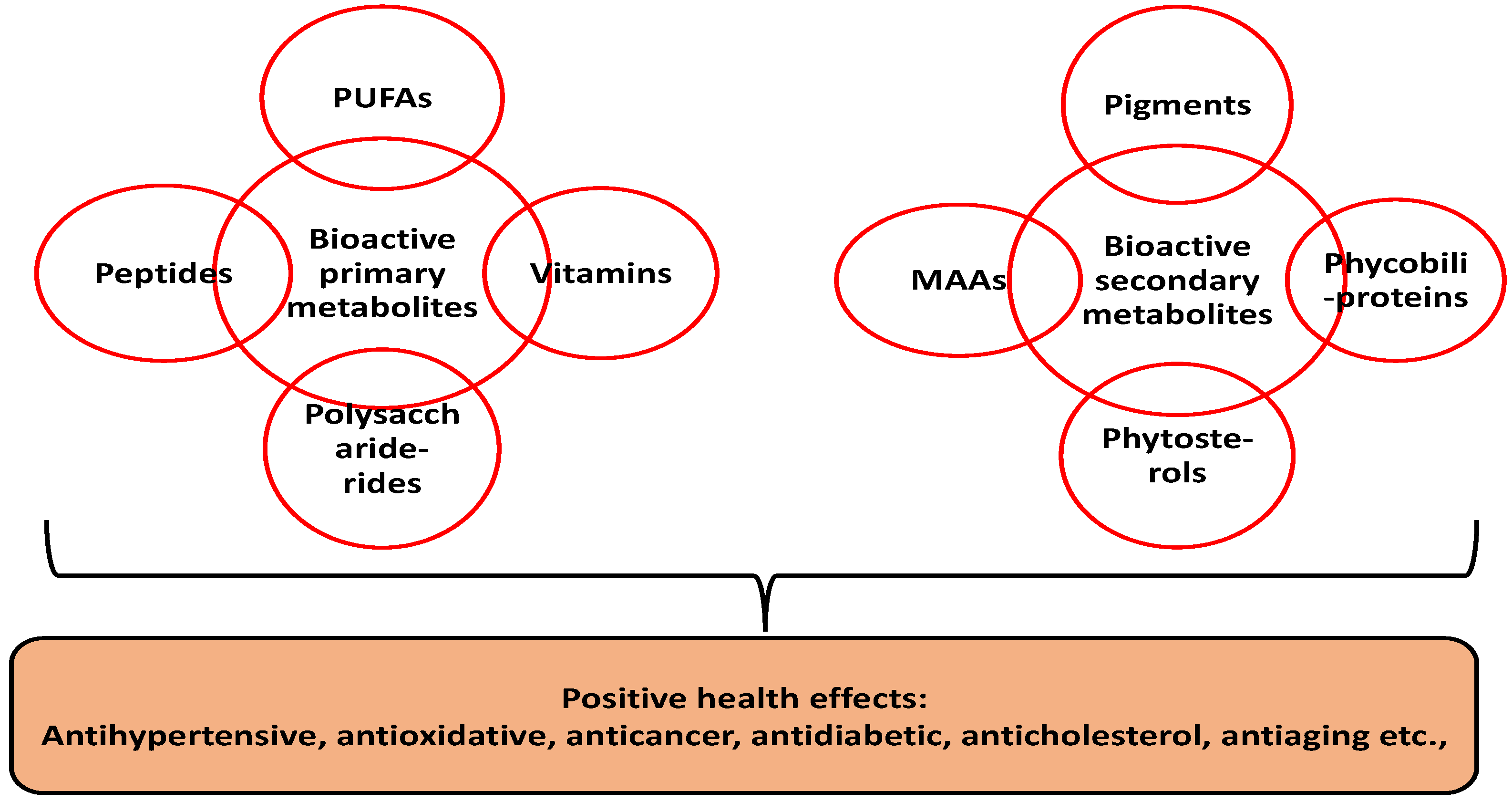
Figure 1.
Bioactive composition of microalgal biomass. PUFA—polyunsaturated fatty acids; MAAs—mycosporine-like amino acids.
2. High-Value Bioactive Primary Metabolites
2.1. Polyunsaturated Fatty Acids (PUFAs)
Polyunsaturated fatty acids (PUFAs) cannot be produced by the human body, hence, the need to obtain them through food consumption. They are divided into two groups namely omega-3 fatty acids (including α-linolenic, ALA; eicosapentaenoic acid, EPA; and docosahexaenoic acid, DHA) and omega-6 fatty acids (including arachidonic acid ARA; linoleic acid, LA; γ-linoleic acid, GLA; and conjugated linoleic acid, CLA) [10][6]. The health value of microalgae can partly be directed to its composition of PUFAs, which have been shown to promote brain and eye health, as well as protect against cardiovascular diseases, obesity, diabetes and arthritis [11][7]. Well-known PUFA microalgal producers include Crypthecodinium, Schizochytrium and Ulkenia sp. although other genera such as Phaeodactylum, Monodus, Nannochloropsis and Porphyridium have also shown considerable levels of DHA and EPA [12][8]. However, it is important to highlight that a majority of research data on microalgal PUFAs are reported with reference to their biofuel applications, with very limited reports on food, health and pharmaceutical applications. Considering the health benefits of PUFAs, coupled with the low consumer acceptance of fish oil PUFAs (i.e., low oxidative stability and high off-flavors), it can be postulated that there is the demand for alternative PUFAs. Hence, scientific data on microalgal PUFA profile, their bioactive properties and stabilities under different processing conditions will be very crucial in helping promote their applications in the functional food industry. In the study of Aussant et al. [13][9], eight species of microalgae were cultivated under different conditions (temperature—8, 14, 20 and 26 °C; time—5, 10 and 14 days). Of the eight investigated species, Nannochloropsis oculata and Isochrysis galbana reported the highest concentration of EPA (2.52 mg/L) and DHA (1.08 mg/L) at 20 °C/day 5 and 14 °C/day 5, respectively, with their investigated in vitro nutritional indices (i.e., hypocholesterolemic, atherogenic and thrombogenic indices) falling within accepted health ranges. Similarly, an increase in bicarbonate concentration from 2 to 8 mM increased the total PUFA content by 5.6% in Pavlova lutheri [14][10]. However, increasing light intensity from 37.7 to 100.0 µmol/m2/s in Chlorella vulgaris reduced DHA and EPA levels by 50 and 70%, respectively [15][11].
2.2. Polysaccharides
From the review of Mourelle et al. [16][12], microalgal polysaccharides are largely exploited from the genera Porphyridium, Phaeodactylum, Chlorella, Tetraselmis, Isochrysis and Rhodella. In microalgae, polysaccharides function as protection agents, energy reservoirs and structural molecules, and are divided into pectins, glycol-protein, sulfated polysaccharides (SPS) and homo- and hetero-polysaccharides [17,18][13][14]. Among these polysaccharide groups, the most widely reported is the sulfated group with findings mainly reported on their anti-inflammatory benefits. In the study of Matsui et al. [19][15], extracts of sulfated polysaccharides from Porphyridium showed in vitro migratory inhibition of leukocytes to inflammation sites. These authors also observed in vivo microalgal inhibition against erythema development. Few antioxidant studies have also been reported with microalgae polysaccharides, as further discussed in Section 4 of the text. It is also important to highlight that microalgal polysaccharides are often exploited for their techno-functional applications, compared to their health benefits, thus, making it difficult to correlate their structure with health effects. Therefore, future studies focusing on the structure–activity relationship between microalgal polysaccharides and potential bioactive effects are highly recommended.
2.3. Vitamins
Although vitamins are essential elements required for proper human development, they can only be obtained through diets or supplements. Microalgae are excellent potential source of vitamins, compared to some well-known sources such as orange, carrot and soy flour [20][16]. Although microalgae are not natural producers of vitamin A, it is interesting to note that microalgae can accumulate vitamin A precursors such as carotenes (i.e., α- and β-carotenes) and retinol, which have been demonstrated to protect against the development of some cancer types [21][17]. The recent study of Ljubic et al. [22][18] investigated the accumulation of vitamin D3 (cholecalciferol) in Nannochloropsis oceanica, Arthrospira maxima, Rhodomonas salina and Chlorella minutissima upon exposure to different doses of ultraviolet B (0, 15, 22 and 36 kJ/m2/day) for 7 days. The authors observed the highest level of vitamin D3 (1 µ/g dry weight) with Nannochloropsis oceanica at UV-B dose of 36 kJ/m2/day, compared to the control (< 0.004 µ/g DW). Edelmann et al. [23][19] reported vitamin B9 contents in formulated powders of Chlorella sp. and Nannochloropsis sp. to be 25.9 and 20.8 µg/g, respectively. It can therefore be postulated that the consumption of about 5 g of Chlorella and Nannochloropsis microalgae powder can provide a quarter of the recommended daily intake (i.e., 400 µg/d) of vitamin B9. According to Tarento et al. [24][20], cyanobacteria has about 200 µg/g of vitamin K1, being about six times higher than levels reported for parsley (i.e., 37 µg/g), a well-known vitamin K1 food source. Hence, adult daily consumption of 1 g cyanobacteria will provide three times their daily needs for vitamin K1. Another crucial vitamin imperative for good health, especially among the aged is vitamin B12, although it is limited in plant foods [25][21]. Nevertheless, Edelmann et al. [23][19] observed Chlorella sp. to contain 2.4 µg/g of vitamin B12, concluding that 5 g Chlorella powder will provide five times the daily requirement of vitamin B12. A further important observation is that literature on bioaccessibility and bioavailability of microalgal vitamins is very limited. Thus, the need for deeper studies to enable health-regulating agencies and food industries to approve and include microalgae in the formulation of functional foods.
2.4. Peptides
Peptides are short chain amino acids (i.e., 20–50 units) linked together by peptide bonds [26][22]. According to Khanra et al. [27][23], 50% of the global protein and peptide market is currently sourced from terrestrial plants and may be replaced by proteins from microalgae and insects by 2054. Considering this trend, microalgal peptides has been exploited from Chlorella, Navicula, Tetraselmis and Nitzschia [2]. Ko et al. [26][22] isolated a pentapeptide with the amino acid sequence Leu-Asn-Gly-Asp-Val-Trp from Chlorella ellipsiodea, and reported appreciable peroxyl radical, 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and hydroxyl radical scavenging capacities with half maximal inhibitory concentration values (IC50) of 0.02, 0.92 and 1.42 mM, respectively. Two isolated peptides from Nannochloropsis oculata with an amino acid sequence of Gly-Met-Asn-Asn-Leu-Thr-Pro and Leu-Glu-Gln were found to possess anti-hypertensive properties by inhibiting the activity of angiotensin-converting enzyme (ACE) at IC50 values of 123 and 173, respectively [28][24]. According to these authors, microalgal peptides can exhibit antihypertensive properties through the (i) inhibition of ACE, the main enzyme responsible for vasoconstriction of veins and arteries (ii) triggering of vasodilation effect, i.e., capacity to increase nitric oxide levels through the stimulation of the endothelial nitric oxide synthase pathway.
References
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.Y. Food flavonols: Nutraceuticals with Complex Health Benefits and Functionalities. Trends Food Sci. Technol. 2021, 117, 194–204.
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-added Molecules Production by Microalgae in Light of the Classification. Biotechnol. Adv. 2020, 41, 1–21.
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with Microalgae and Seaweeds Fostering Consumers Health: A Review on Scientific and Market Innovations. J. Appl. Phycol. 2020, 32, 1789–1802.
- Ampofo, J.O.; Ngadi, M. Ultrasonic Assisted Phenolic Elicitation and Antioxidant Potential of Common Bean (Phaseolus vulgaris) Sprouts. Ultrason. Sonochem. 2020, 64, 1–11.
- Sidari, R.; Tofalo, R. A Comprehensive Overview on Microalgal Fortified/based Food and Beverages. Food Rev. Int. 2019, 35, 778–805.
- Buckley, M.T.; Racimo, F.; Allentoft, M.E.; Jensen, M.K.; Jonsson, A.; Huang, H.; Hormozdiari, F.; Sikora, M.; Marnetto, D.; Eskin, E.; et al. Selection in Europeans on Fatty Acid Desaturases Associated with Dietary Changes. Mol. Biol. Evol. 2017, 34, 1307–1318.
- Dolganyuk, V.; Andreeva, A.; Budenkova, E.; Sukhikh, S.; Babich, O.; Ivanova, S.; Prosekov, A.; Ulrikh, E. Study of Morphological Features and Determination of the Fatty Acid Composition of the Microalgae Lipid Complex. Biomolecules 2020, 10, 1571.
- Sahin, D.; Tas, E.; Altindag, U.H. Enhancement of Docosahexaenoic Acid (DHA) Production from Schizochytrium sp. S31 Using Different Growth Medium Conditions. AMB Express 2018, 8, 1–8.
- Aussant, J.; Guiheneuf, F.; Stengel, B.D. Impact of Temperature on Fatty Acid Composition and Nutritional Value in Eight Species of Microalgae. Appl. Microbiol. Biotechnol. 2018, 102, 5279–5297.
- Freddy, G.; Dagmar, B.S. LC-PUFA-Enriched Oil Production by Microalgae: Accumulation of Lipid and Triacylglycerols Containing n-3 LC-PUFA Is Triggered by Nitrogen Limitation and Inorganic Carbon Availability in the Marine Haptophyte Pavlova lutheri. Mar. Drugs 2013, 11, 4246–4266.
- Khoeyi, Z.A.; Seyfabadi, J.; Ramezanpour, Z. Effect of Light Intensity and Photoperiod on Biomass and Fatty Acid Composition of the Microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49.
- Mourelle, M.L.; Gomez, C.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46.
- Nguyen, V.D. Marine Glycans in Relationship with Probiotic Microorganisms to Improve Human and Animal Health. In Marine Glycobiology: Principles and Applications; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 67–84.
- Chandrarathna, H.P.S.U.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S.; Thulshan, E.H.T.; Nikapitiya, C.; Oh, C.; Kang, D.H.; De Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175.
- Matsui, S.M.; Muizzudin, N.; Arad, S.M.; Marenus, K. Sulfated Polysaccharides from Red Microalgae Anti-inflammatory Properties In-vitro and In-vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22.
- Grossman, A. Nutrient Acquisition: The generation of Bioactive Vitamin B12 by Microalgae. Curr. Biol. 2016, 26, R319–R337.
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 80, 16–24.
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a Future New Natural Source of Vitamin D3. Food Chem. 2020, 320, 1–7.
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, Niacin, Folate and Vitamin B12 in Commercial Microalgae Powders. J. Food Compos. Anal. 2019, 82, 1–10.
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a Source of Vitamin K1. Algal Res. 2018, 36, 77–87.
- Amorim, M.L.; Soares, J.; dos Reis Coimbra, J.S.; de Oliveira Leite, M.; Teixeira Albino, L.F.; Arêdes, M. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2020, 61, 1976–2002.
- Ko, S.C.; Kang, N.; Kim, E.A.; Kang, M.C.; Lee, S.H.; Kang, S.M.; Lee, J.B.; Jeon, B.T.; Kim, S.K.; Park, S.J.; et al. A Novel Angiotensin-I-Converting Enzyme (ACE) Inhibitory Peptide from a Marine Chlorella ellipsoidea and Its Antihypertensive Effect in Spontaneously Hypertensive Rats. Process Biochem. 2012, 470, 2005–2011.
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Food Bioprod. Process. 2018, 110, 60–84.
- Ying, K.; Gilmour, D.J.; Zimmerman, W.B. Effects of CO2 and pH on Growth of the Microalga Dunaliella salina. J. Microb. Biochem. Technol. 2014, 6, 167–173.
More
