Covalent organic frameworks (COFs) are defined as crystalline organic polymers with programmable topological architectures using properly predesigned building blocks precursors. Since the development of the first COF in 2005, many works are emerging using this kind of material for different applications, such as the development of electrochemical sensors and biosensors. COF shows superb characteristics, such as tuneable pore size and structure, permanent porosity, high surface area, thermal stability, and low density. Apart from these special properties, COF’s electrochemical behaviour can be modulated using electroactive building blocks. Furthermore, the great variety of functional groups that can be inserted in their structures makes them interesting materials to be conjugated with biological recognition elements, such as antibodies, enzymes, DNA probe, aptamer, etc. Moreover, the possibility of linking them with other special nanomaterials opens a wide range of possibilities to develop new electrochemical sensors and biosensors.
- COF
- electrochemical biosensors
- electrochemical sensors
1. Introduction
- Introduction
Covalent organic frameworks (COFs) are a type of polymer,s which connect organic molecules in two (2D) or three dimensions (3D) via covalent bonds [1][2][1,2]. Their most noteworthy feature is their intrinsic order, which is predetermined by the monomers, or linkers, employed in the polymerization, building crystalline structures with pre-designable architectures [3][4][5][3,4,5]. The design of the network’s topology lies in the control of the direction of covalent bond formation during the polymerization [1]. To ensure the directionality of growth, the monomers must be formed by relatively rigid bonds and present the functionalities in specific positions [6][7][6,7]. To do this, the design is approached from a simplified block model, where each monomer is represented with a specific geometric shape that symbolizes the relative positions of the reactive points [6][7][8][6,7,8]. In this way, as depicted in Figure 1 Figure 1A, a linear monomer could generate hexagonal networks by cyclotrimerization (for example boroxine formation) or produce square COFs if it is combined with a C4 linker. Following the pioneering work of Yaghi and the co-workers of Yaghi [9][9], several structural motifs have been described, including hexagonal [10][11][10,11], kagome [12][13][14][12,13,14] or square [15][16][15,16] two-dimensional networks (2D-COFs), and diamonoid [17][18][17,18], Cubic [19] [19],[19] or PtS [20] PtS [20] three-dimensional architectures (3D-COFs). It is worth pointing out that the dimensionality of the COF network is also determined by the linkers employed in the polymerization. 2D-COFs are generally built from flat molecules, while 3D-COFs are usually based on linkers endowed with sp3 centres [18] or a geometry significantly distorted from planarity via steric tuning [20].
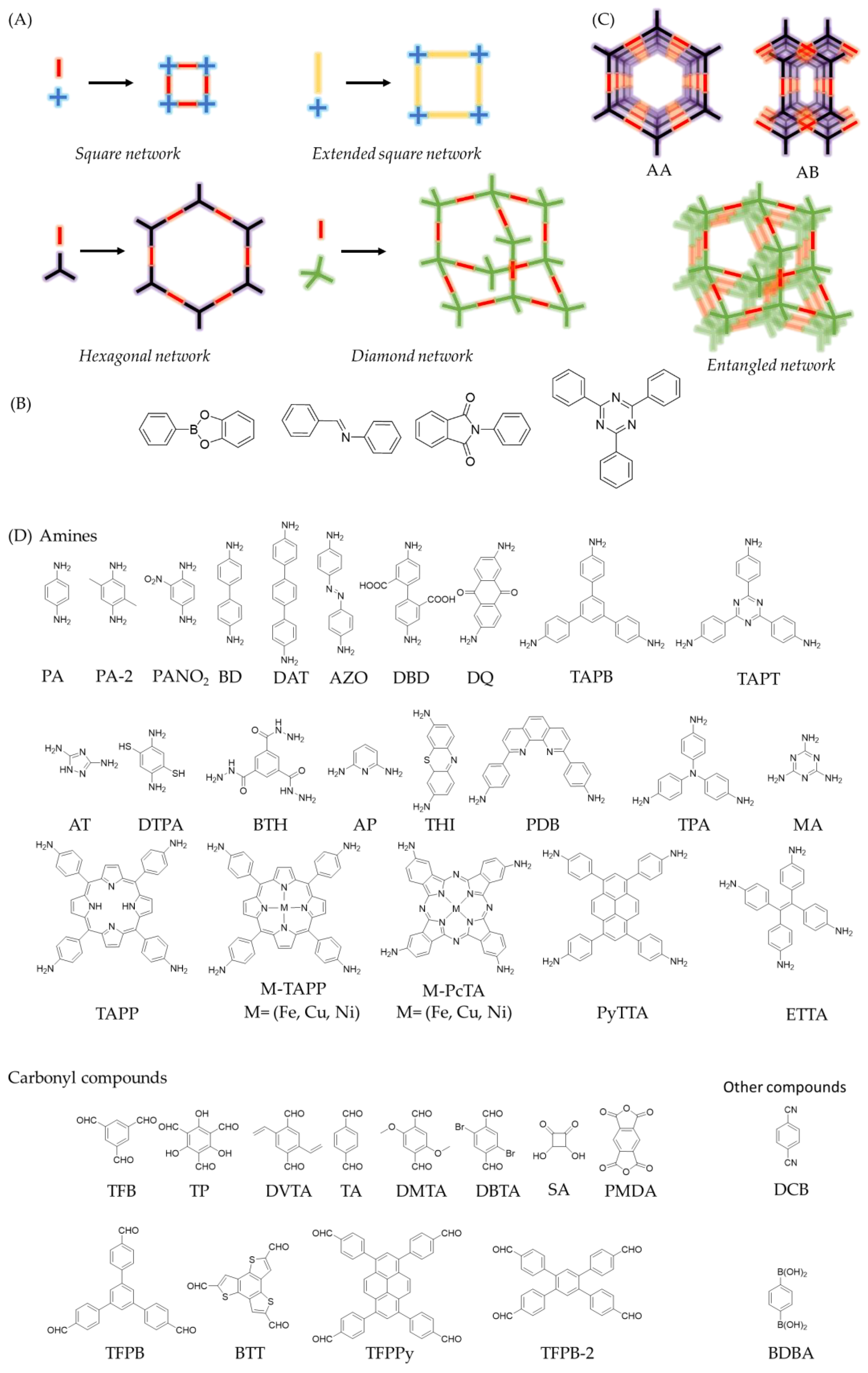
Figure 1. A) Examples of COFs topology diagrams. B) Examples of linkages commonly used for the synthesis of COFs (from left to right: boronate ester, imine, imide and triazine. C) Examples of arrangements of COF’s networks. D) List of linkers named in this rentryview.
The type of bond used to connect the building blocks, commonly known as linkage, is crucial during the formation of the framework as multiple covalent structures with different degrees of crystallinity can be produced during the polymerization reactions [6][7][6,7]. In fact, the main difference between COFs and pPorous oOrganic pPolymers (POPs) is the crystalline order. The generally most widespread strategy to prevent the formation of amorphous products and favour the COF’s crystallization is the use of reversible reactions [6][7][6,7]. In this way, the balance between reactants and products allows curing and error correction of the amorphous network, usually associated to kinetic control products, until the COF crystallizes as the thermodynamically more stable product [7]. These are the characteristic features of dynamic covalent chemistry (DCC) [21][22][21,22]. In simple terms, in a system where DCC operates, it is the stability of the products that decides the final distributions [2]. Thus, several linkages have been used for the formation of COFs, such as boroxine, boronate esterification, imine, azine, imide, or triazine (Figure 1B) [6]. Other approaches to enhance the crystallinity of the systems includes the addition of monofunctional modulators [18], competitors[23] [23], and protected monomers [24] as strategies to control the nucleation and growth of the crystalline phases [25][26][25,26]. Unfortunately, the crystallization conditions to obtain the more thermodynamically more stable products are not known a priori, a priori and the COF crystallization is usually achieved via iterative screening methodology. Nevertheless, a plethora of methodologies have been reported in the last years to enhance the crystallinity of COFs, including the solvothermal synthesis [10], microwave-assisted methods [27] [27], ionothermal synthesis [28], interfacial synthesis [29], repolymerization methods [30],[30] or on-surface synthesis [31]. Furthermore, screening of experimental conditions and structure–-property relationships are being accelerated through computational calculations and automated machine learning [32][33][34][32,33,34], which are emerging as powerful tools to obtain tailor-made materials in record times.
Another distinctive characteristic of COFs is their inherent porosity [35][36][37][35,36,37]. On the one hand, the overlap of the 2D-COFs layers can build up a three-dimensional structure, traversed by unidirectional cavities also known as pores, which can mainly present as two itwo mainly isomers. The first one is an eclipsed structure (AA), composed by adjacent layers, which are located in the same position. The second one, is a staggered structure (AB or ABC), where every COF layer is shifted with respect to the previous one. It is worth pointing out that the staggered structure in 2D-COFs also presents pores traversing the three-dimensional structure. However, in terms of porosity, the structure thatwhich presents the highest surface area is the eclipsed isomer network [1][2][1,2]. On the other hand, networks entanglements can be produced in 3D-COFs, thus lowering the theoretical surface area (Figure 1C) [18].
COFs presents high thermal, mechanical, and chemical stabilities, with differences depending on the linkage and/or the monomers used in the polymerization reaction [6]. The above-mentioned characteristics, together with their inherent insolubility [1], place COFs as ideal materials to explore their applicability in heterogeneous phases. In this way, COFs have already been used in different applications, such as catalysis [38][39][40][38,39,40], solar cells [41], batteries [42][43][42,43], gas storage and separation [44][45][44,45], water remediation [39][46][39,46] and sensing [47].
Concerning with the integration in devices, COFs must face the doble edged sword of one of their main attributes, their inherent insolubility. In this way, the most common procedure to achieve tailor-made devices are two depending on the followed path: (i) Top-down protocol involves the destruction of the granular COF structure, achieving the sheet separation in a process known as exfoliation producing covalent organic nanosheets (CONs) [48]. To address this end, several methods have been developed. The most common procedure is liquid phase exfoliation, which consists in the use of different solvents to produce layer slipping and the subsequent delamination in an ultrasonic bath [49]. Another method is the mechanical delamination, which produces the exfoliation by using an external force over the bulk COF [50]. Chemical or acid spontaneous exfoliations are receiving a great deal of attention because theyit causes the COF delamination using chemical energy, reducing the costs for obtaining CONs [49]. Finally, nitrogen delamination consists in the gentley heating of the bulk COF and subsequent addition of liquid nitrogen, which intercalates between the COF layers and produces exfoliation by the thermal expansion in the liquid-to- to gas phase transition [51]. (ii) The use of bottom-up methods involves the formation of the CONs or the tailor-made COF directly from the monomers. On the one hand, for the synthesis of nanosheets its usually requiresd the use of additives, such as tetra-n-butylammonium fluoride (TBAF) micelles or surfactants mediators producing monomodal distribution of CONs by the restriction of the growth dimensions. Thus, mi-cellar synthesis allows the growing of large few-layered COFs in the interphase between two immiscible solvents [29][52][29,52]. On the other hand, tailor-shaped COFs are obtained by using either the 3D-printing technique, which involves the preparation of a COF “ink” and the subsequent printing following a layer-by-layer protocol [53][54][53,54] or the formation of monolithic COF aerogels by crystallization in moulds to produce tailor-made macroscopic objects [55][56][55,56].
Concerning sensing applications, between all the COF-based sensor assemblies, electrochemical sensors present important advantages, such as their high porosity, which results ion increased sensibility, and the high specificity and fantastic biocompatibility that improves the stability of the electrochemical sensors [57]. The most common procedure followed to achieve these devices is the use of COFs as supports to anchor the active species (or guests) towards the recognition [58][59][58,59] . In this way, the COF enhances the electrochemically active surface area of the electrode by preventing the agglomeration of the active species [59][60][59,60]. There are mainly three main methods to anchor the active species into COFs. The first one involves the coordination of the metallic species in Lewis acid or basic positions of the COF [61]. The second one involves the active specie anchorage by other non-covalent interactions, such as π-interactions [59]. Finally, covalent immobilization can be also be achieved by chemical reactions involving backbone modification or pendant groups reactions, highlighting Hüisgen’s the Hüisgen’s azide–-alkyne cycloaddition as the most prominent example of these guests’ immobilization methods [5].
To achieve electrochemical sensing, great conductivity is desired. However, despite COFs present an ordered and columnar π-skeleton, the electrochemical sensing is still limited due to the polygranular COF’s morphology, leading to insignificant hopping-type electrical conductivity in most of the examples [62. Electrochemical Sensors
]. In the past few years, several strategies have been developed to address this problem. To avoid this problem, conductive additives can be added during the sensor assembly. The most common example is the in situ reduction of metallic precursors to produce metallic nanoparticles (MNPs), which can be anchored to the COF pore walls ha by coordinative interactions preventing the NPs agglomeration [63,64]. Other common conductive additives are carbeonaceous compounds such as carbon black (CB) [65], pyrolyzed three-dimen sional kenaf stem (3D-KSC) [66,67,68] or multi-wall carbon nanotubes (MWCNTs) [69] .
Sensor assemblies are usually achievedely u by different strategies. One of them involves the mixing of the COF with the conductive additives in a hollow glass tube [70,71]. A second in the develostrategy involves the electrode modification with Covalent Organic Nanosheets (also known as CONs [72,73]), produced by delament of ination of COFs usually by liquid phase exfoliation (LPE) and subsequent drop casting of CONs colloids [74,75,76]. Finally, the sensor’s design can be based on simple electrodes [58,61] of dual systems, highlighting the sandwich type electrodes [77,78].
Different examples of electrochemical sensor. Researches and biosensors are exposed in this review, showing the great versatility of functions and capabilities of materials based on COFs, resulting in interesting approaches that can enhance the sensibility and selectivity of the final devices.
- Electrochemical Sensors
COFs have been widely used in the development of electrochemical s ensor. We have classified COFs in three different groups attending to their main role in electrochemical sensor.
2.1. COFs acting as electrocatalysts
The easiest configuration for the use of COFs in the development of electrochemical sensors involves the direct deposition of the COF as modifier on top of a working electrode surface. The high porosity of COFs-based materials and their crystalline structure make them interesting materials for that purpose as shown in the work developed by Y.-H. Pang et al. [79].
2.2. COFs acting as support of Electrocatalyst and/or recognition elements.
One of the main functions of COFs in this context is their role as recognition element in the sensor. The high porosity of COFs together with the possibility of delamination of 2D-COFs allow the production of 2D-nanomaterials with enhanced surface-volume ratio which allows an increment of the sensor recognition element or the electroactive centre exposition to the measuring solution media.
2.3. COFs chelating properties for anodic stripping analysis.
The capability of COFs to include multiple functional groups in their structures allows the development of COFs endowed with chelating groups. There are several examples in the literature showing the use of COFs as chelating agents to promote pre-concentration of metal cations for differential pulse anodic stripping voltammetry.
3. Electrochemical biosensors
- Electrochemical biosensors
COF skeletons are superior scaffolds to allow charge migration and improve the signal amplification in electrochemical sensors. The large specific surface area and tuneable pore structure of COFs make of them good candidates for the development of functional biosensors.
3.1. Enzymatic Biosensors
The structure tunability, porosity, crystallinity, and stability of COFs are promising characteristics for their use envisaged as host arrays for the immobilization of enzymes, among them tunability [80][110]. Their customizable composition through the proper choosing of their precursor, which also determine the functional groups on their surfaces, can be tailored to favour some specific interactions between them and enzymes. Furthermore, the regular distribution of nanopores in the COFs is beneficial to provide a high surface area interface for the adsorption and desegregation of enzymes and to allow a rapid movement of reagents. Moreover, the structural robustness of COFs represents an important attribute to stabilize enzymes during the biosensor manufacture process. Some reported works have used COFs to encapsulate multiple enzymes simultaneously [81][112].
3.2. Immunosensors
COFs have also been used as support material for the development of immunosensors. COFs can be also used to develop electroactive labels linked with secondary antibodies. The high porosity together with the high amount of adsorption sites along the COFs nanostructure make them excellent candidates to guest electroactive molecules, which can be reduced or oxidized during the measuring step, obtaining an electrochemical signal proportional to the concentration of the antigen being analysed [82][114]. A different strategy to obtain a labelling agent is the use of COFs together with peroxidase enzymes, which are adsorbed on the COFs nanostructures [83][117].
3.3. Genosensors
COFs can be used as structural materials to adsorb oligonucleotides and their further use as DNA probes for the development of genosensors [84][119].
3.4. Aptasensors
COFs can be also used as supports to adsorb oligonucleotides, which are capable of recognizing target proteins with an affinity and specificity rivalling that of antibodies [85][122]. The combination of COFs with carbon nanomaterials has been also used for the development of aptasensors as recently reported by He and co-workers [86][126]. COFs have been also used in combination with metal nanoparticles for the development of aptasensors. Metal nanoparticles can supply the limited electrical conductivity of COFs [87][128]. COFs have been also combined with MOFs for the development of aptasensors [59].
Table 1. Examples of COFs application in different samples.
| COF |
|---|
|
COF |
| Modifier |
|---|
Modifier |
| Analyte |
|---|
Analyte |
| Sample |
|---|
Sample |
| Electrode |
|---|
Electrode |
| LOD |
|---|
LOD |
| Linear Range |
|---|
Linear Range |
| Sensitivity |
|---|
Sensitivity |
| Ref. |
|---|
Ref |
β-ketoenamine | |||||||||
|
Bisphenol A, and S |
Food packages |
Graphite |
0.15 and 0.15 µM |
0.5-30 and 0.5-30 µM |
0.239 and 0.150 µA/µM |
[80] |
|||
|
TPA-COF |
Composite Carbon Black |
Dopamine |
Medical injections |
Glassy Carbon |
0.17 µM |
20-1000 µM |
0.023 µA/µM |
[65] |
|
|
TAPB-DMTP-COF@PANI |
polyanyline |
Acetaminophen |
tablets, human blood, serum and urine |
Glassy Carbon |
0.032 µM |
0.10-500 µM |
0.1229 µA/µM |
[84] |
|
|
Fe3O4@AT-COF |
Fe3O4 |
p-nitrophenol and o-nitrophenol |
lake and tap water |
Magnetic beads |
0.2278 and 0.5925 μM |
10-3000 and 10-3000 µM |
0.7588 and 0.7799 µA/µM |
[85] |
|
|
FeTAPP-TA-COF |
Composite graphene aerogel |
NO |
Complex biological system |
Glassy carbon |
0.030 μM |
0.18-400 μM |
8.8 μA/‧μM·cm2 |
[86] |
|
|
MA-TP-COF |
β-Ketoamine |
Cd2+, Cu2+, Pb2+, Hg2+ and Zn2+ |
Drinking water |
Glassy Carbon |
0.922, 0.450, 0.309, 0.208 and 0.526 nM |
- |
17.8, 36.6, 53.2, 79.1 and 31.3 μA/μM cm2 |
[106] |
|
|
DTPA-TFB-COF |
kenaf stem-derived macroporous carbon |
Cd2+, Pb2+, Cu2+ and Hg2+ |
Soil and sewage |
|
12.3 , 11.8 , 18.6 and 21.4 nM |
0.0369-18.0, 0.0356-19.0, 0.0536-19.0, and 0.0503-18.0 µM |
1337.4 , 1389.0 886.2 and 770.0 μA/μM cm2 |
[108] |
|
|
GOD/DMFc/ PA-TFB-COF/CFMEs) |
DMFc and GOD |
Glucose |
Rats’ brains |
Carbon fibre microelectrode |
0.36 µM |
1.08 μM to 8.5 mM |
46.55 mV/ mM cm 2 |
[111] |
|
|
enzyme@ZIF-8@COF |
GOD, HRP, AChE |
Glucose, H2O2 and malathion |
|
Glassy Carbon |
0.85 μM, 2.81 nM, 3.0×10−13 g/L |
2.83 μM–8.0 mM, 9.53 nM-7.0 μM, 10−12 g/L-10−8 g/L |
-
|
[75] |
|
|
COFs-AuNPs |
AuNPs, Capture antiKIM-1 |
KIM-1 |
Plasma samples |
Glassy Carbon |
2.00 fg/ mL |
0.01–50.00 pg/ mL |
1.8981 µA·mL/pg |
[113] |
|
|
AuPt@MnO2@COF |
AuNPs, PSA affinity peptide |
PSA |
Human serum |
|
16.7 fg/mL |
0.00005–10 ng/mL |
2.237 µA/log(ng/mL) |
[115] |
|
|
TP-PANO2-COF |
AuNPs, CYFRA21-1 antibodies |
CYFRA21-1 |
Human serun |
|
0.1 pg/mL |
0.5–1.0 × 104 pg/mL |
6.3 µA/log(pg/mL |
[116] |
|
|
Cu-MOF@Cu-PcTA-COF |
Cu-MOF, HIV-1 DNA probe strands |
HIV-1 DNA |
Human serun |
Glassy carbon |
0.07 fM |
1 fM to 1 nM |
- |
[119] |
|
|
Acronyms |
|
||||||||
|
1,3,5-triformylphloroglucinol (TP) and 2,6-diaminoanthraquinone (DQ) |
|
||||||||
|
TPA-COF triphenylamine-based covalent-organic framework |
|
||||||||
|
TAPB, 1,3,5-tris(4-aminophenyl)benzene; DMTA, 2,5-dimethoxyterephaldehyde |
|
||||||||
|
1,3,5-tris(4-aminophenyl) benzene (TAPB) and 1,3,5-benzenetricarboxaldehyde (TFB) |
|
||||||||
|
5,10,15,20-tetrakis (4-aminophenyl) porphinato]-iron (Fe-TAPP) and terephthalaldehyde (TA), |
|
||||||||
|
2,4,6-triformylphloroglucinol and melamine (MA-TP-COF). |
|
||||||||
|
1,4-benzenedithiol-2,5-diamino-hydrochloride (DTPA) and 1,3,5-triformylbenzene (TFB) |
|
||||||||
|
1,3,5 -Triformylbenzene (TFB) and 1,4-diaminobenzene (PA) |
|
||||||||
|
1,3,5-tris(p-formylphenyl) benzene (TFPB) and 4,4′-diaminobiphenyl-2,2′-dicarboxylic acid (DBD) in the presence of enzyme@ZIF-8 |
|
||||||||
|
Triformylphloroglucinol and 2-nitrobenzene-1,4-diamine (PANO2), |
|
||||||||
|
Copper-phthalocyaninetetra-amine (Cu-PcTA) and 2,9-bis[p-(formyl)phenyl]-1,10-phenanthroline (PDB) |
|
||||||||
| DQTP-COF | β-ketoenamine | Bisphenol A, and S | Food packages | Graphite | 0.15 and 0.15 µM |
0.5–30 and 0.5–30 µM | 0.239 and 0.150 µA/µM | [88] |
| TPA-COF | Composite Carbon Black | Dopamine | Medical injections | Glassy Carbon | 0.17 µM | 20–1000 µM | 0.023 µA/µM | [65] |
| TAPB-DMTP-COF@PANI | polyanyline | Acetaminophen | Tablets, human blood, serum and urine | Glassy Carbon | 0.032 µM | 0.10–500 µM | 0.1229 µA/µM | [89] |
| Fe3O4@AT-COF | Fe3O4 | p-nitrophenol and o-nitrophenol | Lake and tap water | Magnetic beads | 0.2278 and 0.5925 μM | 10–3000 and 10–3000 µM | 0.7588 and 0.7799 µA/µM | [90] |
| FeTAPP-TA-COF | Composite graphene aerogel | NO | Complex biological system | Glassy carbon | 0.030 μM | 0.18–400 μM | 8.8 μA/μM·cm2 | [91] |
| MA-TP-COF | β-Ketoamine | Cd2+, Cu2+, Pb2+, Hg2+ and Zn2+ | Drinking water | Glassy Carbon | 0.922, 0.450, 0.309, 0.208 and 0.526 nM | - | 17.8, 36.6, 53.2, 79.1 and 31.3 μA/μM cm2 | [92] |
| DTPA-TFB-COF | kenaf stem-derived macroporous carbon | Cd2+, Pb2+, Cu2+ and Hg2+ | Soil and sewage | 12.3, 11.8, 18.6 and 21.4 nM | 0.0369–18.0, 0.0356–19.0, 0.0536–19.0, and 0.0503–18.0 µM | 1337.4, 1389.0, 886.2 and 770.0 μA/μM cm2 | [93] | |
| GOD/DMFc/PA-TFB-COF/CFMEs | DMFc and GOD | Glucose | Rats’ brains | Carbon fibre microelectrode | 0.36 µM | 1.08 μM to 8.5 mM | 46.55 mV/mM cm2 | [94] |
| enzyme@ZIF-8@COF | GOD, HRP, AChE | Glucose, H2O2 and malathion | Glassy Carbon | 0.85 μM, 2.81 nM, 3.0 × 10−13 g/L | 2.83 μM–8.0 mM, 9.53 nM–7.0 μM, 10−12 g/L–10−8 g/L | - | [75] | |
| COFs-AuNPs | AuNPs, Capture antiKIM-1 | KIM-1 | Plasma samples | Glassy Carbon | 2.00 fg/mL | 0.01–50.00 pg/mL | 1.8981 µA·mL/pg | [95] |
| AuPt@MnO2@COF | AuNPs, PSA affinity peptide | PSA | Human serum | 16.7 fg/mL | 0.00005–10 ng/mL | 2.237 µA/log (ng/mL) | [96] | |
| TP-PANO2-COF | AuNPs, CYFRA21-1 antibodies | CYFRA21-1 | Human serum | 0.1 pg/mL | 0.5–1.0 × 104 pg/mL | 6.3 µA/log (pg/mL) | [97] | |
| Cu-MOF@Cu-PcTA-COF | Cu-MOF, HIV-1 DNA probe strands | HIV-1 DNA | Human serum | Glassy carbon | 0.07 fM | 1 fM to 1 nM | - | [84] |
| Acronyms | ||||||||
| 1,3,5-triformylphloroglucinol (TP) and 2,6-diaminoanthraquinone (DQ) | ||||||||
| TPA-COF triphenylamine-based covalent-organic framework | ||||||||
| TAPB, 1,3,5-tris(4-aminophenyl)benzene; DMTA, 2,5-dimethoxyterephaldehyde | ||||||||
| 1,3,5-tris(4-aminophenyl) benzene (TAPB) and 1,3,5-benzenetricarboxaldehyde (TFB) | ||||||||
| 5,10,15,20-tetrakis [(4-aminophenyl) porphinato]-iron (Fe-TAPP) and terephthalaldehyde (TA) | ||||||||
| 2,4,6-triformylphloroglucinol and melamine (MA-TP-COF) | ||||||||
| 1,4-benzenedithiol-2,5-diamino-hydrochloride (DTPA) and 1,3,5-triformylbenzene (TFB) | ||||||||
| 1,3,5-Triformylbenzene (TFB) and 1,4-diaminobenzene (PA) | ||||||||
| 1,3,5-tris(p-formylphenyl) benzene (TFPB) and 4,4′-diaminobiphenyl-2,2′-dicarboxylic acid (DBD) in the presence of enzyme@ZIF-8 | ||||||||
| Triformylphloroglucinol and 2-nitrobenzene-1,4-diamine (PANO2) | ||||||||
| Copper-phthalocyaninetetra-amine (Cu-PcTA) and 2,9-bis[p-(formyl)phenyl]-1,10-phenanthroline (PDB) | ||||||||
|
DQTP- COF. |
4. Conclusions
COFs are new and emerging materials with multiple application fields. Among them, sensor and biosensor development is a wide area of application of these materials, as a consequence of their extraordinary properties. In particular, it is worth highlighting their high porosity, appropriate pore sizes for the immobilization of specific recognition elements and post-modification capacity. In addition, the possibility of predesigning chemical structures according to the correct selection of their precursors is opening the door to interesting sensors and biosensors developments. COF can be used in the sensor platforms in order to improve specific area, serving as supportive material for electrocatalysts, enzymes, antibodies, oligonucleotides…etc. COF can also have a specific function related with their structure, acting as electrocatalyst, recognizing element and offering the functional groups needed for specific adsorption or chelating process. Innovative strategies are using COFs as label agent developments, linking them with specific antibodies, aptamer, enzymes, oligonucleotides etc. The great variety of COFs structures and the enormous amount of new COF designs would have a starring role in the next generation of electrochemical sensors and biosensors. Finally, prospects of sensors assemblies should address the problems produced from the inherent insolubility of the COFs. Thus, the use of dynamic covalent chemistry to produce CONs with monomodal distributions through bottom-up strategies may improve the sensor quality, since the top-down strategies may be very destructive, yielding uncontrolled distribution of nanosheets sizes. Furthermore, to ensure a sustainable future, the inclusion of metallic additives on the sensor composition should be avoided. In addition, other additives could produce pore blockage limiting the analyte diffusion to the active sites. Furthermore, the characteristic electrical insulator behaviour of COFs must be ad-dressed by either developing conductive COFs or by using few-layer COFs ensuring close contact between COFs and electrodes. Finally, the new trends on tailor-made COFs by 3D-printing techniques and shapeable monolithic aerogels will pave the way for the production of tailor-made sensors based on COFs to ensure proper device integrations and scalability.
