1. Functions of SA in Mitigating Abiotic Stresses
1.1. Heat
Global warming is causing a serious threat to plant growth and food security. Heat stress disturbs plant cellular homeostasis, retards development, and causes sterility and reduced yield
[1][18]. It has been reported that the application of exogenous SA enhances rice yield under high-temperature conditions
[2][19], while inhibiting the synthesis of SA markedly reduced the level of thermotolerance in pea plants
[3][20]. Furthermore, the biosynthesis of SA was increased under heat stress, as observed in many plant species, such as mustard
[4][21], creeping bentgrass
[5][22], grape
[6][23], and melon
[7][24].
Photosystem II, which functions as an electron transport chain in chloroplasts, is one of the most thermosensitive structures in plants
[8][25]. A study found that spraying 0.25 mM SA onto alfalfa leaves for 5 days ameliorated the heat damage to PSII and photosynthetic efficiency
[9][12]. This may be because SA improves the antioxidant system and chlorophyll fluorescence
[10][26], thus maintaining the thermo-stability of the electron donor and reaction centres of PSII
[11][27]. Heat stress also disturbs osmotic potential and destroys plasma membranes, thereby leading to ion leakage in plant cells. The application of SA can enhance free proline content, which plays a key role in the osmoregulation of plant cells. This phenomenon has been widely observed in wheat
[12][28], cucumber
[10][26], and tomato
[13][14][29,30]. Furthermore, spraying 100 mM SA on grape leaves stabilized the activity of the proton pumps in membranes, including H
+- and Ca
2+-ATPase, which may be another important mechanism for maintaining the integrity of the membrane under heat stress
[15][31]. Activities of SA contribute to better regulation of stomatal aperture along with photosynthetic apparatus, such as PSII and Rubisco activity, and thus increase the capacity of photosynthesis when subjected to stressful temperature conditions
[14][30].
Transcriptome analysis of plants has revealed SA signalling of heat-stress-responsive genes during thermotolerance, such as NPR1 (non-expresser of pathogenesis-related), HSPs (heat shock proteins), MBF1c (multiprotein bridging factor 1c), TGA, and PR-1 (pathogenesis-related protein 1)
[5][16][22,32]. Exogenous application of SA induces the synthesis of heat shock proteins (HSPs), the proteins chiefly responsible for defence against heat stress, as noted in
Arabidopsis thaliana plants
[17][33], tomato
[18][19][34,35], and rice
[20][36]. However, a study with transgenic Arabidopsis obtained the inconsistent results that SA failed to affected the expression of Hsp
[17][33], indicating the molecular mechanism still needs to be further investigated. Endogenous free SA stimulated the production of PIP2-phospholipase C of pea, a lipid-associated enzyme involved in intracellular signalling, in response to heat treatment. In response to heat stress, the pea plant elevated the synthesis of SA initially, which then signalled the production of PIP2-phospholipase C, a lipid-associated enzyme involved in intracellular signalling
[21][37]. SA also increases the expression of the chitinase-1 gene in melons under heat shock
[7][24]. Furthermore, cross–talk between SA and other plant signallings, such as H
2S, Ca
2+, IAA, and ABA, has also been reported
[22][23][24][38,39,40]. For example, treatment with SA increases the activity of L-cysteine desulfhydrase, a key enzyme in H
2S biosynthesis, indicating that H
2S might be a downstream signalling molecule in SA-induced heat tolerance
[22][38].
1.2. Chilling
Chilling injury is one of the main limitations in the growth and productivity of tropical and subtropical crops. The regulatory role of SA in defending against chilling stress has been reported in many plant species, such as maize
[25][41], mountain rye
[26][42], watermelon
[27][43], beans
[28][44], wheat
[29][30][10,45], and barley
[31][46]. Furthermore, low temperatures induced the accumulation of endogenous SA in
Arabidopsis thaliana and wheat plants, which further confirmed the relationship between SA and cold stress responses
[30][32][45,47].
Low temperatures are effective for the storage of fruits and vegetables, but they may also cause chilling injury. SA as a highly efficient buffering agent against cold stress has been widely demonstrated in many fruits. For example, spraying 0.5 mM SA changed H
2O
2 metabolisms and increased the chilling tolerance of banana seedlings
[33][34][48,49]. Similar results have been reported in cut flowers
[35][50], bamboo shoots
[36][51], as well as fruits in lemons
[37][38][52,53], cucumbers
[39][54], bell peppers
[40][55], peaches
[41][42][56,57], pomegranates
[43][44][58,59], and plums
[45][60].
1.3. Salinity
When grown in saline soils, plants may suffer from superabundant ion and osmotic stress, leading to ion imbalance and toxicity in plant cells
[46][75]. It has been found that salt stress can cause a decrease in SA content in plants, such as
Iris hexagona [47][76], tomato
[48][77], and soybean
[49][78], whereas the application of SA increased tolerance to salt toxicity in many plant species, such as pepper
[50][13], cucumber
[51][79], and soybean
[52][80] (See Table 3).
SA is an important regulator of influx and efflux of Na
+. For instance, addition of SA to soil alleviated salt toxicity in maize by decreasing Na
+ accumulation
[53][81]. Exogenous foliar application of 1.5 mM SA reduced osmotic stress and improved the aerial K
+/Na
+ ratio of saffron under saline conditions
[54][82]. Soaking seeds of
Leymus chinensis in SA solution lowered osmotic damage on the plasma membrane by accumulating K
+ and Ca
2+ [55][83]. SA-signalled K
+ accumulation might be due to the activation of H
+-ATPase in the membrane
[56][84], which occurs via guard cells outwardly rectifying K
+ channel (GORK), as noted in
Arabidopsis thaliana under salt stress
[57][85]. The increase in Ca
2+ influx in the cytoplasm may activate the transport system of Na
+/H
+ in the plasma membrane, which is mediated by the salt overly sensitive (SOS) signalling pathway
[58][86]. Furthermore, the application of SA has been shown to maintain the membrane integrity by regulating compatible metabolites such as proline and soluble sugars. Irrigation of the solution with 1 mM SA into soil increased proline content and sustained membrane integrity of pepper cells
[50][13]. Exogenous SA increases proline, soluble carbohydrates, and proteins contents in soybean leaves, thereby adjusting the water content of cells
[56][84]. Pre-treatment of SA might induce a pre-adaptive response through a transient increase in H
2O
2 level, which may act as a second messenger to “set up” the plant to defend the following salt stress that may occur. Pre-treatment with SA enhances the activities of antioxidant enzymes in plants, which in turn decreases stress-induced oxidative stress, as has been noted in
Leymus chinensis [55][83] and
Iris pseudacorus [59][87]. The signalling role of SA is also cross-linked with ABA, glycinebetaine, and ethylene (ET), as they are closely correlated with the synthesis of stress proteins and maintenance of leaf water potential
[60][61][88,89].
Metal phytotoxicity has been a major subject of current plant biology research. Heavy metals can be absorbed easily by plant roots, transported into shoots, and cause various visible toxic symptoms, such as growth retardation, leaf chlorosis, wilting, and cell death. The beneficial role of SA in defence against metal toxicity has been reported in a wide range of plant species
[62][63][96,97]. For instance, application of SA improved the growth and photosynthetic abilities in Pb-stressed rice
[64][98], Cu-stressed
Phaseolus vulgaris [65][99], and Ni-stressed mustard
[66][100]. Recently, the co-reaction of SA with other promoters has also been evaluated. For example, combination exposure of SA and plant-growth-promoting bacteria reduced the Cr-induced oxidative damage in maize
[67][101]. SA in combination with kinetin or calcium ameliorated Ni and Pb stress in
Phaseolus vulgaris plants
[68][102]. The combined supplementation of melatonin and SA effectively detoxified As toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants
[69][103].
Cadmium is one of the most toxic and widespread heavy metals in the world
[70][104]. It is the typical toxic metal that can induce representative symptoms in plants, such as replacing and inactivating essential elements, destroying protein structure, and interfering with photosynthesis, respiration, and cell division
[71][14]. A wide range of plant species have shown that SA is deeply involved in promoting Cd tolerance during processes such as plant growth, element assimilation, Cd translocation, photosynthesis, and senescence
[71][14]. Therefore, this
revie
ntryw on the topic of metal toxicity is focused on the interaction of SA and Cd in plants.
The phytotoxicity of cadmium (Cd) is a major subject of current plant biology research. Recent studies have shown that the synthesis of SA in plants is markedly promoted by Cd stress. For example, after 25 μM Cd treatment, the bound SA of maize was 10 times higher than that of untreated plants
[72][105]. Similar phenomena have been observed in barley
[73][106] and
Pisum sativum [74][107]. Studies on a wide range of plant species have shown that SA is deeply involved in promoting Cd tolerance, including in plant growth, element assimilation, Cd translocation, photosynthesis, and senescence
[71][14].
1.5. Other Stresses
1.5.1. Drought
During drought stress, plants have elevated SA levels, as noted in many plant species, such as barley
[75][135],
Phillyrea angustifolia [76][136], and
Salvia officinalis [77][137]. The alleviation of drought injury by SA goes along with the hardening of the antioxidant system, increasing relative water and proline contents and regulating other phytohormones
[78][79][1,138]. For example, pre-treatment of SA cleared the drought-induced superoxide radical with enhancement of the expression of redox regulating genes and increased proline content with its synthesis-related genes
[80][139].
SA treatments effectively ameliorated the negative effects of drought through not only improving the photosynthetic performance and membrane permeability but also enhancing the activity of antioxidant enzymes. For instance, foliar application of SA substantially decreased the ROS and MDA contents of maize under drought stress
[81][140]. Application of SA at 100 mM enhanced antioxidant enzymatic activities together with other physio-biochemical traits, such as membrane stability, chlorophyl content, and photosynthetic rates in wheat under drought stress
[82][141]. When sprayed with SA at 0.5 mM, wheat seedlings effectively increased the activities of antioxidant enzymes (SOD, CAT, and PPO) to alleviate the drought-stress-induced damage effects
[83][142]. Foliar spray of SA in sweet basil significantly promoted the plant growth and relative water contents under water-deficit conditions
[84][143]. Spraying 2 mM SA into the leaves of
Rosmarinus officinalis L. increased the production of essential oil under the mild drought stress (60% field capacity)
[85][144]. Treatment with SA protected tomato plants from drought stress, mainly by maintaining membrane stability and activities of carbonic anhydrase that directly affect the rate of photosynthetic CO
2 fixation
[86][87][145,146]. Pre-treatment with SA reduced damage to the cell membranes and increased ABA content in the leaves of barley and maize, suggesting that there is cross-talk between SA and ABA during drought stress
[75][88][135,147].
1.5.2. Ozone
Ozone is a powerful oxidising agent that reacts with lipids and proteins in plant cells and causes oxidative damage
[89][90][148,149]. SA deficiency in
NahG plants is sensitive to the ozone, whereas ozone exposure stimulates SA accumulation and promotes virus resistance in tobacco
[91][150]. Further evidence has shown that enhanced accumulation of SA by ozone stress is through the ICS pathway
[92][151]. SA controls ET production of
Salvia officinalis during ozone exposure by balancing cell redox and shrinking chlorosis formation in leaves
[93][152]. However, abnormal levels of SA cause greater ozone injury either in deficiency or superfluousness. Many deficient genotypes, such as
Cvi-0,
NahG,
npr1,
eds5, and
sid2, are sensitive to the ozone stress
[94][153]. Exogenous SA application decreased the stomatal conductance, chlorophyll content, and Mg assimilation of rice under ozone stress
[95][154]. Recently, an interesting study was conducted to test whether the O
3-induced cell death is regulated through SA, JA, or ethylene. The global and targeted analysis of transcriptional changes in single, double, and triple mutants mainly showed that the basal SA levels are essential for plants to defend against ROS-induced cell death, which is in conjunction with ethylene and JA signalling
[96][97][155,156].
1.5.3. Pesticide
Some chemical pesticides, such as herbicide, also directly induced the oxidative damage in plants, as observed in cucumber, pistachio plants (
Pistacia vera L.), and barley
[98][99][100][101][157,158,159,160]. The injury caused by paraquat (a kind of herbicide) continuously generates superoxide in the chloroplasts of plant cells, motivates redox reaction chains, generates various forms of ROS, and leads to oxidative damage
[99][158]. Transgenic
NahG in rice plants causes SA deficiency, with lower glutathione (GSH) content showing great sensitivity to paraquat exposure
[99][158]. SA significantly increases enzymatic parameters and photosynthetic pigments of
Vigna radiata when exposed to fungicide (mancozeb), insecticide (chlorpyrifos), and herbicide (metribuzin)
[102][161]. Pre-treatment with 1 mM SA triggers the activity and expression of pesticide detoxification enzymes (GSTs: glutathione S-transferases; a carbon-monoxide-bound enzyme, P450 (absorption band at 450 nm)) in thiram-treated leaves
[103][162]. Treatment with 1 mM SA promotes the degradation of pesticides and blocks their accumulation in cucumber
[104][163].
1.5.4. Ultraviolet Radiation
UV radiation a key environmental signal that influences plant growth and development and can reduce disease and pest incidence
[105][164]. However, because it is beyond the capacity of sunlight utilisation in plants, excessive exposure can directly induce the ROS production, adversely affects photosynthesis, and damage cell membranes and proteins
[102][161]. It has been shown that SA counteracted the UV-A-, UV-B-, and UV-C-induced oxidative stress on pepper through activating antioxidant enzymes such as POD, APX, CAT, and GR
[106][165]. Furthermore, UV radiation activated SA defences and then enhanced the tomato resistance to pathogen attack in the JA-deficient genotype
[107][166]. Similar to ozone, UV radiation induces SA accumulation in tobacco, which is accompanied by higher activity of benzoic acid 2-hydroxylase, a key enzyme in the catalysis of SA biosynthesis
[91][150]. It has been found that exogenous SA alleviates the damaging effects of UV irradiance in many plant species such as blue grass, soybean, and maize
[108][109][167,168]. The possible roles may include increase in anthocyanin and α-tocopherol content, photochemical efficiency, and activities of antioxidant enzymes.
2. Possible Mechanisms of SA in Mitigating Abiotic Stresses
Intensive research has shown that all abiotic stressors increase the level of endogenous SA, indicating that this simple molecule is involved in stress signalling in plants
[110][111][112][16,169,170]. The regulatory roles of SA are mediated by various physiological processes, including growth development, photosynthesis, ion assimilation, respiration, antioxidant system, and cross-talk with other hormones
[113][3]. The first report on the SA signalling is that it affects ROS production and then provokes pathogenesis-related1 (PR1) expression under pathogenic attack
[114][171]. This discovery sparked the further studies on the complex signalling network between SA and ROS in plants
[115][172]. Thus, the primary mechanism of SA reviewed here is its defensive role through redox signalling.
2.1. Redox Signalling
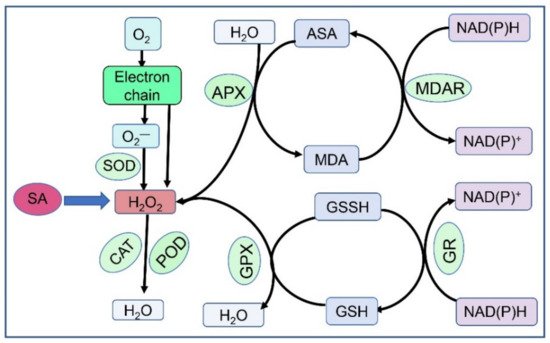
ROS are defined as the inevitable by-products of electron transfer in mitochondria, chloroplasts, and other energy-generating sites of plant cells
[116][173]. Owing to their strong oxidisability, they can interfere with most biochemical metabolic processes, such as enzyme activity, membrane permeability, DNA stability, and protein synthesis. Under normal conditions, ROS are detoxified and maintained at equilibrium by the antioxidant defence system
[117][174]. This system has experienced very complex evolutionary processes for 2.7 billion years. It is estimated that at least 152 genes in plants are involved in this highly dynamic and redundant network, which develops enzymatic and non-enzymatic compounds and encodes ROS-producing and -scavenging proteins
[118][175] (See
Figure 1). In many cases, the capacity to cease production of ROS is an important indicator of plant tolerance. Furthermore, low concentrations of ROS are successfully utilised by plants as a leading signalling pathway in physiological metabolic processes, such as growth development, hormone signalling, programmed cell death, cell cycle, and biotic and abiotic stress responses under normal and stress conditions
[119][176].
Figure 1.
Brief pathways for reactive oxygen species scavenging in plants.
Although the benefits of SA signalling have been thoroughly studied, few studies have reported ambiguous results. SA treatment mitigated Cd toxicity in barley but failed to affect the activity of antioxidant enzymes
[56][84]. High levels of SA promote the generation of H
2O
2 in leaves
[120][15]. The results of studies on SA mutants are still contradictory. High SA levels in
snc1 mutants generated a large amount of ROS, whereas SA deficiency in
NahG lowered Cd-induced oxidative stress
[121][91]. However, this finding is in contrast to the case of
sid2 mutants, in which Cd-induced oxidative damage was aggravated by the SA deficiency
[122][197].
2.2. Cross-Talk with Other Plant Hormones
Besides of ROS, other plant hormones are involved in the SA signal transduction pathway of plants
[123][198]. Most studies have observed a relationship between ABA and SA levels under stress. Treatment with SA induces ABA concentrations in barley and tomato
[124][199]. Exposure of
Arabidopsis thaliana leaves to ABA inhibits SA transduction both upstream and downstream through the SAR signalling pathway, and this suppressive effect is not related to jasmonate (JA)/ET-mediated signalling
[125][200]. Similarly, salt stress increases the content of JA and ABA but decreases the levels of IAA, gibberellic acid (GA), and SA in
Iris hexagona and soybean
[47][49][76,78]. Insect feeding caused a strong accumulation of JA-specific mRNA transcripts, such as GmBPI1, GmKTI1, and GmAAT, but did not influence the free SA or SA-marker gene transcripts accumulation
[126][201]. Drought stress increases the levels of SA and ABA in
Brassica napus, and the effect on ABA is more pronounced
[127][194]. However, the signalling role of SA might be stronger than that of ABA because the inhibition of SA biosynthesis leads to serious heating damage compared to the inhibition of ABA biosynthesis
[128][202]. It seems that the biosynthesis of ABA is a downstream signalling event associated with SA sensing. Treatment with SA in salt-stressed tomato resulted in ABA accumulation in both root and leaf tissues together with upregulation of some ABA biosynthesis genes, such as SlZEP1, SlNCED1, SlAO1, and SlAO2
[129][203]. In pea plants, the activity of SA glucosyl transferase may be inhibited by ABA, thus enhancing the concentrations of free SA
[130][204].
Calcium (Ca
2+) is a key messenger in plants that can induce various defence responses against stress. SA-induced stomatal closure is associated with ABA signalling, and this process is mediated by Ca
2+/Ca
2+-dependent protein kinases (CPK) in
cpk3-2 and
cpk6-1 mutants but not in the Ca
2+-independent protein kinase Open Stomata1 (OST1)
ost1-3 mutant
[131][205]. It was also observed that SA triggered the Ca
2+-sensing receptor in chloroplast thylakoid membranes of
Arabidopsis thaliana [75][135]. Calmodulin, a Ca
2+-binding messenger protein, transduces Ca
2+ signals by binding Ca
2+ and then modifying the target proteins. The biosynthesis of SA is regulated by calmodulin-binding-protein (CBP60g) via the activation of isochorismate synthase 1 (ICS1)
[132][206]. Recent studies in
Arabidopsis thaliana have shown that the SA-signalled plant immunity is associated with calmodulin-binding transcription activators (CAMTA)
[133][207].
Similar to the function of SA, nitric oxide plays a crucial role in controlling redox homeostasis in plant responses to abiotic stresses
[134][208]. The application of SA and SNP (NO donor) significantly improved the heat-stress tolerance of hyacinth bean and Ni tolerance of finger millet
[135][209]. Under As toxicity, the increase in NO concentration in rice is induced by SA through the enhancement of nitrate reductase activity
[136][210]. SA increased As tolerance in maize by activating the antioxidant defence system, but this effect was completely negated when NO synthesis was blocked
[137][211]. Furthermore, NO may act as a downstream signalling molecule that participates in SA-signalled cell wall construction, which could impede Cd influx in Cd-stressed rice seedlings
[138][212]. Both NO and SA are involved in the signal transduction of stomatal closure, and the increase in NO levels is dependent on SA-induced NO synthase-like enzymes
[139][213].
2.3. Mitogen-Activated Protein Kinase
Mitogen-activated protein kinase (MAPK) is a type of protein kinase that is specific to the threonine and amino acids serine, which is involved in cell functions and cellular responses to a diverse array of stimuli
[140][214]. MPK3, MPK4, and MPK6 kinases are the main mediators of plant responses to biotic and abiotic stresses. Studies on
Arabidopsis thaliana have shown that SA is involved in transmitting MAPKs cascade signalling
[141][215]. Compared with the wild-type, approximately 50% of the basal expression level of AtMPK3 was noted in the SA-deficient mutants with low activity of AtMPK3
[142][216]. A 48-kD MAPKs in tobacco was identified by SA activation since it preferentially phosphorylates myelin basic protein (MBP)
[143][217]. Conversely, MAPK regulated the levels of SA in stressed plants
[144][218]. It was reported that SA treatment increased the TaMAPK4 transcripts in wheat under an avirulent race of pathogen attack, whereas knockdown the TaMAPK4 gene downregulated the SA accumulation
[145][219]. Meanwhile, StMKK1 protein negatively regulated SA-related signalling pathways in defence against pathogens in potato
[146][220].
Mpk4 mutant accumulated excessive levels of SA, but this was not the reason for its extreme dwarf phenotype, as knocking down the ICS1 gene (SA synthesis) did not revert mpk4-impaired growth
[147][221]. Furthermore, the accumulation of MPK4 might also be related to SA-regulated redox homeostasis, but this mechanism is still unknown and further study
[148][222].