Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Danushka S Seneviratne.
Boron neutron capture (BNCT) is a biologically targeted, densely ionizing form of radiation therapy that allows for increased tumor cell kill, while reducing toxicity to surrounding normal tissues.
- BNCT
- boron neutron capture therapy
- breast cancer
1. Introduction to BNCT
Boron neutron capture (BNCT) is an emerging radiation treatment modality that is aimed at improving tumor control while limiting damage to normal tissues. BNCT falls under the category of high linear energy transfer (high-LET) radiation, which is a form of densely ionizing radiation that causes clustered, irreparable direct DNA damage in a less oxygen dependent manner, in comparison to traditional low-LET X-ray-based therapies. Treatment with BNCT involves the targeted delivery of boronated compounds to tumor cells, followed by the irradiation of tumors with epithermal neutrons. The interaction of the nonradioactive boron-10 atoms (10B) with thermalized neutrons causes a nuclear capture and fission reaction, leading to the production of a high LET, low energy, alpha particle and a recoil lithium-7 atom (10B + 1n → [11B]* → 4He + 7Li). Given the densely ionizing nature of these high-LET particles, the biological impact of BNCT on tumor cells, compared to a reference radiation type such as X-rays, is expected to be higher. As the high LET particles deposit their energy within a radius of <10 μm, the resulting DNA damage only occurs within the diameter of a single tumor cell, largely sparing adjacent normal tissues. The dense ionization tracks produced along the DNA by high-LET radiation has been shown to produce greater DNA double strand breaks in comparison to low-LET counterparts. Additionally, studies indicate that the clustered DNA damage produced following high-LET radiation is more difficult to repair and is more likely to lead to genomic instability and cell death [1]. In addition to the above described high-LET particles, two other types of directly ionizing radiation are also produced during this reaction and contribute to the overall background radiation dose. These include γ rays formed following capture of the thermal neutrons by the hydrogen atoms in tissue, and the protons created through the scattering of fast neutrons [2,3,4][2][3][4]. The mechanism of action of BNCT is demonstrated in Figure 1.
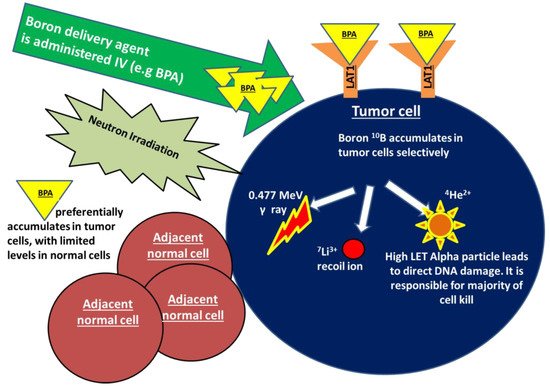
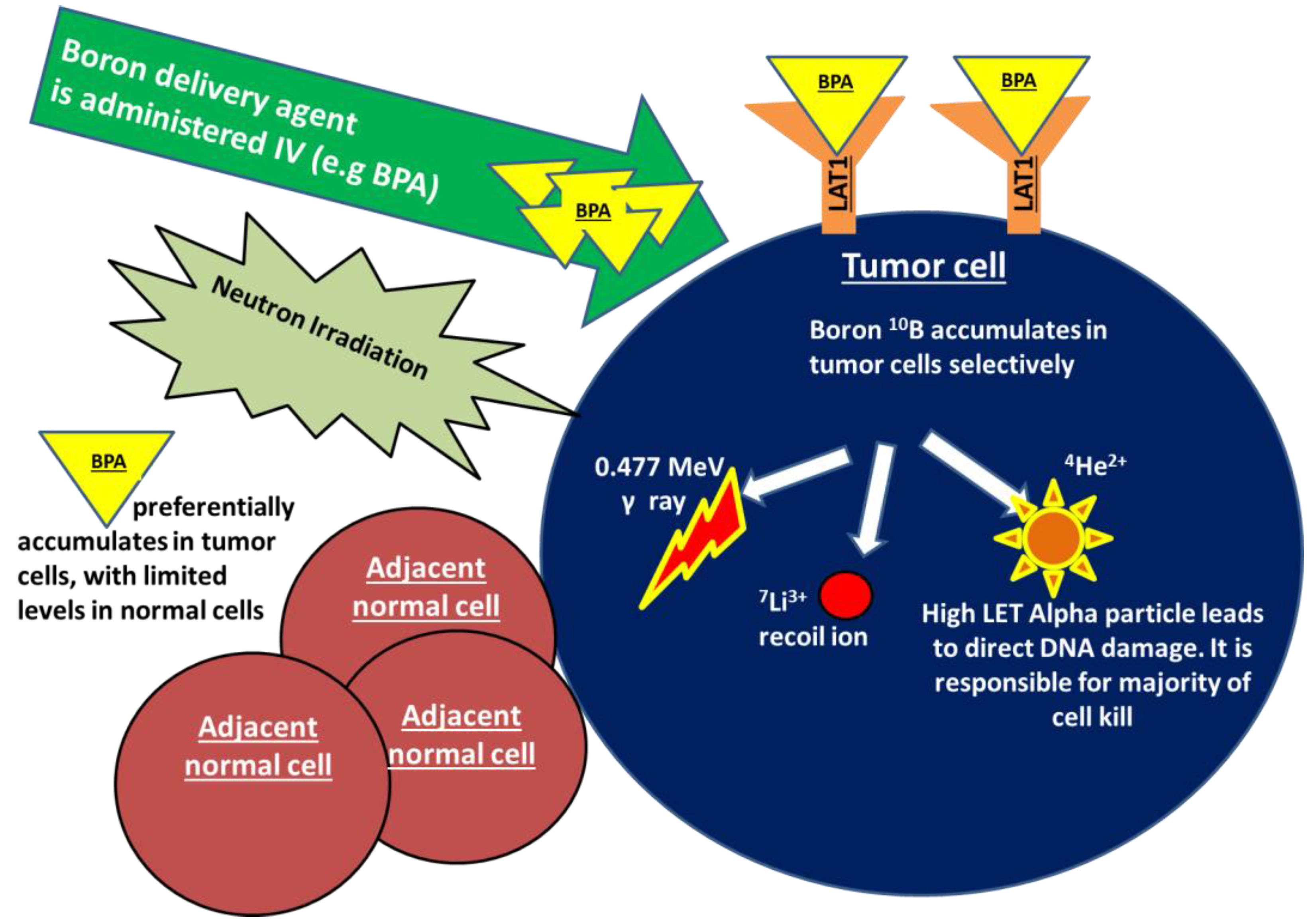 Figure 1. Boron neutron capture (BNCT) is a form of high linear energy transfer (high-LET) radiation. Treatment with BNCT involves the targeted delivery of boronated compounds to tumor cells, followed by irradiation of the tumor with epithermal neutrons. The interaction of the nonradioactive boron-10 atoms (10B) with neutrons causes a nuclear capture and fission reaction, leading to the production of a high LET alpha particle, a recoiling lithium-7, as well as γ rays and protons (10B + 1n → [11B]* → 4He + 7Li).The alpha particle is responsible for a majority of BNCT-induced DNA damage and cell kill. As the high LET particles deposit their energy within a radius of <10 μm, the DNA damage only occurs within the diameter of a single tumor cell, sparing any adjacent normal tissues. BPA in particular shows promise in breast cancer as a boron carrier given that is taken up by the transmembrane LAT-1 amino acid transporter that is highly overexpressed in breast cancer cells.
Figure 1. Boron neutron capture (BNCT) is a form of high linear energy transfer (high-LET) radiation. Treatment with BNCT involves the targeted delivery of boronated compounds to tumor cells, followed by irradiation of the tumor with epithermal neutrons. The interaction of the nonradioactive boron-10 atoms (10B) with neutrons causes a nuclear capture and fission reaction, leading to the production of a high LET alpha particle, a recoiling lithium-7, as well as γ rays and protons (10B + 1n → [11B]* → 4He + 7Li).The alpha particle is responsible for a majority of BNCT-induced DNA damage and cell kill. As the high LET particles deposit their energy within a radius of <10 μm, the DNA damage only occurs within the diameter of a single tumor cell, sparing any adjacent normal tissues. BPA in particular shows promise in breast cancer as a boron carrier given that is taken up by the transmembrane LAT-1 amino acid transporter that is highly overexpressed in breast cancer cells.2. The Potential for BNCT in Breast Cancer
2.1. Current Techniques in the Management of Breast Cancer
A multidisciplinary approach is applied to the management of breast cancer and typically involves breast-conserving surgery (BCS) with radiotherapy, or mastectomy with or without radiotherapy, as well as the use of neoadjuvant or adjuvant systemic therapy in select situations. Breast conserving therapy and mastectomy are both well-established treatment approaches with similar locoregional recurrence rates (LRR). When radiotherapy is delivered following a lumpectomy, a standard treatment field includes the whole breast with or without regional nodes. In the delivery of post-mastectomy radiation therapy (PMRT), the chest wall and regional nodes are usually included in the field [48][5]. The delivery of radiation has been shown to decrease the rate of local failure by 50% and increase the incidence of breast cancer specific survival [49,50][6][7]. Per the Early Breast Cancer Trialists’ Collaborative Group metanalysis, the delivery of PMRT improves LRR and survival in those with node positive disease, particularly if ≥4 pathologically positive nodes were detected at the time of axillary staging [51][8]. Additionally, studies also suggest that PMRT should be offered to patients with larger tumors The delivery of systemic therapy is influenced by the disease subtypes and the stage at presentation. In early-stage, hormone receptor positive tumors, adjuvant estrogen directed therapy is administered. In locally advanced breast cancer neoadjuvant or adjuvant chemotherapy containing both an anthracycline and a taxane are typically delivered. In those with the more aggressive triple negative subtype, following neoadjuvant chemotherapy, surgery, and radiation, further adjuvant chemotherapy with capecitabine is also recommended in attempts to improve long-term disease outcomes [49][6].
2.2. Inoperable Early-Stage Breast Cancer
Although BCS is well tolerated, there is a small subset of early-stage patients who refuse surgery or have extensive medical co-morbidities and thus surgery poses an excessive risk. There are currently very limited treatment options for these patients, which typically include stereotactic ablative body radiotherapy (SBRT), cryoablation, and/or systemic therapy [52,53][9][10]. Given its high-LET characteristics and the potential for increased tumor cell kill, BNCT may offer an aggressive and potentially curative treatment option in these patients through ablation of the tumor without surgery.
2.3. Locally Advanced Breast Cancer
In those with locally advanced breast cancer treated with upfront neoadjuvant chemotherapy, studies indicate that those who achieve a pCR have higher recurrence free survival and breast cancer specific survival (5-year recurrence free survival of 84% vs. 70% favoring the pCR group) [54][11]. Extensive studies conducted over the last decade have demonstrated that both locoregional and distant recurrences are heavily influenced by the tumor subtype and the molecular characteristics of the tumor. LRR and distant metastatic disease are highest in those with TNBC and lowest among those with hormone-receptor positive disease. In fact, TNBC patients who achieved a pCR were shown to have significantly improved recurrence free survival and breast cancer specific survival, while the impact of pCR on patient outcomes was much less pronounced in those with hormone receptor positive disease [49][6]. Given this, those patients who do not achieve a complete radiological response following neoadjuvant chemotherapy, particularly those of the triple negative variety, may be good candidates for pre-surgery BNCT as a window of opportunity trial. In contrast to external beam radiation which is more likely to result in an increased low dose to the surrounding normal tissues and organs at risk, a presurgical tumor targeted BNCT boost may improve the probability of pCR and ultimate disease control in select patient groups, while limiting normal tissue toxicity.
Additionally, in locally advanced breast cancer patients, BNCT may offer an opportunity to mobilize the immune system to improve cancer control. Khan et al. (2019) published methods describing the encapsulation of boron compounds into liposomes. They found that in murine mammary tumor models, uptake of boron containing liposomes modified the peripheral blood mononuclear cells to an antitumor phenotype, contributing to inhibition on tumor growth. This work suggests that in addition to direct tumor cell death following neutron irradiation, boron compounds themselves may be utilized to improve tumor control through immune modulation [34][12].
2.4. Locoregionally Recurrent Breast Cancer
During the treatment of malignancy with external beam radiation, inevitably some doses will be delivered to the surrounding normal tissues. As noted above, a primary advantage of BNCT lies in the fact that it is biologically targeted, thus, normal tissue damage is limited to cells with adequate boron accumulation. This principle is of particular importance in patients with locally recurrent breast cancer following prior irradiation of the chest wall. Further radiation is usually discouraged in this setting due to concerns regarding toxicity. In such locoregionally recurrent breast cancer, BNCT may offer a unique means to deliver ablative radiation doses to the site of recurrence with limited toxicity to adjacent tissues, particularly if surgical resection is not deemed to be favorable. Although it is yet to be clinically investigated, Gadan and colleagues have proposed the use of BNCT to treat locoregional recurrences in HER2+ breast cancers using immunoliposomes labeled with the monoclonal antibody, Trastuzumab. These liposomes may then act as boron carrier nanovehicles to target HER2 overexpressing cells in regions of disease recurrence [55][13]. A similar concept may be applied in other histological/molecular variants of breast cancer to improve disease control following the development of unresectable locoregional recurrences.
2.5. Metastatic Disease
In patients with oligometastatic breast cancer, BNCT may facilitate the ablation of multiple lesions in a targeted manner with curative intent. Given that BNCT allows for the biological targeting of disease based on boron distribution and is less likely to be impacted by internal organ motion, patients with oligometastatic disease within highly motion sensitive organs such as the lung and liver may particularly benefit from this therapy.
Furthermore, leveraging the proposed abscopal effect of BNCT may help improve disease control in widespread metastatic settings. Although this concept is yet to be fully investigated in clinical trials, Trivillin and colleagues described the abscopal effect of BNCT in a proof of principle experiment in xenografted colon cancer rat models. The reseauthorchers found that following subcutaneous injection of BPA and irradiation of right hind leg tumors with neutrons, there was a significant reduction in left leg tumor volumes, indicating that BNCT is capable of inducing abscopal effects following treatment [56][14], due to its immune sensitizing properties.
BNCT may also be advantageous in the treatment of breast cancer associated metastatic brain lesions. Patients with limited intracranial disease are typically treated with radiosurgery due to its physically targeted nature, which allows for a highly favorable dose distribution, limiting damage to the normal adjacent brain. This approach, however, at times requires subjecting the patient to invasive procedures such as an immobilizing head frame, and necessitates the resource heavy process of ensuring precise targeting of radiation within brain lesions. In the setting of many brain lesions or leptomeningeal disease, whole brain radiation is often performed. Although palliative, this treatment is associated with substantial neurocognitive effects and a negative impact on quality of life due to the irradiation of significant amounts of normal brain. While novel techniques such as hippocampal sparing and medications such as memantine have improved the neurocognitive impact of whole brain radiation therapy, approaches to further reduce these side effects are being investigated [57,58][15][16]. In both of the above situations, BNCT may offer an alternative treatment option. Given that boron accumulates primarily in the tumor cells and spares normal brain cells, it will not require precise physical targeting of radiation when treating limited intracranial disease. Similarly, when treating widespread brain lesions, BNCT may have less potential for causing long term neurocognitive impacts given the biological accumulation of boron preferentially within areas of disease.
Based on data on other malignancies, BNCT has been well tolerated with minimal acute or long-term toxicity; however, infusion reactions, skin toxicity, radiation necrosis at the site of treatment, and pseudoprogression of tumors have been described in the literature [17,59,60,61][17][18][19][20]. Further studies are needed to investigate the toxicities associated with BNCT in the treatment of breast cancer.
References
- Maliszewska-Olejniczak, K.; Kaniowski, D.; Araszkiewicz, M.; Tymińska, K.; Korgul, A. Molecular Mechanisms of Specific Cellular DNA Damage Response and Repair Induced by the Mixed Radiation Field During Boron Neutron Capture Therapy. Front. Oncol. 2021, 11, 676575. Available online: https://www.frontiersin.org/article/10.3389/fonc.2021.676575 (accessed on 20 May 2022).
- Barth, R.F.; H Vicente, M.; Harling, O.K.; Kiger, W.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146.
- Current Status of Neutron Capture Therapy. Published February 28, 2019. Available online: https://www.iaea.org/publications/6168/current-status-of-neutron-capture-therapy (accessed on 22 November 2021).
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421.
- Linko, S.; Revitzer, H.; Zilliacus, R.; Kortesniemi, M.; Kouri, M.; Savolainen, S. Boron detection from blood samples by ICP-AES and ICP-MS during boron neutron capture therapy. Scand. J. Clin. Lab. Investig. 2008, 68, 696–702.
- Moo, T.-A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354.
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241.
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135.
- Niu, L.; Zhou, L.; Xu, K. Cryosurgery of breast cancer. Gland Surg. 2012, 1, 111–118.
- Shibamoto, Y.; Murai, T.; Suzuki, K.; Hashizume, C.; Ohta, K.; Yamada, Y.; Niwa, M.; Torii, A.; Shimohira, M. Definitive Radiotherapy with SBRT or IMRT Boost for Breast Cancer: Excellent Local Control and Cosmetic Outcome. Technol. Cancer Res. Treat. 2018, 17, 1533033818799355.
- LeVasseur, N.; Sun, J.; Gondara, L.; Diocee, R.; Speers, C.; Lohrisch, C.; Chia, S. Impact of pathologic complete response on survival after neoadjuvant chemotherapy in early-stage breast cancer: A population-based analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 529–536.
- Khan, A.A.; Maitz, C.; Quanyu, C.; Hawthorne, F. BNCT induced immunomodulatory effects contribute to mammary tumor inhibition. PLoS ONE 2019, 14, e0222022.
- Gadan, M.A.; González, S.J.; Batalla, M.; Olivera, M.S.; Policastro, L.; Sztejnberg, M.L. Reprint of Application of BNCT to the treatment of HER2+ breast cancer recurrences: Research and developments in Argentina. Appl. Radiat. Isot. 2015, 106, 260–264.
- Trivillin, V.A.; Pozzi, E.C.C.; Colombo, L.L.; Thorp, S.I.; Garabalino, M.A.; Monti Hughes, A.; González, S.J.; Farías, R.O.; Curotto, P.; Santa Cruz, G.A.; et al. Abscopal effect of boron neutron capture therapy (BNCT): Proof of principle in an experimental model of colon cancer. Radiat. Environ. Biophys. 2017, 56, 365–375.
- Han, Y.-M.; Cai, G.; Chai, W.-M.; Xu, C.; Cao, L.; Ou, D.; Chen, J.-Y.; Kirova, Y.M. Radiological distribution of brain metastases and its implication for the hippocampus avoidance in whole brain radiotherapy approach. Br. J. Radiol. 2017, 90, 20170099.
- Kühnöl, J.; Kühnöl, C.; Vordermark, D. Radiotherapy of brain metastases from breast cancer: Treatment results and prognostic factors. Oncol. Lett. 2016, 11, 3223–3227.
- Suzuki, M.; Kato, I.; Aihara, T.; Hiratsuka, J.; Yoshimura, K.; Niimi, M.; Kimura, Y.; Ariyoshi, Y.; Haginomori, S.; Sakurai, Y.; et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J. Radiat. Res. 2014, 55, 146–153.
- Hiratsuka, J.; Kamitani, N.; Tanaka, R.; Tokiya, R.; Yoden, E.; Sakurai, Y.; Suzuki, M. Long-Term Outcome of Cutaneous Melanoma Patients Treated with Boron Neutron Capture Therapy (BNCT). J. Radiat. Res. 2020, 61, 945–951.
- Miyatake, S.-I.; Kawabata, S.; Nonoguchi, N.; Yokoyama, K.; Kuroiwa, T.; Matsui, H.; Ono, K. Pseudoprogression in Boron Neutron Capture Therapy for Malignant Gliomas and Meningiomas. Neuro Oncol. 2009, 11, 430–436.
- Moss, R.L. Critical Review, with an Optimistic Outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11.
More
