Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ariadna Delgado-Bermúdez and Version 2 by Lindsay Dong.
Aquaporins (AQPs) are a family of transmembrane channels that allow the transport of water and small solutes across cell membranes. Different members of this family have been identified in gametes. In sperm, they are relevant to osmoadaptation after entering the female reproductive tract, which is crucial for sperm motility activation and capacitation and, thus, for their fertilizing ability. In addition, they are relevant during the cryopreservation process, since some members of this family are also permeable to glycerol, one of the most frequently used cryoprotective agents in livestock. Regarding oocytes, AQPs are very important in their maturation but also during cryopreservation.
- mammals
- oocyte
- sperm
- water channels
- cryopreservation
1. The Family of Aquaporins
Aquaporins (AQPs) are a family of transmembrane proteins that are present ubiquitously in all species and cell types whose main function is to allow the transport of water across cell membranes [1][5].
The members of the AQP family present a highly conserved gene sequence, structure and function. In terms of structure, AQPs present six transmembrane α-helices (TM1-6; Figure 1A) surrounding a central pore that, when resolved through X-ray, shows an “hourglass” shape (Figure 1B) [2][3][11,12]. Transmembrane segments are connected by loops (A–E); loops B and E each present an NPA motif (asparagine, proline, alanine), which is the typical AQP signature motif [4][13]. The two loops that present an NPA motif are half-transmembrane helices that locate towards the center of the channel and create a broken seventh-transmembrane helix (Figure 1A,B). Water molecules cross AQPs via a single-molecule well and interact with the lateral chains of the amino acids that form the channel through hydrogen bonds. In this sense, the NPA motif disrupts any potential proton conduction locking the central molecule of water in a conformation that avoids molecular reorientation [5][14]. Another highly preserved feature in AQP structure is the selectivity filter, which is the narrowest point in the channel and is considered to be essential for channel selectivity [5][14]. The region near these residues is also known as aromatic/arginine (ar/R) constriction region (Figure 1A), since the arginine is a highly conserved residue among all AQPs [6][15].
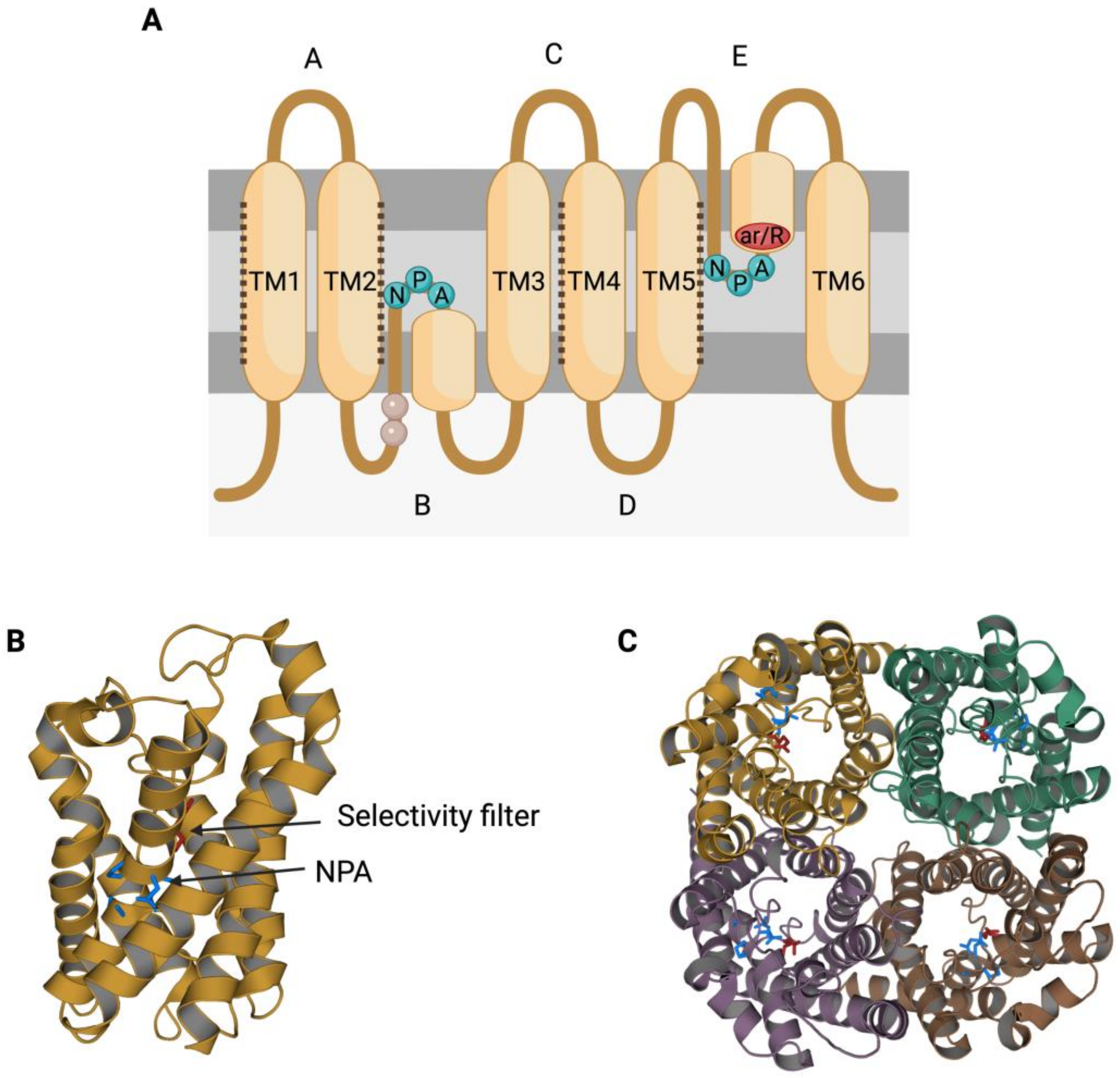

Figure 1. Structural characteristics of the family of aquaporins (AQPs). (A) Aquaporins present six transmembrane α-helices (TM1-6) that are connected through loops (A–E). Loops B and E are half-transmembrane helices oriented towards the center of the pore and present an NPA (asparagine, proline, alanine) motif that is highly conserved. In loop E, there is also a highly conserved arginine (R). Some residues from loop B have been suggested to be involved in AQPs’ mechanosensitivity (light residues). Transmembrane helices TM1, TM2, TM4 and TM5 interact with the TM of the adjacent monomers from an AQP tetramer (dark dot lines). (B) Each monomer folds in an hour-glass conformation, and the region near the R from loop E is formed by aromatic residues. This region is known as the aromatic/arginine (ar/R) region but also as a selectivity filter since it forms the narrowest point of the AQP pore. After folding, the two NPA motifs are in the same region, which is also highly conserved. (C) The quaternary structure of AQPs consists of the formation of tetramers, where each monomer has its own functional pore. This figure is based on PDB structure 6QZJ from AQP7.
Aquaporins (AQPs) function as tetrameric structures formed by four monomers, each with their own permeable pore (Figure 1C). Moreover, tetrameric structures appear to be crucial for AQPs’ mechanosensitivity (Figure 2), as it has been described for AQP1 and AQP4 [7][8][17,18].
In spite of having a highly preserved sequence and structure, the separate members of the AQP family differ in certain structural features and specific permeability to water and other molecules. AQPs can, therefore, be classified into three different groups based on variations in their structure and permeability: orthodox AQPs, aquaglyceroporins (GLPs) and superAQPs (Figure 3).
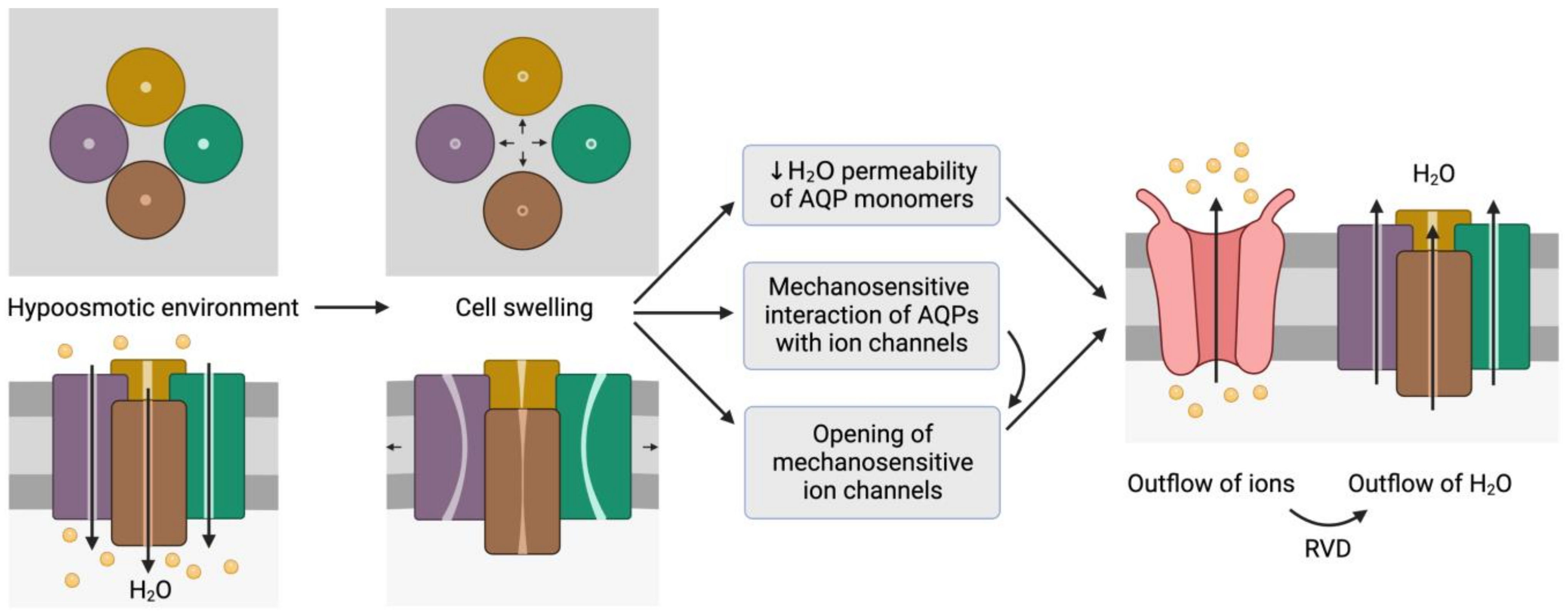
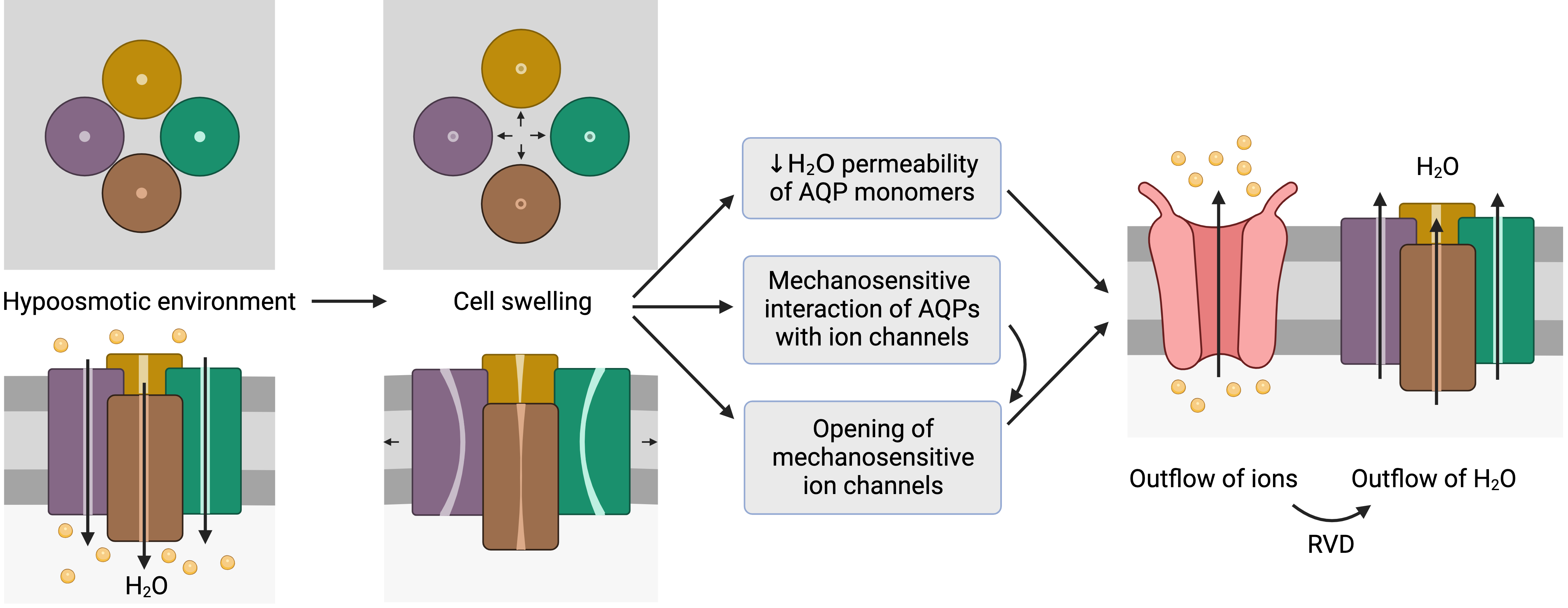


Figure 2. Representation of the mechanosensitive mechanism of aquaporins (AQPs). When cells are in a hypoosmotic environment, water enters them in response to the concentration gradient of solutes. Due to the entry of water, cells may undergo swelling, which can cause the distortion of AQP structures and thus compromise water permeability. In addition, AQPs may have mechanosensitive interactions with ion channels that can trigger their opening to trigger the outflow of ions. In fact, some ion channels are mechanosensitive and open in response to cell swelling, regardless of AQP signaling. The outflow of ions generates a driving force that elicits the outflow of water; this process is also known as regulatory volume decrease (RVD). This schematic representation is inspired by the model proposed by Hill et al. [9][20] and its representation by Ozu et al. [10][16].
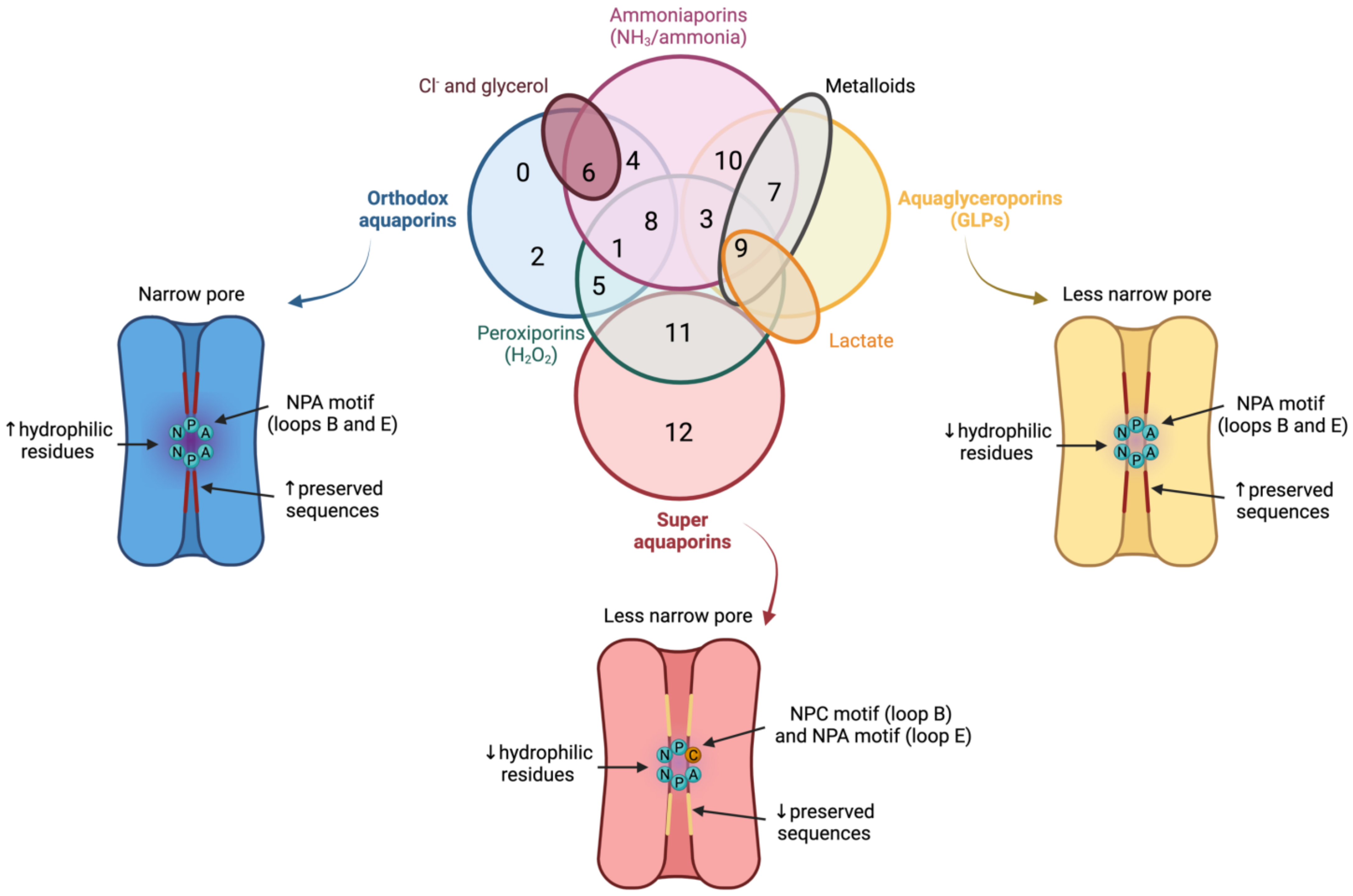
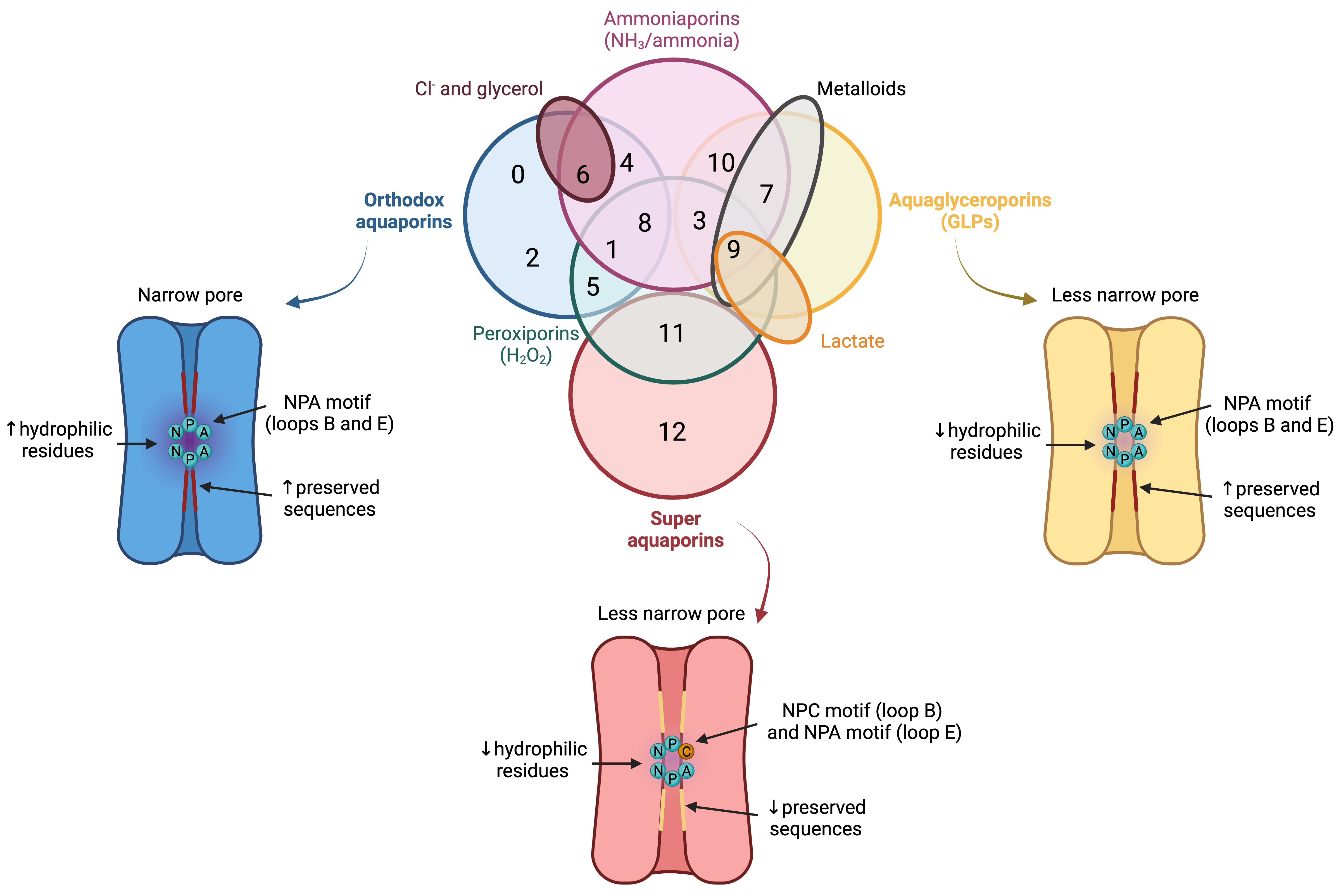


Figure 3. Classification of aquaporins (AQPs) based on their homology and specific permeability to different molecules. The three main groups into which AQPs are classified are: orthodox AQPs (blue), aquaglyceroporins (GLPs; yellow) and superaquaporins (superAQPs; red). The structural characteristics of these three groups are graphically represented. In addition to the classical classification, some AQPs present permeability to H2O2 and they are considered peroxiporins (green). Similarly, some members of this family of proteins are considered ammoniaporins (pink) since they are permeable to ammonia and/or to NH3. Finally, certain members present permeability to other molecules, such as Cl− (dark red), metalloids (grey) or lactate (orange). NPA motif (asparagine, proline, alanine); NPC motif (asparagine, proline, cysteine). Image based on [11][12][13]. (dark red), metalloids (grey) or lactate (orange). NPA motif (asparagine, proline, alanine); NPC motif (asparagine, proline, cysteine). Image based on [21,22,23].
1.1. Orthodox Aquaporins (AQPs)
The group of orthodox AQPs includes AQP0, AQP1, AQP2, AQP4, AQP5, AQP6 and AQP8. Orthodox AQPs present the smallest channel size among the different groups of AQPs and their selectivity filter has a highly hydrophilic nature [5][14]. These two characteristics are the reason for the exclusive permeability of this group of AQPs to water through a steric mechanism of selectivity. In addition, the NPA motif also plays a function in selectivity because, in orthodox AQPs, the side chains of the amino acids of this motif narrow the pore to a smaller diameter and are more hydrophilic than those of the other family members [5][14]. The only exception in this group is AQP6, which also presents anion permeability at low pH [14][25] and localizes in the membrane of intracellular organelles; remarkably, this differs from the other orthodox AQPs that localize in the plasma membrane [15][26].
1.2. Aquaglyceroporins (GLPs)
The group of GLPs includes AQP3, AQP7, AQP9 and AQP10. Water molecules cross GLPs through the establishment of the same interactions as with orthodox AQPs and also form a single-molecule well. In GLPs, nevertheless, the diameter of the pore, which is limited by the NPA motif, is larger than that of orthodox AQPs. In addition, the selectivity filter has a hydrophobic patch of residues and the region near the NPA motif is less hydrophilic than in orthodox AQPs, which determines the lower hydrophilicity of this group of AQPs [5][14].1.3. Superaquaporins (superAQPs)
The last group, also known as superAQPs, includes AQP11 and AQP12, which are expressed in intracellular membranes [16][17][30,31]. In fact, they present an endoplasmic reticulum (ER) retention signal at the carboxy-terminal region, and AQP11 has been identified in the ER when exogenously expressed in mammalian cells [16][30].2. Aquaporins in Mammalian Sperm
2.1. Sperm Aquaporins (AQPs) and Osmoregulation
3.1. Sperm Aquaporins (AQPs) and Osmoregulation
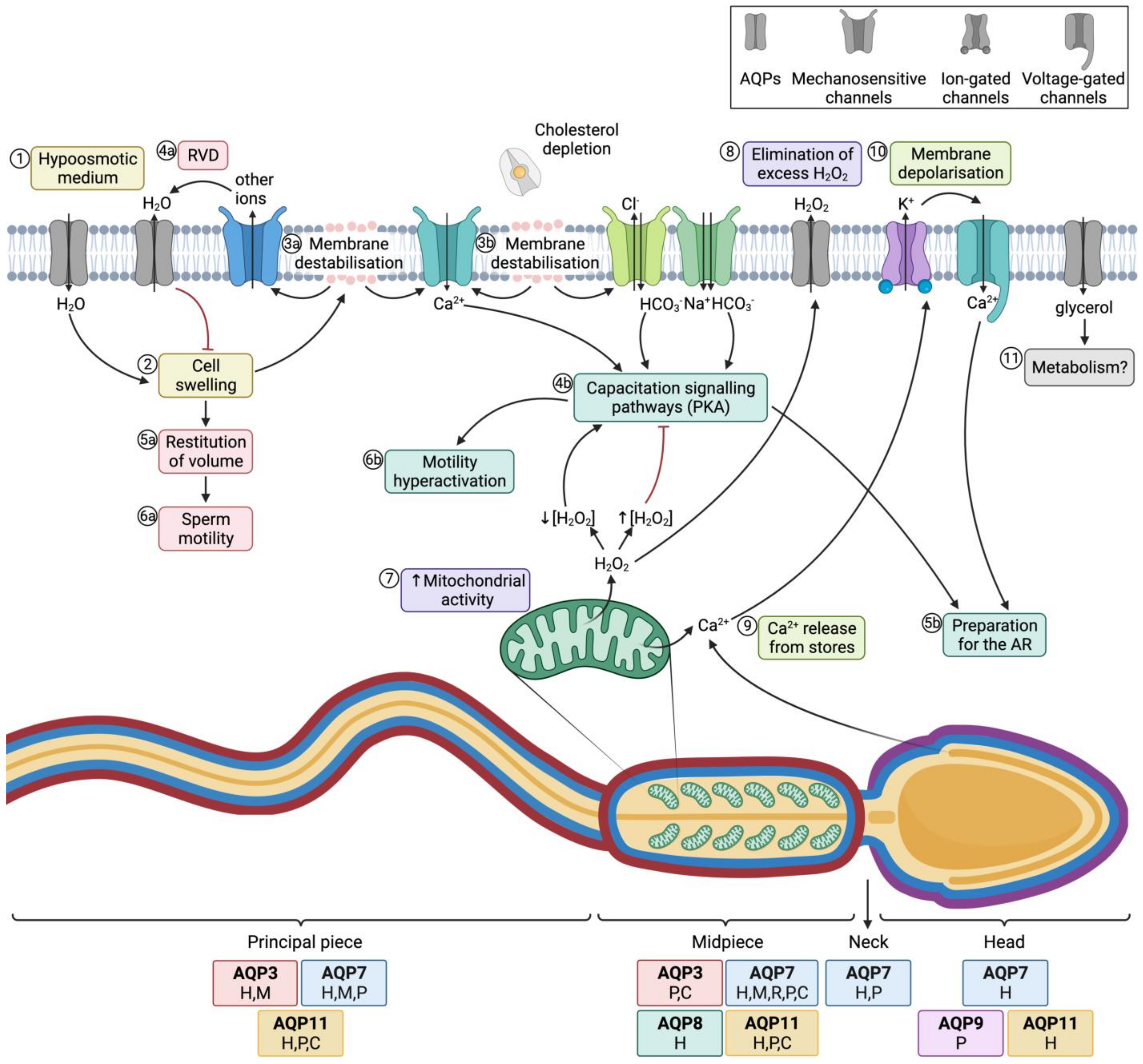
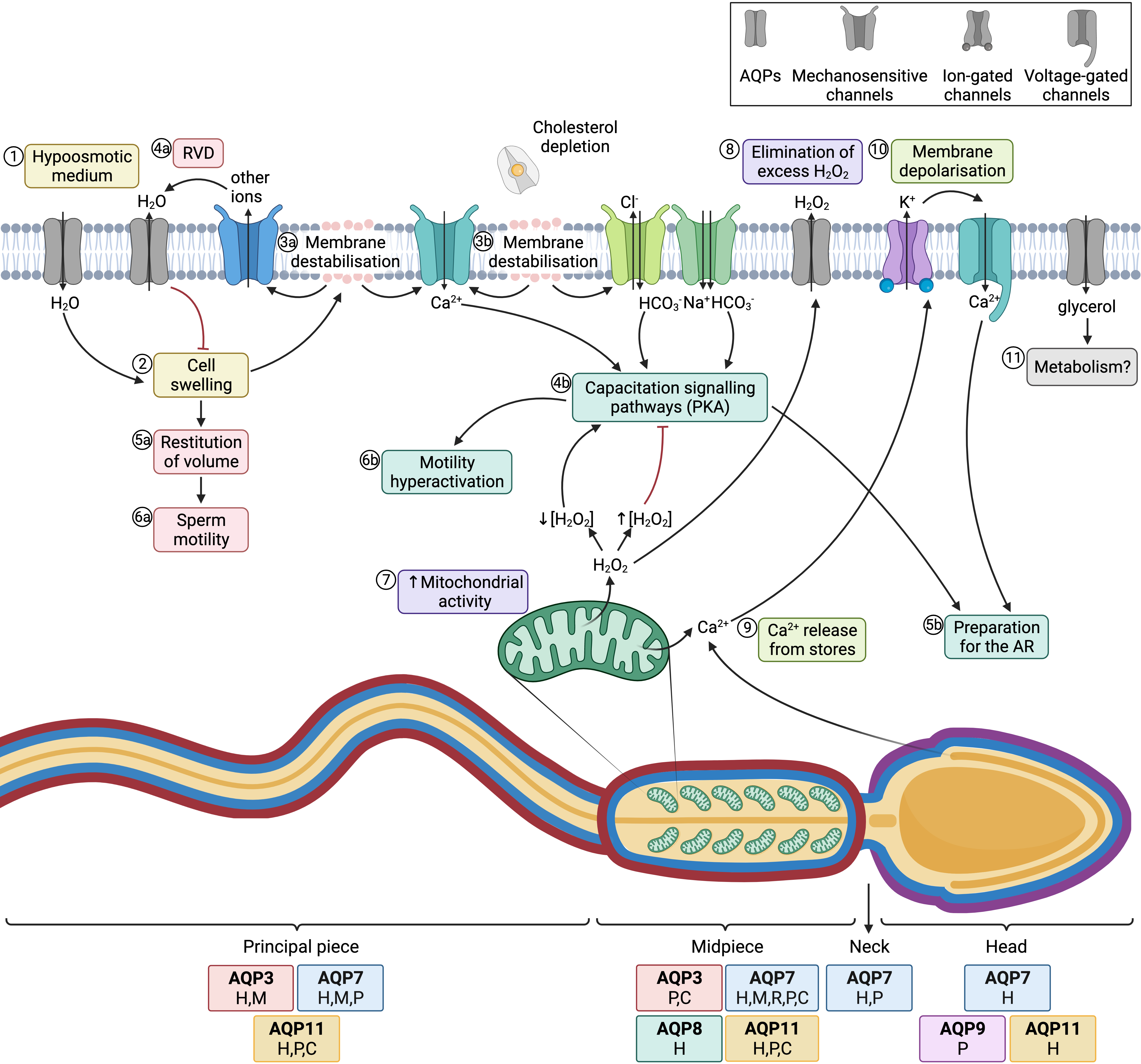
The most important role of AQPs in sperm is strictly related to osmoregulation. When sperm enter the female reproductive tract after ejaculation, they undergo a severe osmotic stress, since osmolality in the cauda epididymis is higher than in the female reproductive tract. In fact, during their transit through the epididymis, sperm acquire their osmoadaptability because the extracellular medium is progressively more hyperosmotic from the caput to the cauda. For this purpose, sperm uptake osmolytes from the epidydimal plasma, which allows the counteraction of the lower osmolality in the female reproductive tract after ejaculation. One must also consider that, at the time of ejaculation, sperm encounter the seminal plasma. There are differences between separate species of mammals in terms of differential osmolality between cauda epididymis, seminal plasma and the oviduct. On the one hand, in bovines, the epididymis, uterine and oviductal environments are hyperosmotic compared to the isoosmotic seminal plasma. In humans, mice, and rats, osmolality is progressively lower from the epididymis to the seminal plasma and then to the uterus, whereas in cattle and sheep the uterus and the epididymis are relatively hyperosmotic to seminal plasma [18][68]. Hypoosmotic shock causes sperm swelling due to an excessive uptake of water, which ends up impairing the normal movement of the sperm tail, which becomes coiled. In addition, plasma membrane function is highly compromised and if the critical volume is reached the membranes may experience ruptures [19][69]. Under physiological conditions, hypoosmotic stress initiates the signaling pathway involved in RVD, which activates an osmolyte efflux that drives water movement and restores cell volume [20][67]. Aquaporins (AQPs) are therefore crucial to allow the rapid trafficking of water across the plasma membrane, which, in turn, regulates cell volume (Figure 4; 1–4a).

Figure 4. Aquaporins (AQPs) and sperm function after ejaculation. (1) After entering the female tract, sperm encounter a hypoosmotic medium, which causes water influx and thus (2) cell swelling. (3a) The increase of cell volume causes membrane destabilization (which affects the sperm plasma membrane, but also alters mitochondrial and acrosomal membranes). (4a) As a consequence, mechanosensory ion channels are open and outflowing ion currents activate regulatory volume decrease (RVD) events, such as (4a) water outflow. (5a) Cell volume is then restituted, which allows (6a) normal sperm motility. (3b) In the female reproductive tract, membrane destabilization occurs in conjunction with cholesterol depletion. The resulting membrane reorganization elicits the entrance of calcium and bicarbonate through different ion channels, which activates capacitation signaling pathways (4b) that involve the activation of protein kinase A (PKA). (5b) Downstream events prepare the spermatozoon for the acrosome reaction (AR) and (6b) drive hyperactivated motility. (7) In this context, mitochondrial activity is elevated and reactive oxygen species (ROS), such as H2O2, are produced. This molecule at low concentrations is essential to elicit capacitation, but at high concentrations it acts as an inhibitor of this process. (8) Aquaporins (AQPs) play an essential role, allowing the outflow of the excess amounts of H2O2 towards the extracellular medium. (9) Another downstream event of the capacitation signaling pathway consists of the release of Ca2+ from intracellular stores, which triggers the opening of calcium-activated K+ channels that causes, in turn, plasma membrane hyperpolarization (10); membrane hyperpolarization subsequently opens voltage-gated Ca2+ channels. This increase in intracellular Ca2+ is crucial for acrosome reaction [21][22][64,65] (11) Glycerol transport through GLPs can be used by metabolic pathways. The sperm regions where AQPs have been identified in different mammalian species are highlighted in the diagram. Boxes indicate the species in which each AQP has been identified (C, cattle; P, pig; H, human; M, mouse; R, rat).
Aquaporin 8 (AQP8) is also relevant in osmoregulation, as its levels in human sperm have been found to be inversely correlated with the presence of sperm with coiled tails, which is indicative of osmotic stress [23][45]. The importance of AQP8 in osmoregulation has also been explored through its inhibition through HgCl2, which blocks quinine-induced swelling in both human [23][45] and mouse sperm [24][25][47,74].
2.2. Aquaporins, Sperm Functionality and Male Fertility
3.2. Aquaporins, Sperm Functionality and Male Fertility
Essentially, sperm’s capacity to regulate volume is crucial for fertility, since cell swelling in response to osmotic stress causes tail bending, which can, in turn, affect sperm motility. Nevertheless, osmotic changes in response to the hypotonic shock that sperm undergo when they enter the female reproductive tract are essential to elicit sperm capacitation. On the one hand, changes in sperm volume in response to osmotic shock trigger the opening of mechanosensitive calcium channels and thus elicit calcium influx, which is one of the first events that occurs during capacitation [26][77]. On the other hand, acrosomal swelling is required for a physiological acrosome reaction [27][78]. In addition, strict regulation of H2O2 concentration is essential for motility hyperactivation and acrosome reaction during capacitation, despite the mechanism remaining unknown [28][79]. Since some members of this family are peroxiporins, their potential involvement in acrosome reaction can be suggested. In addition, the permeability of GLPs to glycerol has been proposed to be relevant for the use of this molecule in metabolic pathways and as a source of energy in sperm [23][45]. The role of AQPs in sperm fertility is, therefore, evidenced by their involvement in both motility and capacitation-associated events, which are crucial for sperm to successfully fertilize the oocyte (Figure 4).
