You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Ariadna Delgado-Bermúdez.
Aquaporins (AQPs) are a family of transmembrane channels that allow the transport of water and small solutes across cell membranes. Different members of this family have been identified in gametes. In sperm, they are relevant to osmoadaptation after entering the female reproductive tract, which is crucial for sperm motility activation and capacitation and, thus, for their fertilizing ability. In addition, they are relevant during the cryopreservation process, since some members of this family are also permeable to glycerol, one of the most frequently used cryoprotective agents in livestock. Regarding oocytes, AQPs are very important in their maturation but also during cryopreservation.
- mammals
- oocyte
- sperm
- water channels
- cryopreservation
1. The Family of Aquaporins
Aquaporins (AQPs) are a family of transmembrane proteins that are present ubiquitously in all species and cell types whose main function is to allow the transport of water across cell membranes [5][1].
The members of the AQP family present a highly conserved gene sequence, structure and function. In terms of structure, AQPs present six transmembrane α-helices (TM1-6; Figure 1A) surrounding a central pore that, when resolved through X-ray, shows an “hourglass” shape (Figure 1B) [11,12][2][3]. Transmembrane segments are connected by loops (A–E); loops B and E each present an NPA motif (asparagine, proline, alanine), which is the typical AQP signature motif [13][4]. The two loops that present an NPA motif are half-transmembrane helices that locate towards the center of the channel and create a broken seventh-transmembrane helix (Figure 1A,B). Water molecules cross AQPs via a single-molecule well and interact with the lateral chains of the amino acids that form the channel through hydrogen bonds. In this sense, the NPA motif disrupts any potential proton conduction locking the central molecule of water in a conformation that avoids molecular reorientation [14][5]. Another highly preserved feature in AQP structure is the selectivity filter, which is the narrowest point in the channel and is considered to be essential for channel selectivity [14][5]. The region near these residues is also known as aromatic/arginine (ar/R) constriction region (Figure 1A), since the arginine is a highly conserved residue among all AQPs [15][6].
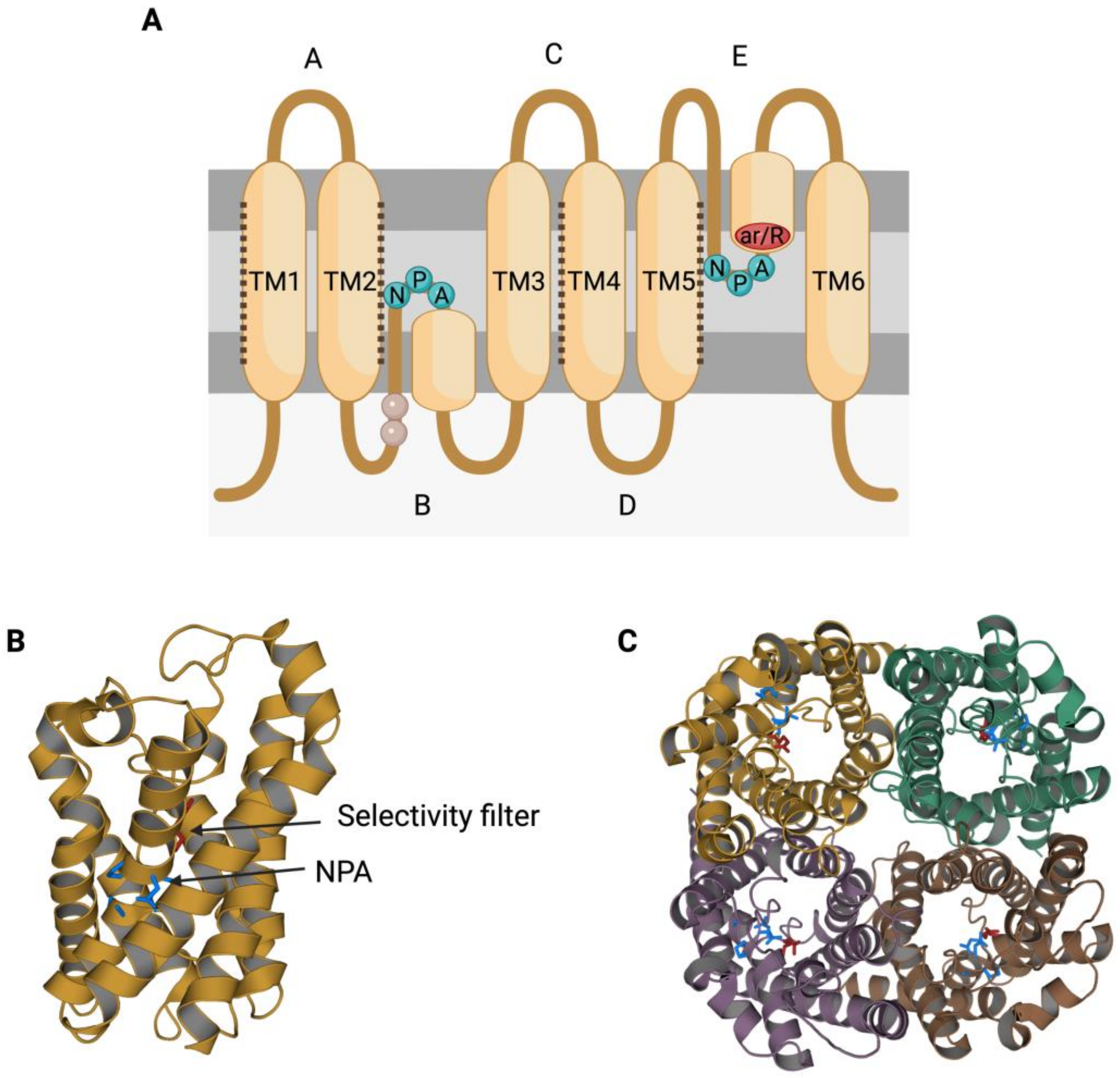

Figure 1. Structural characteristics of the family of aquaporins (AQPs). (A) Aquaporins present six transmembrane α-helices (TM1-6) that are connected through loops (A–E). Loops B and E are half-transmembrane helices oriented towards the center of the pore and present an NPA (asparagine, proline, alanine) motif that is highly conserved. In loop E, there is also a highly conserved arginine (R). Some residues from loop B have been suggested to be involved in AQPs’ mechanosensitivity (light residues). Transmembrane helices TM1, TM2, TM4 and TM5 interact with the TM of the adjacent monomers from an AQP tetramer (dark dot lines). (B) Each monomer folds in an hour-glass conformation, and the region near the R from loop E is formed by aromatic residues. This region is known as the aromatic/arginine (ar/R) region but also as a selectivity filter since it forms the narrowest point of the AQP pore. After folding, the two NPA motifs are in the same region, which is also highly conserved. (C) The quaternary structure of AQPs consists of the formation of tetramers, where each monomer has its own functional pore. This figure is based on PDB structure 6QZJ from AQP7.
Aquaporins (AQPs) function as tetrameric structures formed by four monomers, each with their own permeable pore (Figure 1C). Moreover, tetrameric structures appear to be crucial for AQPs’ mechanosensitivity (Figure 2), as it has been described for AQP1 and AQP4 [17,18][7][8].
In spite of having a highly preserved sequence and structure, the separate members of the AQP family differ in certain structural features and specific permeability to water and other molecules. AQPs can, therefore, be classified into three different groups based on variations in their structure and permeability: orthodox AQPs, aquaglyceroporins (GLPs) and superAQPs (Figure 3).
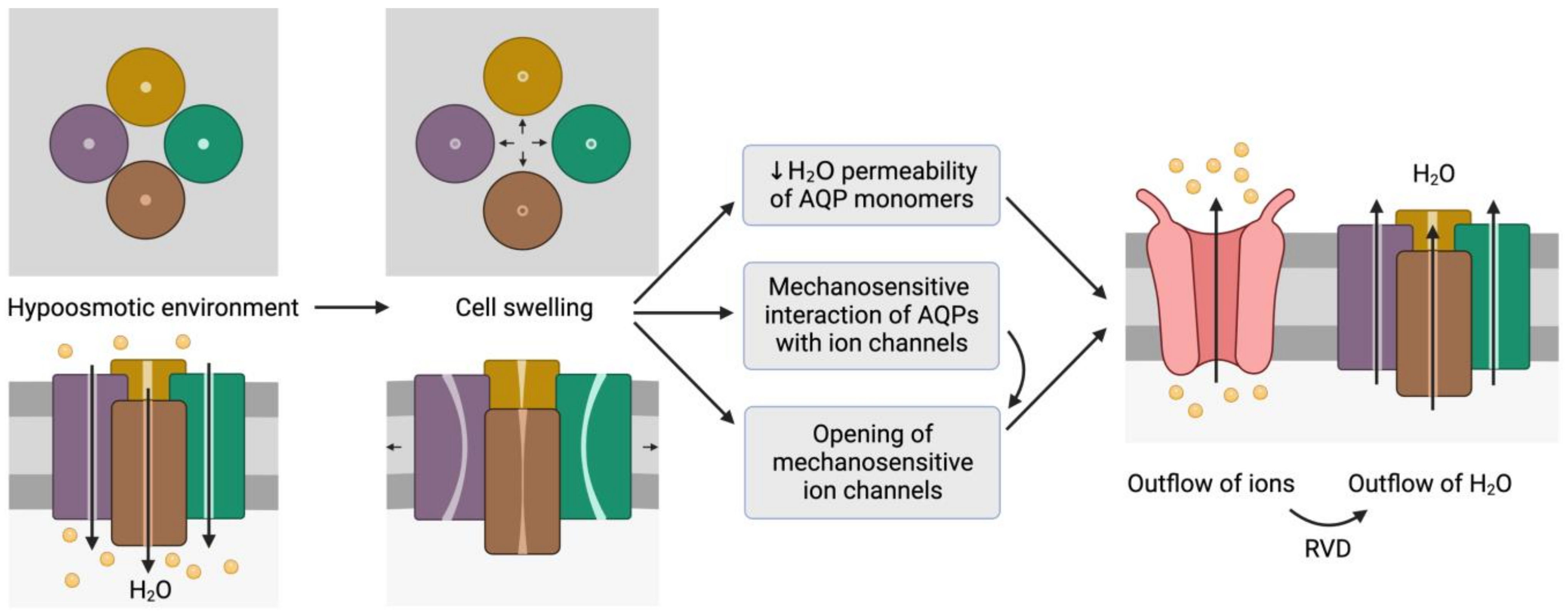
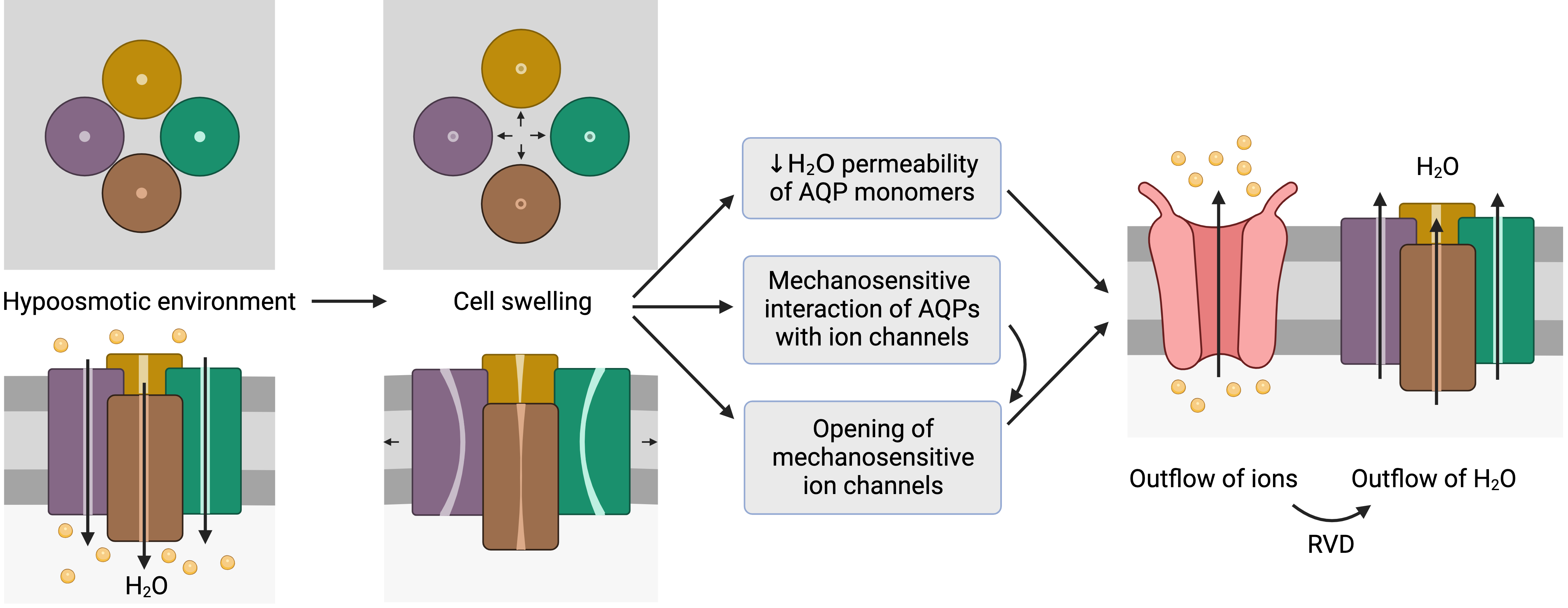


Figure 2. Representation of the mechanosensitive mechanism of aquaporins (AQPs). When cells are in a hypoosmotic environment, water enters them in response to the concentration gradient of solutes. Due to the entry of water, cells may undergo swelling, which can cause the distortion of AQP structures and thus compromise water permeability. In addition, AQPs may have mechanosensitive interactions with ion channels that can trigger their opening to trigger the outflow of ions. In fact, some ion channels are mechanosensitive and open in response to cell swelling, regardless of AQP signaling. The outflow of ions generates a driving force that elicits the outflow of water; this process is also known as regulatory volume decrease (RVD). This schematic representation is inspired by the model proposed by Hill et al. [20][9] and its representation by Ozu et al. [16][10].
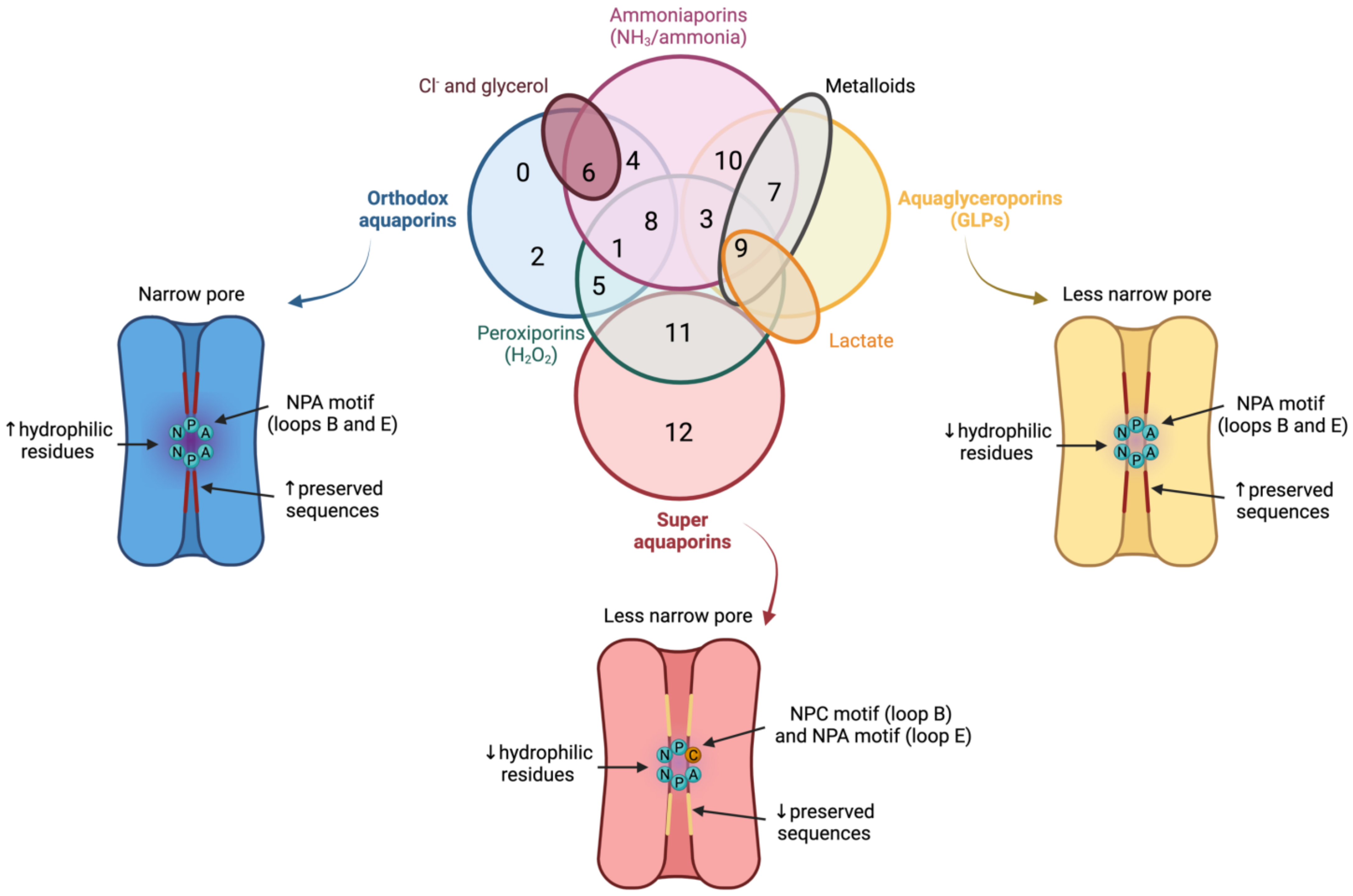
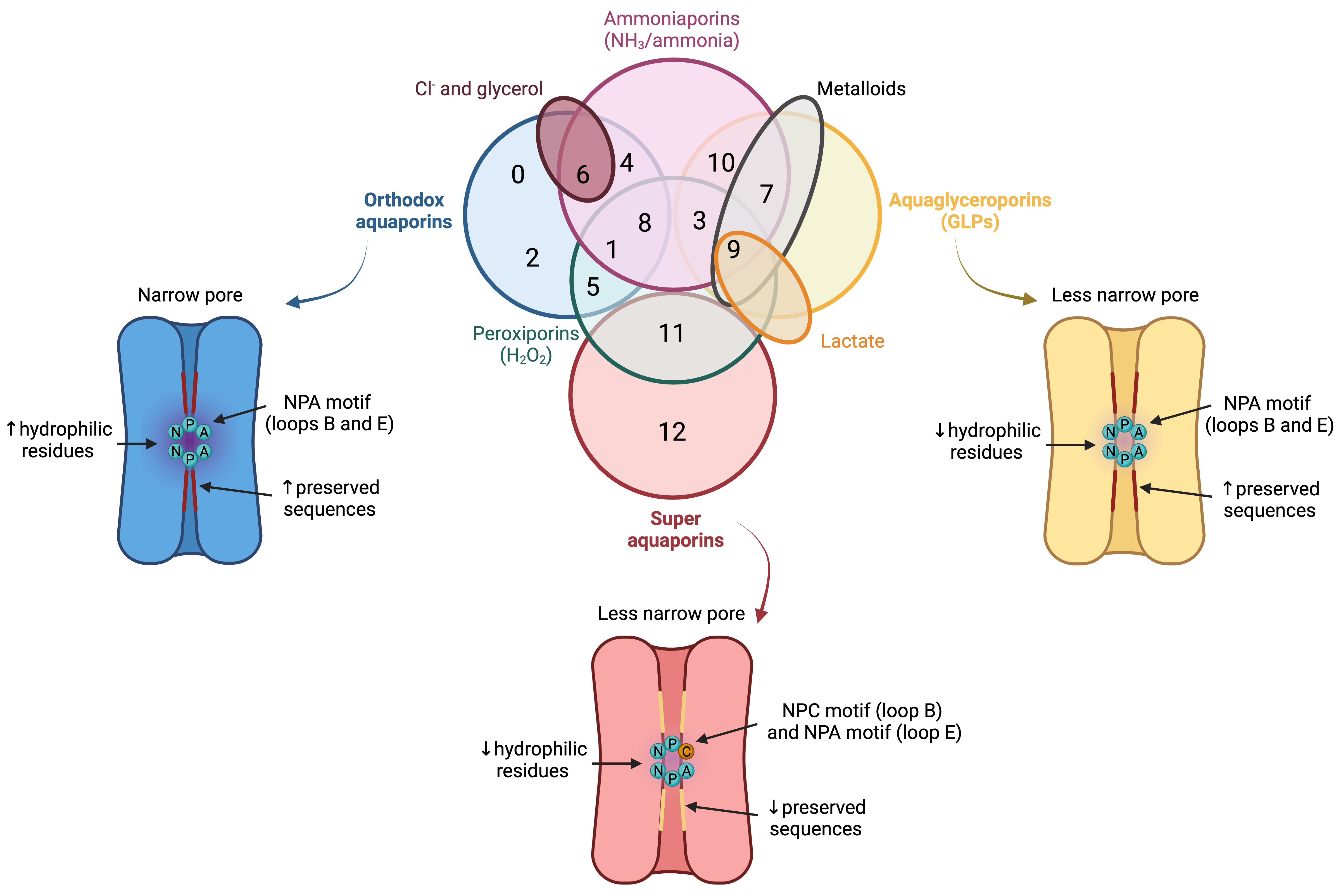


Figure 3. Classification of aquaporins (AQPs) based on their homology and specific permeability to different molecules. The three main groups into which AQPs are classified are: orthodox AQPs (blue), aquaglyceroporins (GLPs; yellow) and superaquaporins (superAQPs; red). The structural characteristics of these three groups are graphically represented. In addition to the classical classification, some AQPs present permeability to H2O2 and they are considered peroxiporins (green). Similarly, some members of this family of proteins are considered ammoniaporins (pink) since they are permeable to ammonia and/or to NH3. Finally, certain members present permeability to other molecules, such as Cl− (dark red), metalloids (grey) or lactate (orange). NPA motif (asparagine, proline, alanine); NPC motif (asparagine, proline, cysteine). Image based on [21,22,23]. (dark red), metalloids (grey) or lactate (orange). NPA motif (asparagine, proline, alanine); NPC motif (asparagine, proline, cysteine). Image based on [11][12][13].
1.1. Orthodox Aquaporins (AQPs)
The group of orthodox AQPs includes AQP0, AQP1, AQP2, AQP4, AQP5, AQP6 and AQP8. Orthodox AQPs present the smallest channel size among the different groups of AQPs and their selectivity filter has a highly hydrophilic nature [14][5]. These two characteristics are the reason for the exclusive permeability of this group of AQPs to water through a steric mechanism of selectivity. In addition, the NPA motif also plays a function in selectivity because, in orthodox AQPs, the side chains of the amino acids of this motif narrow the pore to a smaller diameter and are more hydrophilic than those of the other family members [14][5]. The only exception in this group is AQP6, which also presents anion permeability at low pH [25][14] and localizes in the membrane of intracellular organelles; remarkably, this differs from the other orthodox AQPs that localize in the plasma membrane [26][15].
1.2. Aquaglyceroporins (GLPs)
The group of GLPs includes AQP3, AQP7, AQP9 and AQP10. Water molecules cross GLPs through the establishment of the same interactions as with orthodox AQPs and also form a single-molecule well. In GLPs, nevertheless, the diameter of the pore, which is limited by the NPA motif, is larger than that of orthodox AQPs. In addition, the selectivity filter has a hydrophobic patch of residues and the region near the NPA motif is less hydrophilic than in orthodox AQPs, which determines the lower hydrophilicity of this group of AQPs [14][5].1.3. Superaquaporins (superAQPs)
The last group, also known as superAQPs, includes AQP11 and AQP12, which are expressed in intracellular membranes [30,31][16][17]. In fact, they present an endoplasmic reticulum (ER) retention signal at the carboxy-terminal region, and AQP11 has been identified in the ER when exogenously expressed in mammalian cells [30][16].2. Aquaporins in Mammalian Sperm
3.1. Sperm Aquaporins (AQPs) and Osmoregulation
2.1. Sperm Aquaporins (AQPs) and Osmoregulation
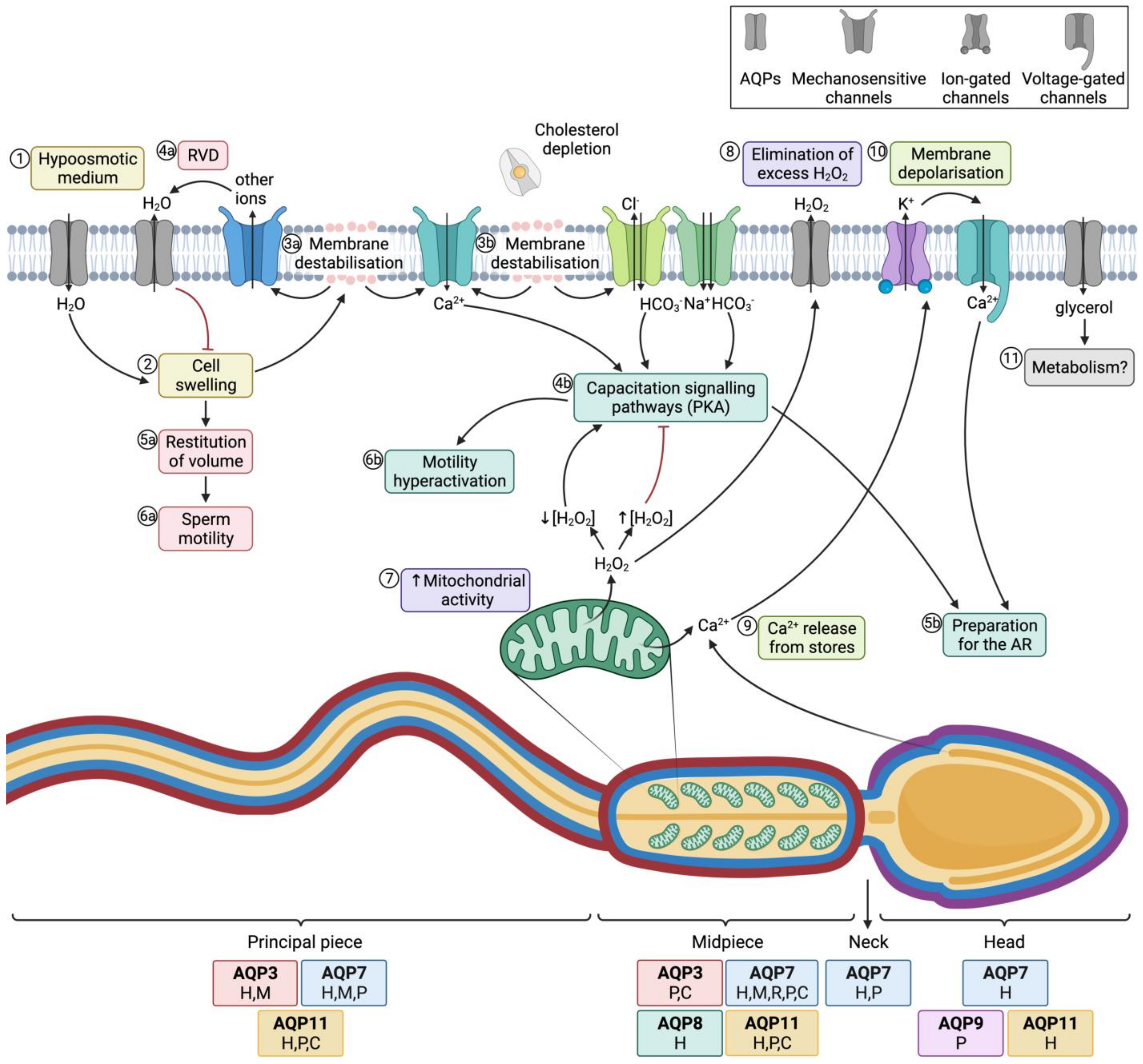
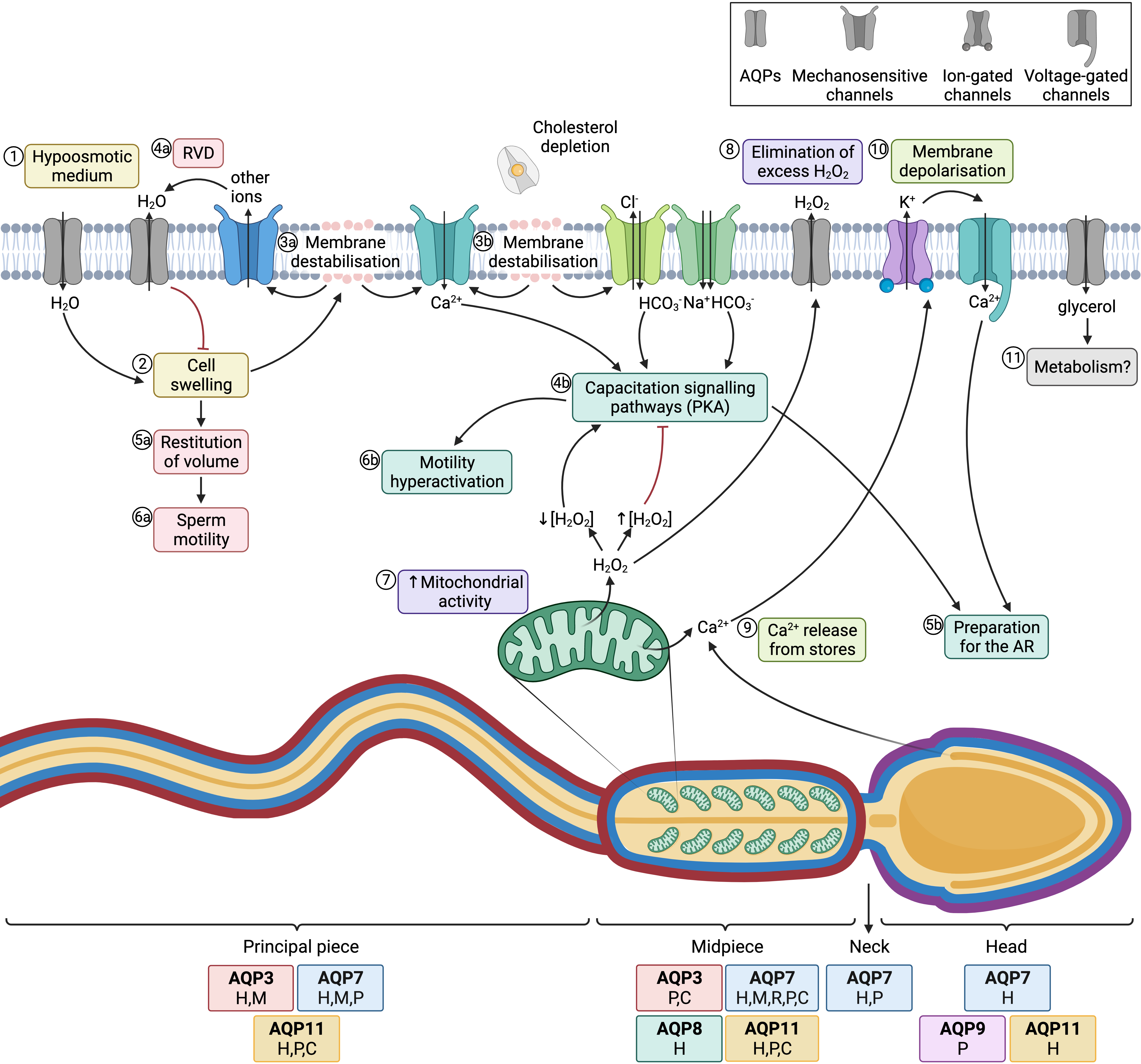
The most important role of AQPs in sperm is strictly related to osmoregulation. When sperm enter the female reproductive tract after ejaculation, they undergo a severe osmotic stress, since osmolality in the cauda epididymis is higher than in the female reproductive tract. In fact, during their transit through the epididymis, sperm acquire their osmoadaptability because the extracellular medium is progressively more hyperosmotic from the caput to the cauda. For this purpose, sperm uptake osmolytes from the epidydimal plasma, which allows the counteraction of the lower osmolality in the female reproductive tract after ejaculation. One must also consider that, at the time of ejaculation, sperm encounter the seminal plasma. There are differences between separate species of mammals in terms of differential osmolality between cauda epididymis, seminal plasma and the oviduct. On the one hand, in bovines, the epididymis, uterine and oviductal environments are hyperosmotic compared to the isoosmotic seminal plasma. In humans, mice, and rats, osmolality is progressively lower from the epididymis to the seminal plasma and then to the uterus, whereas in cattle and sheep the uterus and the epididymis are relatively hyperosmotic to seminal plasma [68][18]. Hypoosmotic shock causes sperm swelling due to an excessive uptake of water, which ends up impairing the normal movement of the sperm tail, which becomes coiled. In addition, plasma membrane function is highly compromised and if the critical volume is reached the membranes may experience ruptures [69][19]. Under physiological conditions, hypoosmotic stress initiates the signaling pathway involved in RVD, which activates an osmolyte efflux that drives water movement and restores cell volume [67][20]. Aquaporins (AQPs) are therefore crucial to allow the rapid trafficking of water across the plasma membrane, which, in turn, regulates cell volume (Figure 4; 1–4a).

Figure 4. Aquaporins (AQPs) and sperm function after ejaculation. (1) After entering the female tract, sperm encounter a hypoosmotic medium, which causes water influx and thus (2) cell swelling. (3a) The increase of cell volume causes membrane destabilization (which affects the sperm plasma membrane, but also alters mitochondrial and acrosomal membranes). (4a) As a consequence, mechanosensory ion channels are open and outflowing ion currents activate regulatory volume decrease (RVD) events, such as (4a) water outflow. (5a) Cell volume is then restituted, which allows (6a) normal sperm motility. (3b) In the female reproductive tract, membrane destabilization occurs in conjunction with cholesterol depletion. The resulting membrane reorganization elicits the entrance of calcium and bicarbonate through different ion channels, which activates capacitation signaling pathways (4b) that involve the activation of protein kinase A (PKA). (5b) Downstream events prepare the spermatozoon for the acrosome reaction (AR) and (6b) drive hyperactivated motility. (7) In this context, mitochondrial activity is elevated and reactive oxygen species (ROS), such as H2O2, are produced. This molecule at low concentrations is essential to elicit capacitation, but at high concentrations it acts as an inhibitor of this process. (8) Aquaporins (AQPs) play an essential role, allowing the outflow of the excess amounts of H2O2 towards the extracellular medium. (9) Another downstream event of the capacitation signaling pathway consists of the release of Ca2+ from intracellular stores, which triggers the opening of calcium-activated K+ channels that causes, in turn, plasma membrane hyperpolarization (10); membrane hyperpolarization subsequently opens voltage-gated Ca2+ channels. This increase in intracellular Ca2+ is crucial for acrosome reaction [64,65][21][22] (11) Glycerol transport through GLPs can be used by metabolic pathways. The sperm regions where AQPs have been identified in different mammalian species are highlighted in the diagram. Boxes indicate the species in which each AQP has been identified (C, cattle; P, pig; H, human; M, mouse; R, rat).
Aquaporin 8 (AQP8) is also relevant in osmoregulation, as its levels in human sperm have been found to be inversely correlated with the presence of sperm with coiled tails, which is indicative of osmotic stress [45][23]. The importance of AQP8 in osmoregulation has also been explored through its inhibition through HgCl2, which blocks quinine-induced swelling in both human [45][23] and mouse sperm [47,74][24][25].
3.2. Aquaporins, Sperm Functionality and Male Fertility
2.2. Aquaporins, Sperm Functionality and Male Fertility
Essentially, sperm’s capacity to regulate volume is crucial for fertility, since cell swelling in response to osmotic stress causes tail bending, which can, in turn, affect sperm motility. Nevertheless, osmotic changes in response to the hypotonic shock that sperm undergo when they enter the female reproductive tract are essential to elicit sperm capacitation. On the one hand, changes in sperm volume in response to osmotic shock trigger the opening of mechanosensitive calcium channels and thus elicit calcium influx, which is one of the first events that occurs during capacitation [77][26]. On the other hand, acrosomal swelling is required for a physiological acrosome reaction [78][27]. In addition, strict regulation of H2O2 concentration is essential for motility hyperactivation and acrosome reaction during capacitation, despite the mechanism remaining unknown [79][28]. Since some members of this family are peroxiporins, their potential involvement in acrosome reaction can be suggested. In addition, the permeability of GLPs to glycerol has been proposed to be relevant for the use of this molecule in metabolic pathways and as a source of energy in sperm [45][23]. The role of AQPs in sperm fertility is, therefore, evidenced by their involvement in both motility and capacitation-associated events, which are crucial for sperm to successfully fertilize the oocyte (Figure 4).
3.3. Aquaporins and Sperm Cryopreservation
2.3. Aquaporins and Sperm Cryopreservation
Cryopreservation of mammalian sperm is the most efficient method for long-term storage. This procedure is of high importance in livestock production, since cryopreserved sperm can be used for artificial insemination (AI), but they are also important for the establishment of genetic banks for endangered domestic and wild species [92][29]. Furthermore, it is also relevant as a procedure for the management of male fertility before undergoing certain treatments or for storing sperm that are to be used later in assisted reproduction technology (ART) [93][30]. Cryopreservation, however, is a challenging process for sperm integrity because of the osmotic shock these cells endure; under these circumstances, allowing water outflow of the cell is crucial to avoid the formation of intracellular crystal structures. This procedure may affect the integrity of the sperm nucleus, cytoskeleton and plasma membrane, reduce mitochondrial activity and motility and impair protein function [94[31][32][33],95,96], which results in decreased fertilizing ability. To minimize cryoinjuries, freezing media are supplemented with cryoprotecting agents, among which glycerol is used in different livestock species [28][34]. It is worth considering, nevertheless, that, apart from variations in size and morphology, the marked differences in plasma membrane composition and metabolism between sperm from separate species reflect the high variability in their cryotolerance (also known as freezability). In addition, within a given species, samples with similar fresh semen quality present both intra- and inter-individual differences in terms of sperm cryotolerance. Ejaculates can thus be classified into good (GFE) and poor freezability ejaculates (PFE) depending on post-thawing sperm quality [97,98,99][35][36][37]. In this context, the need for cryotolerance biomarkers has become highly apparent. Recent studies have evidenced differences in the transcriptomes [100][38], metabolomes [101][39], antioxidant activity [102[40][41],103], lipidomes [104][42] and proteomes [105,106,107][43][44][45] between GFEs and PFEs. Among the proteins that have considerable relevance as potential cryotolerance biomarkers, levels of AQPs in sperm from different mammalian species have been found to differ between GFEs and PFEs. In bovine sperm, AQP3 content is positively related to motility after thawing [37][46], whereas AQP11 levels are correlated with both cryotolerance and AI outcomes of cryopreserved sperm [63][47]. Also in this species, relative levels of AQP7 in sperm are positively correlated with sperm cryotolerance [36][48], and those of AQP7 mRNA are positively correlated with both their osmoregulation ability and fertility [108][49].3. Aquaporins in Mammalian Oocytes
3.1. Oocyte Aquaporins and Osmoregulation
It has been put forward that the main function of AQPs in sperm is osmoadaptation and the key events in which these proteins are crucial are the entry to the female reproductive tract and during the cryopreservation process. The function of AQPs in oocytes, therefore, seems to be less crucial in their physiological environment because these cells do not undergo major osmolality changes after ovulation unless they are cryopreserved. For this reason, most of the studies aiming to evaluate the characteristics and functional role of AQPs in oocytes are based on the evaluation of their permeability to water and cryoprotectants. In pig oocytes, water and cryoprotectants cross the plasma membrane mainly through simple diffusion [60][50]. However, the expression of exogenous (human and zebrafish) AQP3 also increases their permeability to water and cryoprotectants [119][51]. In bovine oocytes, AQP3 is permeable to water, glycerol and ethylene glycol, but not to DMSO or propylene glycol. In this species, water molecules and cryoprotectants cross the plasma membrane predominantly by simple diffusion, but channel-facilitated transport seems to have a more relevant role in bovine oocytes compared to other species, such as mice [120][52]. Finally, in mouse oocytes, the transport of water and glycerol through the plasma membrane occurs via simple diffusion. While membrane channels are involved in the transit of water and cryoprotectants [121[53][54],122], the fact that mouse oocytes show low permeability to water and glycerol suggests that AQPs are not very abundant in these cells [123][55]. In spite of this, the treatment of mouse oocytes with mercury chloride, which inhibits all AQPs except AQP7, partially blocks water flow through the plasma membrane [124[56][57],125], thus suggesting that not only AQP7 but also other mercury-sensitive AQPs play an important function in water transport in mouse oocytes. This, together with the partial (but not complete) blocking of water flow through the plasma membrane with mercury chloride [124][56], suggests that not only AQP7 is involved in the osmoregulation of mouse oocytes.4.2. Aquaporins, Oocyte Functionality and Female Infertility
3.2. Aquaporins, Oocyte Functionality and Female Infertility
Considering that, in mature oocytes, water seems to cross the plasma membrane mainly through simple diffusion in the different species of mammals that have been studied thus far, AQPs seem to have a less relevant role in this cell type than in sperm. Different studies, however, support the idea that AQPs are crucial during oocyte maturation. This process requires an interaction between granulosa cells and oocytes, and AQPs have been proposed to be involved in these interactions. Jo et al. [126][58] described an increase in Aqp3 mRNA expression during in vivo and in vitro oocyte maturation, which decreased in mature oocytes. Moreover, controlled ovarian hyperstimulation in mouse is known to reduce Aqp3 mRNA and AQP3 protein expression in oocytes [121][53] Furthermore, water permeability and swelling in response to osmotic stress in oocytes from female mice that have undergone controlled ovarian hyperstimulation are impaired, as, too, are fertilization rates, which confirms the importance of AQP3 for the quality of these oocytes [121][53].4.3. Aquaporins and Oocyte Cryopreservation
3.3. Aquaporins and Oocyte Cryopreservation
The importance of oocyte cryopreservation has increased recently because of the need for a standard approach that preserves fertility in women suffering from diseases requiring treatments with detrimental effects on germ cells or with high risk of premature ovarian insufficiency [123][55]. In addition, fertility postponing for social reasons has led many women to preserve oocytes in view of the decline in quality with age due to expectations of lacking a stable partner, career choices or financial issues. Cryopreservation is also useful for long-term storage in human oocyte banks, which makes the process easier by eliminating the need for cycle synchronization between donors and receptors [123][55]. At present, vitrification is considered to be more efficient than slow-freezing when cryopreserving mammalian oocytes [124][56]. Vitrification allows the rapid solidification of cells and the extracellular medium, in which both are converted into a glass-like state that avoids the formation of ice [125][57]. During vitrification, rapid cooling takes place in the presence of high concentrations of permeable and non-permeable cryoprotectants. Permeability to water and cryoprotectants is thus of high importance to minimize the time of exposure to high concentrations of cryoprotectants at temperatures of 25 °C or higher, at which these molecules are toxic for oocytes. Increase of cooling/warming rates and water dehydration to avoid intracellular crystal formation are crucial to boosting the resilience of oocytes to vitrification [125,127][57][59]. Since the cryoprotectants preferably used over the last decades are propylene glycol, ethylene glycol, DMSO and sucrose, and glycerol is not routinely employed [125][57]. the main role of AQPs during oocyte vitrification must be strictly related to their water permeability. Permeability to glycerol, which could be considered a potential alternative cryoprotectant for vitrification, has also been studied in different species. It has been previously stated that, in mouse oocytes, the transport of water and glycerol through the plasma membrane occurs mainly via simple diffusion, which suggests that AQPs are not very abundant in these cells [128][60]. In spite of this, oocytes from an Aqp7 knockout mouse model present reduced survival after vitrification [129][61]. This, together with the partial (but not complete) blocking of water flow through the plasma membrane with mercury chloride [130][62], suggests that AQP7 is not only involved in osmoregulation but also in the cryotolerance of mouse oocytes. In fact, the hyperosmotic stress caused by the presence of different cryoprotectants in the medium, including DMSO, ethylene glycol and sucrose, increases the expression of the AQP7 protein in the plasma membrane, but not that of AQP3 and AQP9 [129,130][61][62]. The molecule with the highest impact on AQP7 expression is DMSO, and its presence in the vitrification medium is associated with a higher water flow through the plasma membrane, which allows the oocyte to achieve the osmotic balance within a reduced time compared to other cryoprotectants [129,130][61][62]. With regard to other AQPs, oocytes from an Aqp3 knockout mouse model present lower resilience to vitrification, although the relevance of this protein in osmoregulation during cryopreservation is substantially more modest than that of AQP7 [129][61]. The exogenous expression of AQP3 in mouse oocytes, nevertheless, improves their survival of cryopreservation and does not alter their capacity to be fertilized [132,133,134][63][64][65]. In human oocytes, cryopreservation causes a decrease in the mRNA levels of AQP1, AQP2 and AQP11, as well as in the expression of AQP1 protein in the plasma membrane [58][66]. The presence of melatonin during the cryopreservation process, however, avoids the decrease in the mRNA and protein levels of these AQPs. This occurs in conjunction with a better oocyte cryotolerance, including the maintenance of morphology, ultrastructure, mitochondrial function and water permeability, and higher fertilization rates, embryo quality and blastocyst rates [58][66].54. Conclusions
Aquaporins (AQPs) are ubiquitously expressed in different species, organs, tissues and cells and they are crucial for the transport of water and small solutes across cell membranes. Focusing on mammalian gametes, AQP1, AQP3, AQP7, AQP8, AQP9 and AQP11 have been identified in sperm, whereas AQP1, AQP2, AQP3, AQP7, AQP9 and AQP11 have been reported to be present in oocytes. In both cell types, AQP3 and AQP7 have been thoroughly studied in terms of physiological relevance, and mounting evidence supports the proposition that AQP3 is crucial for the function of both gametes. It must be highlighted that most of these AQPs belong to the group of GLPs which, in addition to water, are permeable to other solutes, including glycerol and H2O2. It has been suggested that AQP7 may be involved in the influx of glycerol, which would be used as an energy source by sperm, but further research is needed to establish this relationship. In addition, both water and glycerol permeability are highly relevant during cryopreservation because quick efflux of water and influx of cryoprotectants are crucial to reducing the formation of intracellular crystals and shortening cooling time, and the rapid rehydration and outflow of cryoprotectants are vital to avoiding toxic effects on gametes. Specifically in sperm, AQPs are also relevant to overcome the major osmotic stress that they undergo immediately after ejaculation, when they enter the female reproductive tract. This osmoadaptation is required for motility activation. Future research should aim to define the exact sets of AQPs that are present in the oocytes from different species, as the present studies envisage certain AQPs and their roles but do not provide complete information on the entire set of AQPs. This is of high importance because, in sperm, AQPs are known to compensate the role of non-functional members. Since AQPs are present in all species and cells, and appear to be important for gamete cryotolerance, further research aimed at identifying their localization and addressing their involvement in cryotolerance is warranted.References
- Törnroth-Horsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvist-Petersson, K.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010, 584, 2580–2588.
- Jung, J.S.; Preston, G.M.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654.
- Fu, D.; Libson, A.; Miercke, L.J.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000, 290, 481–486.
- Tani, K.; Fujiyoshi, Y. Water channel structures analysed by electron crystallography. Biochim. Biophys. Acta 2014, 1840, 1605–1613.
- Savage, D.F.; Egea, P.F.; Robles-Colmenares, Y.; O’Connell, J.D., 3rd; Stroud, R.M. Architecture and selectivity in aquaporins: 2.5 a X-ray structure of aquaporin Z. PLoS Biol. 2003, 1, e72.
- Sui, H.; Han, B.-G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878.
- Ozu, M.; Dorr, R.A.; Gutiérrez, F.; Politi, M.T.; Toriano, R. Human AQP1 is a constitutively open channel that closes by a membrane-tension-mediated mechanism. Biophys. J. 2013, 104, 85–95.
- Tong, J.; Briggs, M.M.; McIntosh, T.J. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys. J. 2012, 103, 1899–1908.
- Hill, A.E.; Shachar-Hill, Y. Are Aquaporins the Missing Transmembrane Osmosensors? J. Membr. Biol. 2015, 248, 753–765.
- Ozu, M.; Galizia, L.; Acuña, C.; Amodeo, G. Aquaporins: More Than Functional Monomers in a Tetrameric Arrangement. Cells 2018, 7, 209.
- Carrageta, D.F.; Bernardino, R.L.; Soveral, G.; Calamita, G.; Alves, M.G.; Oliveira, P.F. Aquaporins and male (in)fertility: Expression and role throughout the male reproductive tract. Arch. Biochem. Biophys. 2020, 679, 108222.
- Ribeiro, J.C.; Alves, M.G.; Yeste, M.; Cho, Y.S.; Calamita, G.; Oliveira, P.F. Aquaporins and (in)fertility: More than just water transport. Biochim. Biophys. Acta-Mol. Basis Dis. 2021, 1867, 166039.
- Michalek, K.; Oberska, P.; Malkowska, P.; Bartkiene, E. In search of new potential markers for male fertility and semen quality control. Aquaporins in reproductive system and metabolomic profiling of semen. J. Physiol. Pharmacol. 2021, 72, 309–319.
- Yasui, M.; Hazama, A.; Kwon, T.H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184–187.
- Yasui, M.; Kwon, T.H.; Knepper, M.A.; Nielsen, S.; Agre, P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 1999, 96, 5808–5813.
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779.
- Itoh, T.; Rai, T.; Kuwahara, M.; Ko, S.B.H.; Uchida, S.; Sasaki, S.; Ishibashi, K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005, 330, 832–838.
- Lavanya, M.; Selvaraju, S.; Krishnappa, B.; Krishnaswamy, N.; Nagarajan, G.; Kumar, H. Microenvironment of the male and female reproductive tracts regulate sperm fertility: Impact of viscosity, pH, and osmolality. Andrology 2022, 10, 92–104.
- Drevius, L.-O.; Eriksson, H. Osmotic swelling of mammalian spermatozoa. Exp. Cell Res. 1966, 42, 136–156.
- Yeung, C.H.; Barfield, J.P.; Cooper, T.G. Physiological volume regulation by spermatozoa. Mol. Cell. Endocrinol. 2006, 250, 98–105.
- Rossato, M.; Di Virgilio, F.; Rizzuto, R.; Galeazzi, C.; Foresta, C. Intracellular calcium store depletion and acrosome reaction in human spermatozoa: Role of calcium and plasma membrane potential. Mol. Hum. Reprod. 2001, 7, 119–128.
- Yeste, M.; Fernández-Novell, J.M.; Ramió-Lluch, L.; Estrada, E.; Rocha, L.G.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Concha, I.I.; Ramírez, A.; Rodríguez-Gil, J.E. Intracellular calcium movements of boar spermatozoa during ‘in vitro’ capacitation and subsequent acrosome exocytosis follow a multiple-storage place, extracellular calcium-dependent model. Andrology 2015, 3, 729–747.
- Yeung, C.H.; Callies, C.; Tüttelmann, F.; Kliesch, S.; Cooper, T.G. Aquaporins in the human testis and spermatozoa-identification, involvement in sperm volume regulation and clinical relevance. Int. J. Androl. 2010, 33, 629–641.
- Yeung, C.H.; Callies, C.; Rojek, A.; Nielsen, S.; Cooper, T.G. Aquaporin Isoforms Involved in Physiological Volume Regulation of Murine Spermatozoa. Biol. Reprod. 2009, 80, 350–357.
- Callies, C.; Cooper, T.G.; Yeung, C.H. Channels for water efflux and influx involved in volume regulation of murine spermatozoa. Reproduction 2008, 136, 401–410.
- Rossato, M.; Virgilio, F.D.; Foresta, C. Involvement of osmo-sensitive calcium influx in human sperm activation. Mol. Hum. Reprod. 1996, 2, 903–909.
- Zanetti, N.; Mayorga, L.S. Acrosomal Swelling and Membrane Docking Are Required for Hybrid Vesicle Formation During the Human Sperm Acrosome Reaction. Biol. Reprod. 2009, 81, 396–405.
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of Hydrogen Peroxide in Sperm Capacitation and Acrosome Reaction. Biol. Reprod. 2004, 70, 518–522.
- Pickard, A.R.; Holt, W.V. Cryopreservation as a Supporting Measure in Species Conservation: Not the Frozen Zoo! In Life in the Frozen State; Benson, E., Fuller, B., Lane, N., Eds.; CRC Press: Baton Rouge, LA, USA; Boca Raton, FL, USA, 2004; pp. 393–414.
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv. Urol. 2012, 2012, 854837.
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64.
- Peña, F.J.; Macías García, B.; Samper, J.C.; Aparicio, I.M.; Tapia, J.A.; Ortega Ferrusola, C. Dissecting the molecular damage to stallion spermatozoa: The way to improve current cryopreservation protocols? Theriogenology 2011, 76, 1177–1186.
- Sieme, H.; Harrison, R.A.P.; Petrunkina, A.M. Cryobiological determinants of frozen semen quality, with special reference to stallion. Anim. Reprod. Sci. 2008, 107, 276–292.
- Curry, M.R. Cryopreservation of mammalian semen. In Cryopreservation and Freeze-Drying Protocols. Methods in Molecular BiologyTM; Day, J.G., Stacey, G.N., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; Volume 368, pp. 303–311.
- Hoffmann, N.; Oldenhof, H.; Morandini, C.; Rohn, K.; Sieme, H. Optimal concentrations of cryoprotective agents for semen from stallions that are classified “good” or “poor” for freezing. Anim. Reprod. Sci. 2011, 125, 112–118.
- Rego, J.P.A.; Martins, J.M.; Wolf, C.A.; van Tilburg, M.; Moreno, F.; Monteiro-Moreira, A.C.; Moreira, R.A.; Santos, D.O.; Moura, A.A. Proteomic analysis of seminal plasma and sperm cells and their associations with semen freezability in Guzerat bulls. J. Anim. Sci. 2016, 94, 5308–5320.
- Baishya, S.K.; Biswas, R.K.; Kadirvel, G.; Deka, B.C.; Kumar, S.; Sinha, S.; Dutta, D.J.; Saikia, G.K. Effect of conventional and controlled freezing method on the post thaw characteristics of boar spermatozoa. Anim. Reprod. Sci. 2014, 149, 231–237.
- Fraser, L.; Brym, P.; Pareek, C.S.; Mogielnicka-Brzozowska, M.; Paukszto, Ł.; Jastrzębski, J.P.; Wasilewska-Sakowska, K.; Mańkowska, A.; Sobiech, P.; Żukowski, K. Transcriptome analysis of boar spermatozoa with different freezability using RNA-Seq. Theriogenology 2020, 142, 400–413.
- Torres, M.A.; Pedrosa, A.C.; Novais, F.J.; Alkmin, D.V.; Cooper, B.R.; Yasui, G.S.; Fukumasu, H.; Machaty, Z.; de Andrade, A.F.C. Metabolomic signature of spermatozoa established during holding time is responsible for differences in boar sperm freezability. Biol. Reprod. 2021, 106, 213–226.
- Papas, M.; Catalan, J.; Barranco, I.; Arroyo, L.; Bassols, A.; Yeste, M.; Miró, J. Total and specific activities of superoxide dismutase (SOD) in seminal plasma are related with the cryotolerance of jackass spermatozoa. Cryobiology 2020, 92, 109–116.
- Papas, M.; Catalán, J.; Fernandez-Fuertes, B.; Arroyo, L.; Bassols, A.; Miró, J.; Yeste, M. Specific Activity of Superoxide Dismutase in Stallion Seminal Plasma Is Related to Sperm Cryotolerance. Antioxidants 2019, 8, 539.
- Xin, A.-J.; Wu, Y.-C.; Lu, H.; Cheng, L.; Gu, Y.-H.; Diao, H.; Chen, G.-W.; Wu, B.; Li, Z.; Tao, S.-C.; et al. Comparative analysis of human sperm glycocalyx from different freezability ejaculates by lectin microarray and identification of ABA as sperm freezability biomarker. Clin. Proteom. 2018, 15, 19.
- Vilagran, I.; Yeste, M.; Sancho, S.; Casas, I.; del Rivera Álamo, M.M.; Bonet, S. Relationship of sperm small heat-shock protein 10 and voltage-dependent anion channel 2 with semen freezability in boars. Theriogenology 2014, 82, 418–426.
- Pinart, E.; Yeste, M.; Bonet, S. Acrosin activity is a good predictor of boar sperm freezability. Theriogenology 2015, 83, 1525–1533.
- Llavanera, M.; Delgado-Bermúdez, A.; Fernandez-Fuertes, B.; Recuero, S.; Mateo, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 61.
- Fujii, T.; Hirayama, H.; Fukuda, S.; Kageyama, S.; Naito, A.; Yoshino, H.; Moriyasu, S.; Yamazaki, T.; Sakamoto, K.; Hayakawa, H.; et al. Expression and localization of aquaporins 3 and 7 in bull spermatozoa and their relevance to sperm motility after cryopreservation. J. Reprod. Dev. 2018, 64, 327–335.
- Morató, R.; Prieto-Martínez, N.; Muiño, R.; Hidalgo, C.O.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Aquaporin 11 is related to cryotolerance and fertilising ability of frozen–thawed bull spermatozoa. Reprod. Fertil. Dev. 2018, 30, 1099–1108.
- Prieto-Martinez, N.; Morato, R.; Muino, R.; Hidalgo, C.O.; Rodriguez-Gil, J.E.; Bonet, S.; Yeste, M.; Prieto-Martínez, N.; Morató, R.; Muiño, R.; et al. Aquaglyceroporins 3 and 7 in bull spermatozoa: Identification, localisation and their relationship with sperm cryotolerance. Reprod. Fertil. Dev. 2017, 29, 1249–1259.
- Kasimanickam, R.K.; Kasimanickam, V.R.; Arangasamy, A.; Kastelic, J.P. Associations of hypoosmotic swelling test, relative sperm volume shift, aquaporin7 mRNA abundance and bull fertility estimates. Theriogenology 2017, 89, 162–168.
- Jin, B.; Higashiyama, R.; Nakata, Y.; Yonezawa, J.; Xu, S.; Miyake, M.; Takahashi, S.; Kikuchi, K.; Yazawa, K.; Mizobuchi, S.; et al. Rapid Movement of Water and Cryoprotectants in Pig Expanded Blastocysts via Channel Processes: Its Relevance to Their Higher Tolerance to Cryopreservation. Biol. Reprod. 2013, 89, 87.
- Morato, R.; Chauvigne, F.; Novo, S.; Bonet, S.; Cerda, J. Enhanced water and cryoprotectant permeability of porcine oocytes after artificial expression of human and zebrafish aquaporin-3 channels. Mol. Reprod. Dev. 2014, 81, 450–461.
- Jin, B.; Kawai, Y.; Hara, T.; Takeda, S.; Seki, S.; Nakata, Y.-I.; Matsukawa, K.; Koshimoto, C.; Kasai, M.; Edashige, K. Pathway for the movement of water and cryoprotectants in bovine oocytes and embryos. Biol. Reprod. 2011, 85, 834–847.
- Meng, Q.-X.; Gao, H.-J.; Xu, C.-M.; Dong, M.-Y.; Sheng, X.; Sheng, J.-Z.; Huang, H.-F. Reduced expression and function of aquaporin-3 in mouse metaphase-II oocytes induced by controlled ovarian hyperstimulation were associated with subsequent low fertilization rate. Cell. Physiol. Biochem. 2008, 21, 123–128.
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374.
- Dolmans, M.-M.; Donnez, J. Fertility preservation in women for medical and social reasons: Oocytes vs. ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 63–80.
- Kuleshova, L.; Gianaroli, L.; Magli, C.; Ferraretti, A.; Trounson, A. Birth following vitrification of a small number of human oocytes: Case report. Hum. Reprod. 1999, 14, 3077–3079.
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155.
- Jo, J.W.; Jee, B.C.; Suh, C.S.; Kim, S.H.; Choi, Y.M.; Kim, J.G.; Moon, S.Y. Effect of maturation on the expression of aquaporin 3 in mouse oocyte. Zygote 2011, 19, 9–14.
- Vajta, G.; Rienzi, L.; Ubaldi, F.M. Open versus closed systems for vitrification of human oocytes and embryos. Reprod. Biomed. Online 2015, 30, 325–333.
- Pedro, P.B.; Yokoyama, E.; Zhu, S.E.; Yoshida, N.; Valdez, D.M.J.; Tanaka, M.; Edashige, K.; Kasai, M. Permeability of mouse oocytes and embryos at various developmental stages to five cryoprotectants. J. Reprod. Dev. 2005, 51, 235–246.
- Tan, Y.-J.; Zhang, X.-Y.; Ding, G.-L.; Li, R.; Wang, L.; Jin, L.; Lin, X.-H.; Gao, L.; Sheng, J.-Z.; Huang, H.-F. Aquaporin7 plays a crucial role in tolerance to hyperosmotic stress and in the survival of oocytes during cryopreservation. Sci. Rep. 2015, 5, 17741.
- Tan, Y.-J.; Xiong, Y.; Ding, G.-L.; Zhang, D.; Meng, Y.; Huang, H.-F.; Sheng, J.-Z. Cryoprotectants up-regulate expression of mouse oocyte AQP7, which facilitates water diffusion during cryopreservation. Fertil. Steril. 2013, 99, 1428–1435.
- Edashige, K.; Yamaji, Y.; Kleinhans, F.W.; Kasai, M. Artificial Expression of Aquaporin-3 Improves the Survival of Mouse Oocytes after Cryopreservation1. Biol. Reprod. 2003, 68, 87–94.
- Edashige, K.; Ohta, S.; Tanaka, M.; Kuwano, T.; Valdez, D.M.J.; Hara, T.; Jin, B.; Takahashi, S.; Seki, S.; Koshimoto, C.; et al. The role of aquaporin 3 in the movement of water and cryoprotectants in mouse morulae. Biol. Reprod. 2007, 77, 365–375.
- Yamaji, Y.; Seki, S.; Matsukawa, K.; Koshimoto, C.; Kasai, M.; Edashige, K. Developmental ability of vitrified mouse oocytes expressing water channels. J. Reprod. Dev. 2011, 57, 403–408.
- Zhang, Z.; Mu, Y.; Ding, D.; Zou, W.; Li, X.; Chen, B.; Leung, P.C.; Chang, H.-M.; Zhu, Q.; Wang, K.; et al. Melatonin improves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permeability of the oolemma. J. Pineal Res. 2021, 70, e12707.
More
