Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Fanli Meng.
The sensing characteristics of metal oxide semiconductors (MOSs) depend on the carrier concentration, which is relevant to the working temperature. The characteristic parameters commonly used to measure gas-sensing performance include optimal working temperature, sensitivity, selectivity, stability, repeatability, response and recovery time, and the lowest detection limits.
- triethylamine
- metal oxide semiconductor
- sensing performance
1. Introduction
Emissions of noisome gases are ubiquitous in daily life and industrial production. Outdoor fuel combustion and transportation and indoor emissions from building and decorative materials, furniture, household appliances, cleaning agents, and the human body itself all produce noxious fumes. Volatile organic compounds (VOCs) are a common class of hazardous gases in the air that can have a huge negative impact on human health. As a member of VOCs, triethylamine is a colorless oily substance that is slightly soluble in water and easily dissolves in organic solvents such as ethanol; it is toxic and flammable and has a strong ammonia odor [1,2,3][1][2][3].
In many chemical synthesis processes, TEA is considered a multifunctional and efficient organocatalyst and solvent [4]. Because of its relative safety, commercial availability, and low price, it is often used in industrial production as a synthetic dye and preservative, and because of its excellent physical and chemical properties, it is also used in large quantities in chemical experiments [5]. However, when the TEA concentration is too high, it endangers ourthe physical health by causing injuries such as skin burns and headaches as well as pulmonary edema and poisoning by accidental swallowing; its vapor can also strongly irritate the eyelids and mucous membranes [6,7][6][7]. It also has the risk of rapid burning and explosion when exposed to open fire, high temperature, and strong oxidizing agents [8,9][8][9]. Both the European Commission and the American Conference of Governmental Industrial Hygienists have recommended that the threshold concentration of TEA exposed to air be 1 ppm [10,11][10][11]. Therefore, TEA gas sensors with low detection limits that can detect quickly need to be developed.
The gas-detection methods developed so far include quartz crystal microbalance gas sensor [12], visual colorimetric detection [13], headspace gas chromatography [14], electrochemical sensors [15,16[15][16][17],17], and chemiresistive semiconductor gas sensors. The detection methods mentioned above can all detect a certain concentration of TEA, but several of them have a long detection time, high detection cost, and complicated detection operation. Therefore, the chemiresistive semiconductor sensors, which can be fast, accurate, and highly sensitive; have low detection limits; and can be manufactured in batches, have received widespread attention from scientific researchers around the world. To date, semiconductor gas sensors are mostly made of metal oxide semiconductors (MOSs), which have excellent physicochemical properties such as wide bandgap, unique microstructure [18[18][19][20],19,20], higher sensitivity to gases, and fast response time; most importantly, lower fabrication costs make them circulate in the market in large quantities. The most commonly used n-type semiconductor metal oxide materials are ZnO, SnO2, Fe2O3, and MoO3 [21], and p-type semiconductor metal materials are Co3O4, CuO, and NiO [22]. It is found that they all respond to TEA gas, but all have the shortcomings of low detection limit, poor stability, and high operating temperature to be solved. To improve the gas-sensing performance of TEA sensors, experimenters have been studying the uninterrupted optimization of material morphology and the compositions of different materials (MXenes, TMDs, and graphene materials) in terms of all sorts of sensing characteristics.
2. Gas-Sensing Characteristics of Metal Oxide Semiconductor Sensors
2. Gas-Sensing Characteristics of Metal Oxide Semiconductor Sensors
It is usually necessary to use some specific indicators to evaluate the gas-detection ability of a sensor. At present, the characteristic parameters commonly used to measure gas-sensing performance include optimal working temperature, sensitivity, selectivity, stability, repeatability, response and recovery time, and the lowest detection limits.
2.1. Optimal Working Temperature
The sensing characteristics of MOSs depend on the carrier concentration, which is relevant to the working temperature. Only at the optimum operating temperature can the sensitive materials fully stimulate the chemical activity to push the gas sensor to its maximum response. Several response curves are shown in Figure 1 [23], which often show an increase–maximum–decrease trend. When the temperature is too low, TEA molecules are inert and cannot overcome the activation energy barrier to react with the adsorbed oxygen [24]; then, as the temperature increases, the whole reaction will accelerate. However, at higher temperatures, the gas molecules get enough energy to rapidly escape from the material surface without affecting the conductivity of the sensor, resulting in the decline response [25].
At the present time, the optimal working temperature of most semiconductor sensors is high, often in hundreds of degrees Celsius, which causes huge power consumption. Developing a special gas sensor with a low operating temperature is also one of the current challenges. According to research, metals have surface redox reaction or catalytic properties, so noble doped or loaded can reduce the demand for energy input [26]. Additionally, the construction of heterojunctions can regulate carrier transport, or layered or core–shell gas-sensing materials will be more conducive to gas adsorption and desorption because of the porous structure and large specific surface area [27,28,29][27][28][29]. The above three methods are used to reduce the operating temperature of gas sensors.
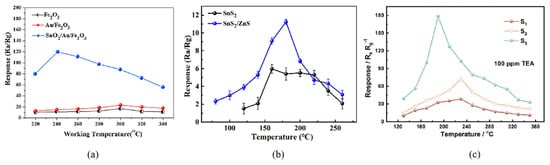
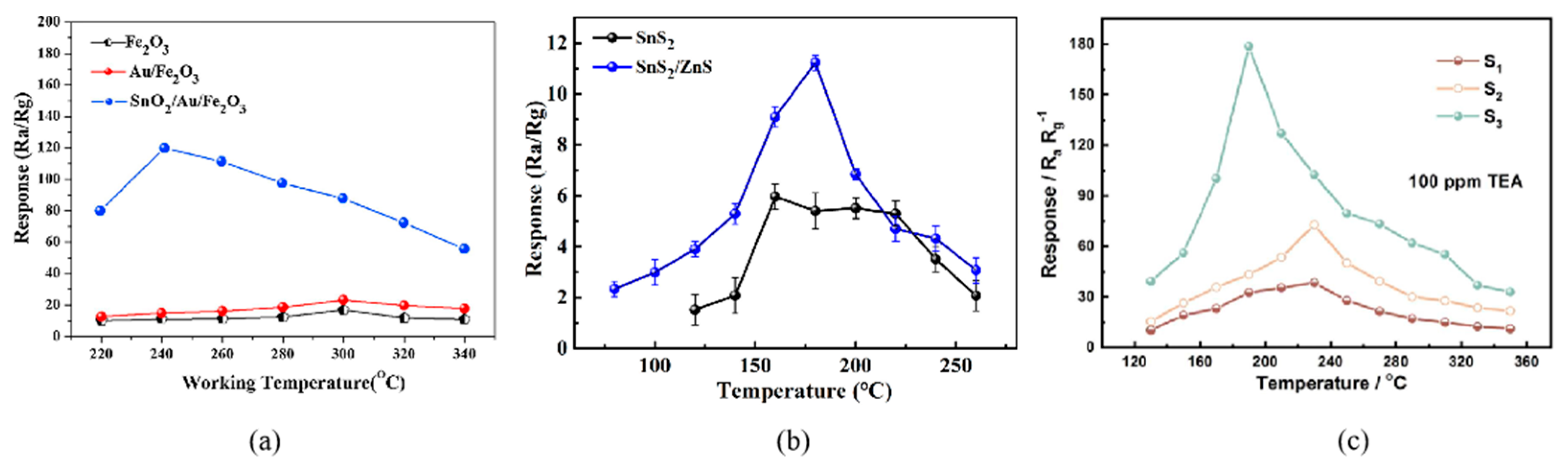 Figure 1. (a) The response curves of SnS2 and SnS2/ZnS to 50 ppm TEA under various working temperatures [23]; (b) The response curves of Fe2O3, Au/Fe2O3, and SnO2/Au/Fe2O3 to 100 ppm TEA at different operating temperatures [30]; (c) The response curves of sample-1, -2, -3 to 100 ppm TEA at various operating temperatures [31].
Figure 1. (a) The response curves of SnS2 and SnS2/ZnS to 50 ppm TEA under various working temperatures [23]; (b) The response curves of Fe2O3, Au/Fe2O3, and SnO2/Au/Fe2O3 to 100 ppm TEA at different operating temperatures [30]; (c) The response curves of sample-1, -2, -3 to 100 ppm TEA at various operating temperatures [31].2.2. Sensitivity
2.2. Sensitivity
The sensitivity K of a gas sensor is an indicator of the responsiveness of a gas sensor to the target gas. It represents the compliance relationship between the electrical parameters of the gas sensor and the target gas concentration. There is no doubt that the greater the K value, the better the performance of the gas-sensing materials. The sensitivity K is usually expressed as K = Ra/Rg (n-type semiconductor) or K = Rg/Ra (p-type semiconductor) [32,33][32][33]. Ra and Rg represent the resistance of the sensor in air and in the target gas, respectively.
2.3. Selectivity
The selectivity of a gas sensor refers to its ability to recognize and measure a gas without interference from non-target gases in multigas environments [34]. In short, selectivity is the ability of a gas sensor to identify the measured gas more accurately in mixed gas. Only when the sensitivity of the target gas is several times or even tens of times higher than that of other interfering gases can a sensor be said to have good selectivity.
2.4. Stability
Stability is an important index for evaluating the property of a sensor. It refers to whether the sensor can work for a long time and still maintain or approach the initial performance within the predetermined working range [35]. Considering the practical application of gas sensors, the sensor’s ability to maintain long-term stability is very necessary. Normally, the response of the prepared sensor over one or several months will be measured to ensure its stability.
2.5. Repeatability
Repeatability is defined as the ability of a semiconductor resistance sensor to restore its resistance to its original value and maintain its high sensing performance after target gas measurement. If the sensor cannot recover the resistance value in the normal gas environment, it maybe that the target gas has an irreversible impact on the sensor that makes it no longer operational [36].
2.6. Response Time (τ
res
) and Recovery Time (τ
rec
)
Response time (τres) is defined as the time required for the resistance value of a sensor in the air to reach 90% of the resistance value in the measured gas. Similarly, recovery time (τrec) is specified as the time required to recover to 90% of the resistance value in the air after removing the target gas [37]. The preparation of sensors that can quickly detect TEA gas is also one of the directions that people are vigorously studying.
2.7. The Lowest Detection Limits
The lowest detection limit is one of the major indexes pursued by many TEA sensors. It refers to the minimum gas concentration that can make the sensor respond under certain conditions, that is, the minimum detection concentration. High-performance sensors with low detection limits can often detect parts from per million (ppm) or even lower to parts per billion (ppb) [38]. This makes it possible to capture mixed harmful gases in the air much earlier when gases leaks.
A comparison of the performance of several TEA sensors is given in Table 1.
Table 1.
A comparison of Tthe sensing characteristicsperformance of several typical triethylamine TEA sensorss is given in Table 1.
Table 1. The sensing characteristics of several typical triethylamine sensors.
| Nanomaterial Shapes | τ | res | /τ | rec | (s) | T (°C) | Conc. (ppm) | Lim. (ppm) | Res. | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SnS | 2 | /ZnS microspheres | 2/8 | 180 | 50 | - | 11.21 | [23] | ||||
| ZIF-67/PBA arrays | 5/182 | 180 | 100 | - | 11.7 | [24] | ||||||
| ZnFe | 2 | O | 4 | –ZnO mesoporous | 0.9/23 | 240 | 50 | - | 21.23 | [25] | ||
| Au−PdO Modified Cu-Doped K | 2 | W | 4 | O | 13 | Nanowires | 17/27 | 120 | 10 | 1 | 282 | [26] |
| mesoporous ZnO/Co | 3 | O | 4 | nanosheets | 17/25 | 240 | 50 | 0.087 | 67.8 | [27] | ||
| COFs@SnO | 2 | @carbon nanospheres |
7/5 | RT | 2 | 0.2 | 95.1 | [28] | ||||
| ZnO/SnO | 2 | micro-camellia |
27/12 | 100 | 100 | 1 | 780 | [29] | ||||
| yolk-shell SnO | 2 | /Au/Fe | 2 | O | 3 | nanoboxes | 7/10 | 240 | 100 | 0.05 | 126.84 | [30] |
| Zn | 2 | SnO | 4 | /ZnSnO | 3 | 19/37 | 190 | 100 | 0.5 | 179.7 | [31] | |
| ZnO/Co | 3 | O | 4 | nanomeshes | 30/55 | 100 | 5 | - | 3.2 | [32] |
Note: τres and τrec represent response times and recovery times, respectively. T and Conc. indicate the optimal working temperature and detection concentration, respectively. Lim. is the abbreviation of the lowest detection limit. And Res. and Ref. represent response and reference, respectively. RT represents room temperature.
References
- Yuan, Z.; Zhao, J.; Meng, F.; Qin, W.; Chen, Y.; Yang, M.; Ibrahim, M.; Zhao, Y. Sandwich-like composites of double-layer Co3O4 and reduced graphene oxide and their sensing properties to volatile organic compounds. J. Alloys Compd. 2019, 793, 24–30.
- Yun, P.; Ma, S.; Xu, X.; Wang, S.; Liu, W.; Wang, L.; Alhadi, A. Bi2WO6 nanoparticles-decorated ZnO nanosheets and their enhanced gas sensing properties. Vacuum 2021, 194, 110627.
- Yun, P.D.; Ma, S.Y.; Xu, X.L.; Wang, S.Y.; Han, T.; Sheng, H.; Pei, S.T.; Yang, T.T. Excellent triethylamine sensor with ultra-fast response and recovery time based on bulk Bi2WO6 material. Mater. Lett. 2021, 285, 129162.
- Li, C.B.; Xiao, F.; Xu, W.; Chu, Y.; Wang, Q.; Jiang, H.; Li, K.; Gao, X.W. Efficient self-photo-degradation of cationic textile dyes involved triethylamine and degradation pathway. Chemosphere 2021, 266, 129209.
- Wang, Z.; Lin, R.; Fang, W.; Li, G.; Guo, Y.; Qin, Z. Triethylamine as an initiator for cracking of heptane. Energy 2006, 31, 2773–2790.
- Yin, M.; Zhang, L.; Qiu, T.; Chen, Y.; Qi, S.; Wei, X.; Tian, X.; Ge, K.; Qiu, J.; Xu, D. Double-layer capsule of mesoporous 2 for sensitive detection of triethylamine. Analyst 2021, 146, 6193–6201.
- Brahmachari, G.; Nayek, N.; Nurjamal, K.; Karmakar, I.; Begam, S. Triethylamine—A Versatile Organocatalyst in Organic Transformations: A Decade Update. Synthesis 2018, 50, 4145–4164.
- Abbas, H.; Marappan, G.; Chidabaram, D.; Govindasamy, S.; Jayaraman Surya, V.; Sivalingam, Y. Graphene Oxide based Gas Sensor for Triethylamine Detection at Room Temperature. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1219, 012031.
- Cai, T.; Chen, L.; Ren, Q.; Cai, S.; Zhang, J. The biodegradation pathway of triethylamine and its biodegradation by immobilized Arthrobacter protophormiae cells. J. Hazard. Mater. 2011, 186, 59–66.
- Xu, Y.; Ma, T.; Zheng, L.; Sun, L.; Liu, X.; Zhao, Y.; Zhang, J. Rational design of Au/Co3O4-functionalized W18O49 hollow heterostructures with high sensitivity and ultralow limit for triethylamine detection. Sens. Actuators B Chem. 2019, 284, 202–212.
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection. Mater. Horiz. 2020, 7, 1519–1527.
- Ayad, M.M.; Torad, N.L. Quartz crystal microbalance sensor for detection of aliphatic amines vapours. Sens. Actuators B Chem. 2010, 147, 481–487.
- Filippo, E.; Manno, D.; Buccolieri, A.; Serra, A. Green synthesis of sucralose-capped silver nanoparticles for fast colorimetric triethylamine detection. Sens. Actuators B Chem. 2013, 178, 1–9.
- Moore, W.M.; Edwards, R.J.; Bavda, L.T. An Improved Capillary Gas Chromatography Method for Triethylamine. Application to Sarafloxacin Hydrochloride and GnRH Residual Solvents Testing. Anal. Lett. 1999, 32, 2603–2612.
- Majder-Łopatka, M.; Węsierski, T.; Dmochowska, A.; Salamonowicz, Z.; Polańczyk, A. The Influence of Hydrogen on the Indications of the Electrochemical Carbon Monoxide Sensors. Sustainability 2019, 12, 14.
- Sun, Y.; Ding, W.; Li, J.; Jia, Y.; Guo, G.; Deng, Z. A novel and simple fluorescent chemical sensor SX based on AIE for relay recognition of Zn2+ and Cu2+ in aqueous system and analysis in logic gates. J. Mol. Struct. 2022, 1252, 132219.
- Promphet, N.; Ummartyotin, S.; Ngeontae, W.; Puthongkham, P.; Rodthongkum, N. Non-invasive wearable chemical sensors in real-life applications. Anal. Chim. Acta 2021, 1179, 338643.
- Gui, Y.; Zhao, J.; Wang, W.; Tian, J.; Zhao, M. Synthesis of hemispherical WO3/graphene nanocomposite by a microwave-assisted hydrothermal method and the gas-sensing properties to triethylamine. Mater. Lett. 2015, 155, 4–7.
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225.
- Zhang, J.; Yu, Y.; Fang, P.; Liu, L.; Yue, H.; Ou, J.; Han, A. Anodization of aluminum in a sealed container. Electrochem. Commun. 2021, 129, 107086.
- Chu, X.; Chen, T.; Zhang, W.; Zheng, B.; Shui, H. Investigation on formaldehyde gas sensor with ZnO thick film prepared through microwave heating method. Sens. Actuators B Chem. 2009, 142, 49–54.
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217.
- Xu, X.; Ma, S.; Xu, X.; Pei, S.; Han, T.; Liu, W. Transformation synthesis of heterostructured SnS2/ZnS microspheres for ultrafast triethylamine detection. J. Alloys Compd. 2021, 868, 159286.
- Xu, K.; Zhan, C.; Zhao, W.; Yu, X.; Zhu, Q.; Yang, L. Tunable resistance of MOFs films via an anion exchange strategy for advanced gas sensing. J. Hazard. Mater. 2021, 416, 125906.
- Yang, T.; Yang, X.; Zhu, M.; Zhao, H.; Zhang, M. Coral-like ZnFe2O4–ZnO mesoporous heterojunction architectures: Synthesis and enhanced sensing properties for triethylamine. Inorg. Chem. Front. 2020, 7, 1918–1926.
- Zeb, S.; Cui, Y.; Zhao, H.; Sui, Y.; Yang, Z.; Khan, Z.U.; Ahmad, S.M.; Ikram, M.; Gao, Y.; Jiang, X. Synergistic Effect of Au-PdO Modified Cu-Doped K2W4O13 Nanowires for Dual Selectivity High Performance Gas Sensing. ACS Appl. Mater. Interfaces 2022, 14, 13836–13847.
- Gong, F.-L.; Peng, M.-X.; Yue, L.-J.; Chen, J.-L.; Xie, K.-F.; Zhang, Y.-H. Design of p-n heterojunction on mesoporous ZnO/Co3O4 nanosheets for triethylamine sensor. Chem. Phys. Lett. 2021, 779, 138891.
- Shao, S.; Xie, C.; Xia, Y.; Zhang, L.; Zhang, J.; Wei, S.; Kim, H.W.; Kim, S.S. Highly conjugated three-dimensional van der Waals heterostructure-based nanocomposite films for ultrahigh-responsive TEA gas sensors at room temperature. J. Mater. Chem. A 2022, 10, 2995–3008.
- Zhang, Y.-H.; Wang, C.-N.; Gong, F.-L.; Chen, J.-L.; Xie, K.-F.; Zhang, H.-L.; Fang, S.-M. Ultra-sensitive triethylamine sensors based on oxygen vacancy-enriched ZnO/SnO2 micro-camellia. J. Mater. Chem. C 2021, 9, 6078–6086.
- Liu, L.; Zhao, Y.; Song, P.; Yang, Z.; Wang, Q. ppb level triethylamine detection of yolk-shell SnO2/Au/Fe2O3 nanoboxes at low-temperature. Appl. Surf. Sci. 2019, 476, 391–401.
- Li, Z.; Xiong, Y.; Bi, D.; Liu, Q.; Yang, C.; Zhang, J. Continuously improved gas-sensing performance of Zn2SnO4 porous octahedrons by structure evolution and further ZnSnO3 nanosheets decoration. J. Alloys Compd. 2022, 901, 163744.
- Xiong, Y.; Liu, W.; Qiao, X.; Song, X.; Wang, S.; Zhang, X.; Wang, X.; Tian, J. Confined synthesis of 2D ultrathin ZnO/Co3O4 nanomeshes heterostructure for superior triethylamine detection at low temperature. Sens. Actuators B Chem. 2021, 346, 130486.
- Liang, X.; Zhang, J.; Zhang, K.; Yang, X.; Zhang, M. The modification effect of Fe2O3 nanoparticles on ZnO nanorods improves the adsorption and detection capabilities of TEA. Inorg. Chem. Front. 2022, 9, 259–266.
- Jin, Q.; Wen, W.; Wang, Z.-X.; Wang, R.-H.; Zheng, S.; Ye, Z.; Wu, J.-M. Nanoarchitectonics of nest-like MnO2/TiO2 thin film for triethylamine sensing. Sens. Actuators B Chem. 2022, 353, 131137.
- Meng, L.; Bu, W.; Li, Y.; Qin, Q.; Zhou, Z.; Hu, C.; Chuai, X.; Wang, C.; Sun, P.; Lu, G. Highly selective triethylamine sensing based on SnO/SnO2 nanocomposite synthesized by one-step solvothermal process and sintering. Sens. Actuators B Chem. 2021, 342, 130018.
- Meng, F.; Liao, Z.; Xing, C.; Yuan, Z.; Zhang, R.; Zhu, H.; Li, J. Preparation of SnO2/SiO2 nanocomposites by sol-gel method for enhancing the gas sensing performance to triethylamine. J. Alloys Compd. 2022, 893, 162189.
- Liu, J.; Zhang, L.; Fan, J.; Yu, J. Semiconductor Gas Sensor for Triethylamine Detection. Small 2022, 18, e2104984.
- Yang, J.; Han, W.; Ma, J.; Wang, C.; Shimanoe, K.; Zhang, S.; Sun, Y.; Cheng, P.; Wang, Y.; Zhang, H.; et al. Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens. Actuators B Chem. 2021, 340, 129971.
More
