In non-small cell lung cancer (NSCLC), there is a pressing need for immunotherapy predictive biomarkers. The processes underlying B-cell dysfunction, as well as their prognostic importance in NSCLC, are unknown. This study presents novel insights on a dysregulated B cell network that promotes proliferation in epithelial cells in NSCLC. Within this network, a nine-gene signature demonstrated prognostic and predictive indications in more than 1400 NSCLC patients using their gene and protein expression profiles in bulk tumors. Multiple genes (HLA-DRA, HLA-DRB1, OAS1, and CD74) differentially expressed in NSCLC B cells, peripheral blood lymphocytes, and tumor T cells had concordant prognostic indications at mRNA and protein expression levels.
- B cells
- T cells
- single-cell RNA sequencing
- CRISPR-Cas9/RNAi screening
- prognostic and predictive biomarkers
- non-small cell lung cancer
- Introduction
Non-small cell lung cancer (NSCLC), accounting for 84% of lung cancer cases [1], is the leading cause of cancer-related mortality. The major histological subtypes that constitute NSCLC are lung adenocarcinoma (40% of NSCLC cases), squamous cell carcinoma (25–30%), and large cell carcinoma (5–10%), and each subtype represents a distinct prognosis for the patients as does the treatment option [2,3][2][3]. Adjuvant chemotherapy of stage II/III NSCLC has resulted in 10–15% increased overall survival [4]. However, the overall 5-year survival rate for NSCLC is less than 15% due to the limited therapeutic response and the resulted tumor recurrence/metastasis [5]. Immunotherapy has shown promising results in NSCLC [6,7][6][7]. The neoadjuvant PD-1 inhibitor nivolumab induced a pathological response in 9 of 20 (45%) resected NSCLC tumors in stages I, II, and IIIA, including both PD-1-positive and -negative tumors, with few side effects [6]. In an open-label phase I clinical trial of chemotherapy-naive NSCLC patients with stage IIIB or stage IV, nivolumab with anti-CTLA4 ipilimumab in first-line therapy had an acceptable safety profile and demonstrated promising clinical performance, with a high response rate and long-term response [7]. Patients with stage IIIB who received anti-PD-L1 durvalumab after chemoradiation had a significant overall survival benefit (median OS not reached in the durvalumab arm compared to 29.1 months in the placebo arm (HR 0.69 [95% CI 0.55–0.86])) in a phase III randomized trial [8,9][8][9]. The overall survival benefit of pembrolizumab monotherapy was demonstrated in untreated stage IV NSCLC patients compared to chemotherapy in patients with tumors expressing a PD-L1 tumor proportion score (TPS) ≥ 50%, TPS ≥ 20%, and TPS ≥ 1% in randomized phase III clinical trials [10-12][10][11][12]. Atezolizumab received FDA approval for stage II and IIIA NSCLC following chemotherapy in patients with PD-L1 > 1% [13,14][13][14]. Nevertheless, only a subset of NSCLC patients responded to immunotherapy due to primary, adaptive, or acquired immune resistance [15,16][15][16]; mutations; and the varying amounts and properties of tumor-infiltrating lymphocytes (TILs) [17-19][17][18][19]. PD-L1 and tumor mutational burdens have not been demonstrated to be reliable predictive biomarkers [20]. There are currently no well-established predictive biomarkers for immunotherapy response. In addition, there are no clinically applied biomarkers to identify early-stage NSCLC patients with all histological subtypes who are at high risk for tumor recurrence and metastasis for adjuvant therapies.
The tumor immune microenvironment is complex, dynamic, and heterogeneous, comprising interweaving signaling pathways and networks of genes and proteins within the immune system, stromal cells, and the host factors [21]. When tertiary lymphoid structures (TLSs) are present in the tumor microenvironment, cancer patients typically have favorable clinical outcomes [22]. TLSs in NSCLC tumors contain follicular B cells and adjacent clusters of dendritic cells and T cells [23]. TLSs and tumor-infiltrating B cells increase immune checkpoint inhibitors’ (ICIs) responses in cancer immunotherapy, which has prognostic implications [24-27][24][25][26][27]. Profiling of the immune cell composition showed that only B cells had a significantly higher presence in tumors compared to the distal lung in an NSCLC patient cohort [28]. A high density of B cells within a TLS is positively correlated with tumor antigen-specific antibody responses and increased intratumor CD4+ T cell clonality as well as early differentiated, activated, and non-regulatory CD4+ T cells, suggesting a central role of B cells in determining protective T cell responses in NSCLC patients [29,30][29][30]. T cell dysfunction and therapy have been established for cancer treatment, including NSCLC [31-37][31][32][33][34][35][36][37]. However, the biology, prognostic significance, and potential benefit of B-cell-based immunotherapy in lung cancer have yet to be deciphered [38-40][38][39][40].
Given this intricacy and heterogeneity, a gene signature integrating various features should be created to identify the right patient for a specific immunotherapy [21], in concert with chemotherapy and/or radiotherapy. Novel computational network modeling is necessary to reveal essential molecular interactions with implications in prognosis, proliferation, and response to therapies for improving NSCLC treatment. Rigorous pathway and network approaches are crucial for the discovery of innovative targeted therapies and repurposing drugs to prolong NSCLC survival [41]. This study utilized Boolean implication networks [42-44] [42][43][44] to identify tumor-specific B cell gene co-expression networks in NSCLC using public single-cell RNA sequencing data. Within the identified B cell networks, proliferation genes were identified from CRISPR-Case9/RNA interference (RNAi) screening assays in human NSCLC cell lines. Differentially expressed genes in B cells and T cells with prognostic implications were selected using bulk NSCLC tumor transcriptome (n = 1313) and proteome profiles (n = 103).
- RNesultw Discoveries
2.1. Tumor-Specific Gene Co-Expression Networks in NSCLC B Cells
Using the Boolean implication network algorithm, whole-genome mRNA co-expression networks were generated with single-cell RNA sequencing data of normal and NSCLC tumor B cells. The constructed tumor and normal B cell gene co-expression networks were compared to identify tumor-specific B cell co-expression networks, i.e., gene co-expression relations that existed only in tumor B cells and not in normal B cells, and vice versa. To obtain a manageable amount of network edges, we selected the significant (p < 0.00005, one-tailed z-tests) gene associations (i.e., implication rules) for further analysis. A total of 232 significant gene co-expression relations (network edges) existed only in the NSCLC tumor B cell network and not in the normal B cell network, and 615,332 significant co-expression relations existed only in the normal B cell network and not in the NSCLC tumor B cell network. These identified tumor-specific B cell gene co-expression networks were included in further analysis.
2.2. DE Genes in NSCLC B Cells and T Cells with Prognostic Implications
We included tThe significant DEdifferentially expressed (DE) genes (p < 0.05, |log2FC| > 1, and FDR < 0.25) in tumor vs. normal B cells were included in the next analysis. A total of 1086 unique genes were selected, including 1082 genes from the network only present in normal B cells and 35 genes from the network only present in NSCLC tumor B cells.
Proliferation and prognostic genes were further selected from these 1086 DE genes. The proliferation genes that had a significant effect in both CRISPR-Cas9 and RNAi screening assays in more than 50% of the tested human NSCLC epithelial cell lines were selected (Figure 1A). The prognostic genes that had a significant hazard ratio (HR, p < 0.05, univariate Cox model) in the survival analysis of an NSCLC cohort (GSE81089) and significant differential expression (p < 0.05, two-sample t-tests) between short- and long-term NSCLC survivors were selected (GSE28571). The selected prognostic genes had a concordant association with patient survival in both cohorts.
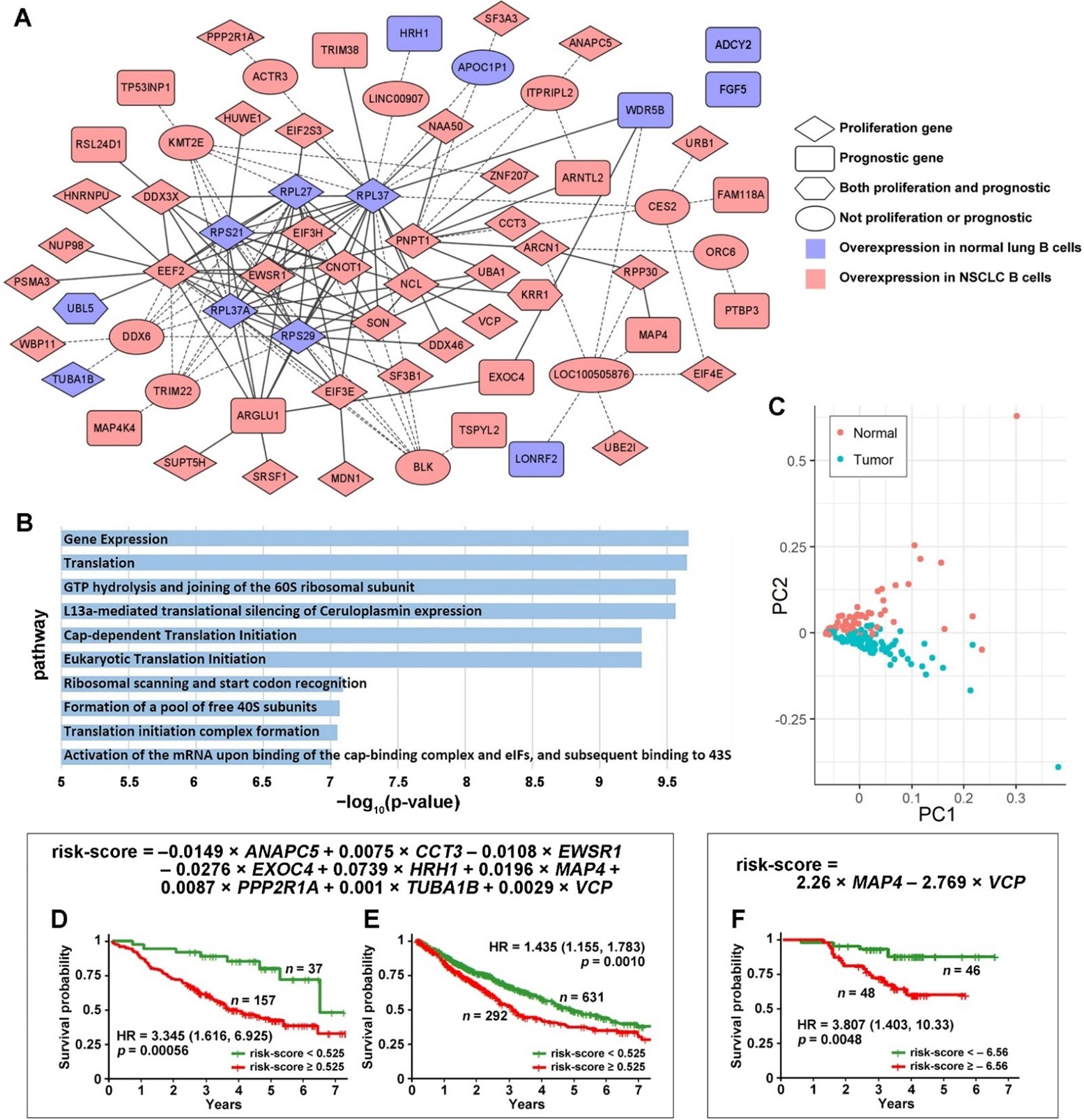
Figure 1. The identified proliferation and prognostic gene co-expression network in NSCLC B cells. (A) The shown gene co-expression network was present in normal B cells and missing in NSCLC tumor B cells. None of the co-expression relations were present in tumor B cells. All genes were significantly differentially expressed in NSCLC tumor-associated B cells vs. B cells in adjacent normal lung tissues. The intermediate genes are in the ellipse circles. These genes were not in the selected proliferation or prognostic gene list, but the selected genes were connected through these intermediate genes. The solid lines indicate direct connections between the selected genes, and the dashed lines indicate connections through intermediate genes. (B) The −log10 (p-value) of the top 10 significantly enriched pathways in the ToppGene functional enrichment analysis of the proliferation and prognostic network. (C) Principal component analysis (PCA) using all the genes shown in Figure 1A in single-cell RNA sequencing data separates normal and NSCLC tumor B cells. Kaplan–Meier analysis of the 9-gene signature using RNA sequencing data in the training set GSE81089 (D) and the TCGA-LUAD and TCGA-LUSC validation set (E). (F) Kaplan–Meier analysis of the 9-gene signature using proteomic data of MAP4 and VCP in LUAD patients.
The identified proliferation and prognostic gene co-expression network (Figure 1A) was only present in the normal B cells and was missing in the NSCLC tumor B cells. Some genes were not directly connected with the network but were connected through one of the intermediate genes differentially expressed in NSCLC tumor B cells. ADCY2 and FGF5 were not connected with the network directly or through any intermediate genes. The B cell proliferation and prognostic network pathway analyses were conducted with ToppGene. The top 10 significantly enriched pathways are shown in Figure 1B. The principal component analysis (PCA) using all the genes shown in Figure 1A generated a clear separation of normal and NSCLC tumor B cells in single-cell RNA sequencing data (Figure 1C).
From the B cell proliferation and prognostic gene co-expression network (Figure 1A), a nine-gene prognostic signature was identified in the multivariate Cox model from the training set (GSE81089) and was validated in the combined TCGA-LUAD and TCGA-LUSC data of NSCLC patients with stage I, II, or IIIA. In the multivariate Cox model analysis, a stepwise gene selection method was used to construct the model in the training set. In each iteration, the least statistically significant gene variable in the multivariate Cox model was dropped. This iteration was repeated until the model with the optimal prognostication in the training set was achieved. This model was then validated on the test set (TCGA data) with all the training parameters fixed. Nine marker genes were selected in this process: ANAPC5, CCT3, EWSR1, EXOC4, HRH1, MAP4, PPP2R1A, TUBA1B, and VCP. The Kaplan–Meier analysis results showed that the patients with a risk score lower than 0.525 survived significantly longer than the patients with a risk score higher than 0.525 in both the training (p = 0.00056; HR: 3.345 [1.616, 6.925]; Figure 1D) and validation cohorts (p = 0.0010; HR: 1.435 [1.155, 1.783]; Figure 1E).
The protein expression of the nine-gene marker panel also provided significant patient stratification in a LUAD patient cohort [45]. Only MAP4 and VCP were included in the proteomic multivariate Cox model due to data availability and the univariate significance. The Kaplan–Meier analysis results showed that the patient group with a risk score lower than –6.56 survived significantly longer than the patient group with a risk score higher than –6.56 (p = 0.0048; HR: 3.807 [1.403, 10.33]; Figure 1F).
Interactions and coordination between B cells and T cells are crucial in antibody generation and immune protection [46]. To gain a better understanding of genes important in NSCLC immunogenetics, we next examined DE genes in T cells in NSCLC PBL and tumors. Firstly, significant DE genes (p < 0.05, |log2FC| > 1, and FDR < 0.25) between normal and NSCLC PBL T cells (GSE151531) were identified. Secondly, this list of DE genes was compared with two T cell DE gene lists from published studies [47,48][47][48]. One published gene list [48] [48] contained DE genes (p < 0.05, two-sided moderated t-tests) between suppressive tumor Tregs of CD4-C9-CTLA4 cells (n = 868) vs. other tumor-infiltrating Tregs of CD4-C8-FOXP3 cells (n = 122) as well as DE genes (p < 0.05, two-sided moderated t-tests) between activated tumor Tregs of CD4-C9-CTLA4 (TNFRSF9+, n = 519) vs. non-activated tumor Tregs of CD4-C9-CTLA4 (TNFRSF9−, n = 420). The other published gene list [47] [47] consisted of the DE genes in each of the 14 T cell clusters. Eleven genes, including CCND2, CD74, DUSP2, GBP4, HLA-DRA, HLA-DRB1, IL2RA, LMNA, OAS1, TIGIT, and TNIP3, were common DE genes in all three lists. Two genes, HSD17B13 and TSC22D3, had concordant significant DE (p < 0.05, |log2FC| > 1, and FDR < 0.25) between NSCLC vs. normal PBL T cells (GSE151531) and B cells in the lung tissues (GSE84789). These selected 22 genes, including 13 DE genes and the nine-gene prognostic marker panel identified from the B-cell network (Figure 1A), and 4 ICIs (CD27, CTLA4, PD1, and PDL1) were visualized in heatmaps (Figure 2). CTLA4, PD1, and PD-L1 are well-established immunotherapy targets in NSCLC [49]. CD27 is a new ICI [50] [50] that is being investigated in phase I/II clinical studies for a variety of cancer types, with encouraging results [51,52][51][52]. CD27 was also included in a seven-gene prognostic and chemopredictive assay we identified previously, showing concordant prognostic indications at the mRNA and protein expression levels in NSCLC tumors [44,53][44][53]. Here, we sought to examine the expression of these ICIs in NSCLC tumor B cells, T cells, and PBL T cells in single-cell RNA sequencing profiles. The average expression of these genes in the 14 NSCLC T cell clusters [47] [47] is shown in Figure 2A, and their DE patterns in NSCLC T cells and B cells are shown in Figure 2B. HRH1 and HSD17B13 were not available in the NSCLC tumor T cell dataset [47] [47] and are therefore not included in Figure 2A.
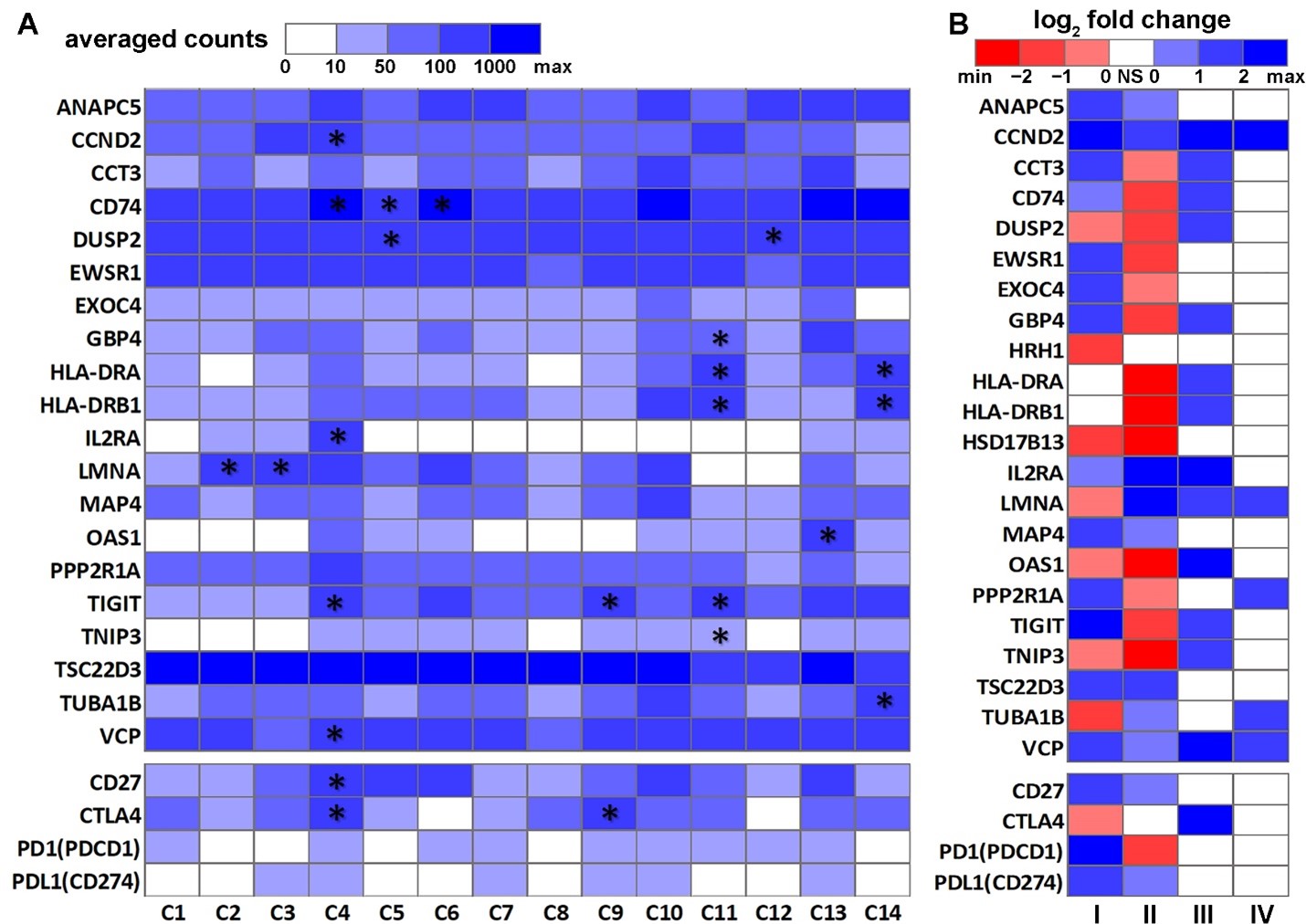
.Figure 2. Differential expression patterns of the selected genes in NSCLC T cells and B cells. (A) Heatmap of the average expression of the selected genes in 14 NSCLC tumor T cell clusters [47]. The asterisk (*) indicates that the gene was significantly differentially expressed in the corresponding T cell cluster [47][47] . (B) Heatmap of the log2FC patterns of the selected genes in this study. I: NSCLC tumor B cells (n = 96) vs. normal B cells (n = 96) [54]. II: Peripheral blood lymphocyte T cells from NSCLC patients (n = 531) vs. healthy donors (n = 92) [47]. III: NSCLC suppressive tumor Tregs of CD4-C9-CTLA4 cells (n = 868) vs. other tumor-infiltrating Tregs of CD4-C8-FOXP3 cells (n = 122) [48]. IV: NSCLC activated tumor Tregs of CD4-C9-CTLA4 (TNFRSF9+, n = 519) vs. non-activated tumor Tregs of CD4-C9-CTLA4 (TNFRSF9−, n = 420) [48]. NS: not significant.
The 14 T cell clusters presented in Figure 2A were published by Chiou et al. [47]. In this analysis of the Stanford cohort, 14 major distinct T cell states of activation/exhaustion were identified [47], of which 13 (C1 to C13) could be linked to the cell states reported by Guo et al. [48] [48] using a different patient cohort. Cluster C14 from Chiou et al. [47] [47] represented CD4+ and CD8+ T cells in the cell cycle, which was not reported by Guo et al. [48]. Clusters C5, C6, and C12 were CD8+ T cells with effector phenotypes. Clusters C7 and C10 were CD8+ T cells with a resident memory phenotype. C11 [47]/CD8-C6-LAYN [48] [48] (CD8+ exhausted T cells) and C4 [47]/CD4-C9-CTLA4 [48] [48] (CD4+ Tregs) consisted almost entirely of cells originated from tumors. Clusters C5, C6, C7, and C12 were inferred to exhibit virus-specific T cell states [47].
Among the selected genes (Figure 2), HLA-DRA, HLA-DRB1, OAS1, and CD74 had concordant prognostic indications at the mRNA and protein expression levels in bulk NSCLC tumors with stage I, II, or IIIA (Figure 3). Figure 3A presents their mRNA expression in 2950 single NSCLC T cells across 14 clusters (GSE151537) illustrated in the UMAP layout. HLA-DRA and HLA-DRB1 were both DE genes in C11 [47]/CD8-C6-LAYN [48] [48] (CD8+ exhausted T cells) and the C14 cluster of CD4+ and CD8+ cells in the cell cycle [47] [47] (Figure 2A). OAS1 was a DE gene in C13 [47]/C4-GZMK and C4-CD69 [48] [48] CD4+ cells. CD74 was a DE gene in C4 [47]/C9-CTLA4 [48] [48] CD4+ cells and the C5 and C6 clusters of CD8+ effector T cells. The results showed that the patients with a higher expression of HLA-DRA, HLA-DRB1, and CD74 survived significantly longer (p < 0.05, Kaplan–Meier analysis) than those with a lower expression of these genes at both the mRNA (Figure 3B) and protein levels [45] (Figure 3C). When OAS1 was expressed more highly at both the mRNA and protein levels, patients survived for a significantly shorter duration (p < 0.05, Kaplan–Meier analysis) in both TCGA-LUAD and TCGA-LUSC cohorts (Figure 3B) and the proteomic LUAD cohort from Xu et al. [45] [45] (Figure 3C). Although different patient cohorts were used in the single-cell and prognostic analysis, these results indicate the potential utility of the identified genes in single B cell and T cell analysis of NSCLC patient tumors and PBLs for diagnosis and prognosis.
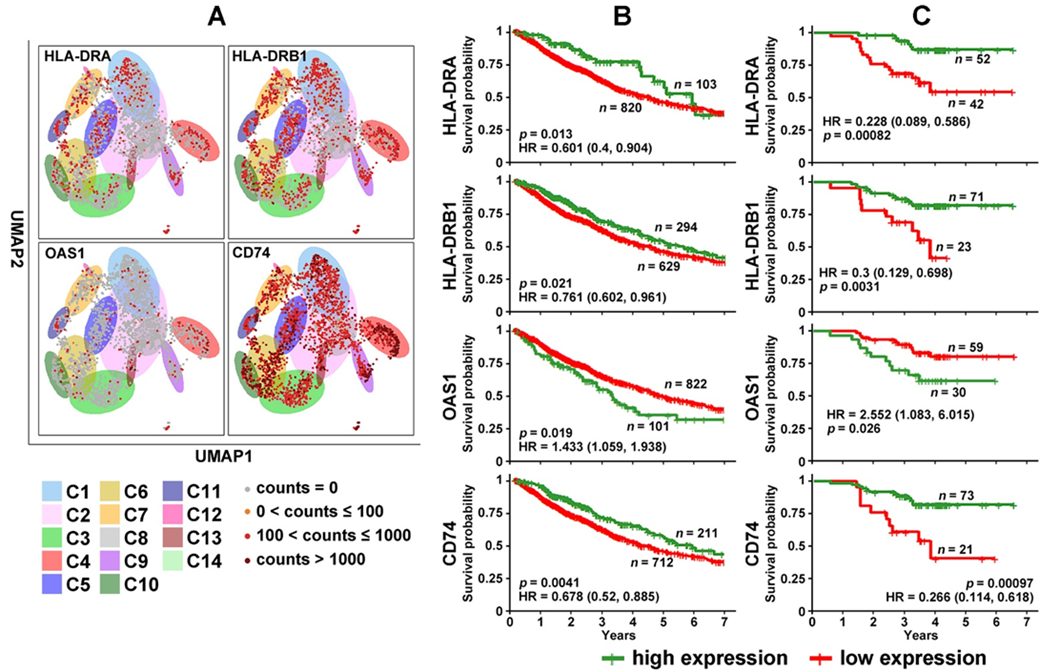
Figure 3. Differentially expressed genes in NSCLC T cells with prognostic indications. (A) Expression of HLA-DRA, HLA-DRB1, OAS1, and CD74 in 2950 single T cells across 14 clusters illustrated in the UMAP layout. (B) Kaplan–Meier analysis of TCGA-LUAD and TCGA-LUSC patients stratified based on mRNA expression of HLA-DRA, HLA-DRB1, OAS1, and CD74 in the RNA-sequencing data. (C) Kaplan–Meier analysis of patients in the Xu cohort [45] [45] stratified based on the protein expression of HLA-DRA, HLA-DRB1, OAS1, and CD74.
- Conclusions
NSCLC remains the leading cause of cancer-related mortality, despite the promising results from immunotherapy. There are currently no biomarkers to identify early-stage NSCLC patients of all histology who are at risk for tumor recurrence/metastasis and benefit from adjuvant therapies. There is a pressing demand for predictive biomarkers of immunotherapy in NSCLC. The mechanisms underlying B cell dysfunction and their prognostic significance in NSCLC are not well understood. To meet these critical needs, this study identified a tumor-specific B cell proliferation and prognostic gene co-expression network in NSCLC using Boolean implication modeling of single-cell RNA sequencing data. A nine-gene marker panel within this network provided accurate prognostic stratification for early-stage NSCLC patients using RNA sequencing and proteomic profiles. DE genes in NSCLC tumor B cells, PBLs, and tumor T cells with prognostic implications were garnered. These rigorous analyses of extensive public data generated solid results and hypotheses for future clinical investigations and will aid the development of novel therapies to improve NSCLC patient outcomes.
References
- Spira, A.; Wood, A.J.J.; Ettinger, D.S. Multidisciplinary Management of Lung Cancer. New England Journal of Medicine 2004, 350, 379-392, doi:10.1056/NEJMra035536.
- Ho, C.; Tong, K.M.; Ramsden, K.; Ionescu, D.N.; Laskin, J. Histologic classification of non-small-cell lung cancer over time: reducing the rates of not-otherwise-specified. Current oncology (Toronto, Ont.) 2015, 22, e164-170, doi:10.3747/co.22.2339.
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: current treatment and future advances. Translational lung cancer research 2016, 5, 288-300, doi:10.21037/tlcr.2016.06.07.
- Crino, L.; Weder, W.; Van, M.J.; Felip, E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2010, 21 Suppl 5, v103-v115.
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. Molecular histology of lung cancer: from targets to treatments. Cancer Treat Rev 2015, 41, 361-375, doi:10.1016/j.ctrv.2015.02.008.
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England journal of medicine 2018, doi:10.1056/NEJMoa1716078.
- Hellmann, M.D.; Rizvi, N.A.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; Ready, N.E.; Gerber, D.E.; Chow, L.Q.; Juergens, R.A.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. The Lancet. Oncology 2017, 18, 31-41, doi:10.1016/s1470-2045(16)30624-6.
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2020, 15, 288-293, doi:10.1016/j.jtho.2019.10.002.
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. The New England journal of medicine 2017, 377, 1919-1929, doi:10.1056/NEJMoa1709937.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 2016, 375, 1823-1833, doi:10.1056/NEJMoa1606774.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019, 37, 537-546, doi:10.1200/jco.18.00149.
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 2019, 393, 1819-1830, doi:10.1016/s0140-6736(18)32409-7.
- FDA approves atezolizumab as adjuvant treatment for non-small cell lung cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-adjuvant-treatment-non-small-cell-lung-cancer (accessed on October 18, 2021).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D'Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. Journal of the National Comprehensive Cancer Network : JNCCN 2021, 19, 254-266, doi:10.6004/jnccn.2021.0013.
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Frontiers in cell and developmental biology 2020, 8, 672, doi:10.3389/fcell.2020.00672.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707-723, doi:10.1016/j.cell.2017.01.017.
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568-571, doi:10.1038/nature13954.
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.) 2015, 348, 124-128, doi:10.1126/science.aaa1348.
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60-65, doi:10.1038/nature22079.
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clinical cancer research : an official journal of the American Association for Cancer Research 2019, 25, 4592-4602, doi:10.1158/1078-0432.ccr-18-1538.
- Emens, L.A. Predictive Biomarkers: Progress on the Road to Personalized Cancer Immunotherapy. Journal of the National Cancer Institute 2021, doi:10.1093/jnci/djab068.
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nature reviews. Cancer 2019, 19, 307-325, doi:10.1038/s41568-019-0144-6.
- Dieu-Nosjean, M.-C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L. Long-term survival for patients with non–small-cell lung cancer with intratumoral lymphoid structures. Journal of Clinical Oncology 2008, 26, 4410-4417.
- Xia, L.; Guo, L.; Kang, J.; Yang, Y.; Yao, Y.; Xia, W.; Sun, R.; Zhang, S.; Li, W.; Gao, Y.; et al. Predictable Roles of Peripheral IgM Memory B Cells for the Responses to Anti-PD-1 Monotherapy Against Advanced Non-Small Cell Lung Cancer. Front Immunol 2021, 12, 759217, doi:10.3389/fimmu.2021.759217.
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561-565, doi:10.1038/s41586-019-1914-8.
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549-555, doi:10.1038/s41586-019-1922-8.
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556-560, doi:10.1038/s41586-019-1906-8.
- Stankovic, B.; Bjørhovde, H.A.K.; Skarshaug, R.; Aamodt, H.; Frafjord, A.; Müller, E.; Hammarström, C.; Beraki, K.; Bækkevold, E.S.; Woldbæk, P.R.; et al. Immune Cell Composition in Human Non-small Cell Lung Cancer. Front Immunol 2018, 9, 3101, doi:10.3389/fimmu.2018.03101.
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. American journal of respiratory and critical care medicine 2014, 189, 832-844, doi:10.1164/rccm.201309-1611OC.
- Germain, C.; Devi-Marulkar, P.; Knockaert, S.; Biton, J.; Kaplon, H.; Letaïef, L.; Goc, J.; Seguin-Givelet, A.; Gossot, D.; Girard, N.; et al. Tertiary Lymphoid Structure-B Cells Narrow Regulatory T Cells Impact in Lung Cancer Patients. Front Immunol 2021, 12, 626776, doi:10.3389/fimmu.2021.626776.
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer cell 2018, 33, 547-562, doi:10.1016/j.ccell.2018.03.012.
- Labanieh, L.; Majzner, R.G.; Mackall, C.L. Programming CAR-T cells to kill cancer. Nature biomedical engineering 2018, 2, 377-391, doi:10.1038/s41551-018-0235-9.
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. 'Off-the-shelf' allogeneic CAR T cells: development and challenges. Nature reviews. Drug discovery 2020, 19, 185-199, doi:10.1038/s41573-019-0051-2.
- Manfredi, F.; Cianciotti, B.C.; Potenza, A.; Tassi, E.; Noviello, M.; Biondi, A.; Ciceri, F.; Bonini, C.; Ruggiero, E. TCR Redirected T Cells for Cancer Treatment: Achievements, Hurdles, and Goals. Front Immunol 2020, 11, 1689, doi:10.3389/fimmu.2020.01689.
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nature reviews. Cancer 2020, 20, 218-232, doi:10.1038/s41568-019-0235-4.
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, doi:10.3390/cells9051305.
- Singh, A.K.; McGuirk, J.P. CAR T cells: continuation in a revolution of immunotherapy. The Lancet. Oncology 2020, 21, e168-e178, doi:10.1016/s1470-2045(19)30823-x.
- Wang, S.S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cellular & molecular immunology 2019, 16, 6-18, doi:10.1038/s41423-018-0027-x.
- Patel, A.J.; Richter, A.; Drayson, M.T.; Middleton, G.W. The role of B lymphocytes in the immuno-biology of non-small-cell lung cancer. Cancer immunology, immunotherapy : CII 2020, 69, 325-342, doi:10.1007/s00262-019-02461-2.
- Leong, T.L.; Bryant, V.L. B cells in lung cancer-not just a bystander cell: a literature review. Translational lung cancer research 2021, 10, 2830-2841, doi:10.21037/tlcr-20-788.
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: progress, challenges and recommendations. Nature reviews. Drug discovery 2019, 18, 41-58, doi:10.1038/nrd.2018.168.
- Guo, L.; Cukic, B.; Singh, H. Predicting Fault Prone Modules by the Dempster-Shafer Belief Networks. Proceedings of 18th IEEE International Conference on Automated Software Engineering (ASE'03) 2003, 249-252.
- Ye, Q.; Singh, S.; Qian, P.R.; Guo, N.L. Immune-Omics Networks of CD27, PD1, and PDL1 in Non-Small Cell Lung Cancer. Cancers (Basel) 2021, 13, doi:10.3390/cancers13174296.
- Ye, Q.; Falatovich, B.; Singh, S.; Ivanov, A.V.; Eubank, T.D.; Guo, N.L. A Multi-Omics Network of a Seven-Gene Prognostic Signature for Non-Small Cell Lung Cancer. International journal of molecular sciences 2021, 23, doi:10.3390/ijms23010219.
- Xu, J.Y.; Zhang, C.; Wang, X.; Zhai, L.; Ma, Y.; Mao, Y.; Qian, K.; Sun, C.; Liu, Z.; Jiang, S.; et al. Integrative Proteomic Characterization of Human Lung Adenocarcinoma. Cell 2020, 182, 245-261.e217, doi:10.1016/j.cell.2020.05.043.
- Petersone, L.; Edner, N.M.; Ovcinnikovs, V.; Heuts, F.; Ross, E.M.; Ntavli, E.; Wang, C.J.; Walker, L.S.K. T Cell/B Cell Collaboration and Autoimmunity: An Intimate Relationship. Frontiers in Immunology 2018, 9, doi:10.3389/fimmu.2018.01941.
- Chiou, S.H.; Tseng, D.; Reuben, A.; Mallajosyula, V.; Molina, I.S.; Conley, S.; Wilhelmy, J.; McSween, A.M.; Yang, X.; Nishimiya, D.; et al. Global analysis of shared T cell specificities in human non-small cell lung cancer enables HLA inference and antigen discovery. Immunity 2021, 54, 586-602 e588, doi:10.1016/j.immuni.2021.02.014.
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med 2018, 24, 978-985, doi:10.1038/s41591-018-0045-3.
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897-907, doi:10.1007/s00408-020-00407-5.
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunology 2020, 20, 651-668, doi:10.1038/s41577-020-0306-5.
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. Journal of hematology & oncology 2018, 11, 39, doi:10.1186/s13045-018-0582-8.
- Burris, H.A.; Infante, J.R.; Ansell, S.M.; Nemunaitis, J.J.; Weiss, G.R.; Villalobos, V.M.; Sikic, B.I.; Taylor, M.H.; Northfelt, D.W.; Carson, W.E., 3rd; et al. Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients With Advanced Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017, 35, 2028-2036, doi:10.1200/jco.2016.70.1508.
- Guo, N.L.; Dowlati, A.; Raese, R.A.; Dong, C.; Chen, G.; Beer, D.G.; Shaffer, J.; Singh, S.; Bokhary, U.; Liu, L.; et al. A Predictive 7-Gene Assay and Prognostic Protein Biomarkers for Non-small Cell Lung Cancer. EBioMedicine 2018, 32, 102-110, doi:10.1016/j.ebiom.2018.05.025.
- Lizotte, P.H.; Ivanova, E.V.; Awad, M.M.; Jones, R.E.; Keogh, L.; Liu, H.; Dries, R.; Almonte, C.; Herter-Sprie, G.S.; Santos, A.; et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight 2016, 1, e89014, doi:10.1172/jci.insight.89014.
References
- Spira, A.; Wood, A.J.J.; Ettinger, D.S. Multidisciplinary Management of Lung Cancer. New England Journal of Medicine 2004, 350, 379-392, doi:10.1056/NEJMra035536.
- Ho, C.; Tong, K.M.; Ramsden, K.; Ionescu, D.N.; Laskin, J. Histologic classification of non-small-cell lung cancer over time: reducing the rates of not-otherwise-specified. Current oncology (Toronto, Ont.) 2015, 22, e164-170, doi:10.3747/co.22.2339.
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: current treatment and future advances. Translational lung cancer research 2016, 5, 288-300, doi:10.21037/tlcr.2016.06.07.
- Crino, L.; Weder, W.; Van, M.J.; Felip, E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2010, 21 Suppl 5, v103-v115.
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. Molecular histology of lung cancer: from targets to treatments. Cancer Treat Rev 2015, 41, 361-375, doi:10.1016/j.ctrv.2015.02.008.
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England journal of medicine 2018, doi:10.1056/NEJMoa1716078.
- Hellmann, M.D.; Rizvi, N.A.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; Ready, N.E.; Gerber, D.E.; Chow, L.Q.; Juergens, R.A.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. The Lancet. Oncology 2017, 18, 31-41, doi:10.1016/s1470-2045(16)30624-6.
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2020, 15, 288-293, doi:10.1016/j.jtho.2019.10.002.
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. The New England journal of medicine 2017, 377, 1919-1929, doi:10.1056/NEJMoa1709937.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 2016, 375, 1823-1833, doi:10.1056/NEJMoa1606774.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019, 37, 537-546, doi:10.1200/jco.18.00149.
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 2019, 393, 1819-1830, doi:10.1016/s0140-6736(18)32409-7.
- FDA approves atezolizumab as adjuvant treatment for non-small cell lung cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-adjuvant-treatment-non-small-cell-lung-cancer (accessed on October 18, 2021).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D'Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. Journal of the National Comprehensive Cancer Network : JNCCN 2021, 19, 254-266, doi:10.6004/jnccn.2021.0013.
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Frontiers in cell and developmental biology 2020, 8, 672, doi:10.3389/fcell.2020.00672.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707-723, doi:10.1016/j.cell.2017.01.017.
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568-571, doi:10.1038/nature13954.
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.) 2015, 348, 124-128, doi:10.1126/science.aaa1348.
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60-65, doi:10.1038/nature22079.
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clinical cancer research : an official journal of the American Association for Cancer Research 2019, 25, 4592-4602, doi:10.1158/1078-0432.ccr-18-1538.
- Emens, L.A. Predictive Biomarkers: Progress on the Road to Personalized Cancer Immunotherapy. Journal of the National Cancer Institute 2021, doi:10.1093/jnci/djab068.
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nature reviews. Cancer 2019, 19, 307-325, doi:10.1038/s41568-019-0144-6.
- Dieu-Nosjean, M.-C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L. Long-term survival for patients with non–small-cell lung cancer with intratumoral lymphoid structures. Journal of Clinical Oncology 2008, 26, 4410-4417.
- Xia, L.; Guo, L.; Kang, J.; Yang, Y.; Yao, Y.; Xia, W.; Sun, R.; Zhang, S.; Li, W.; Gao, Y.; et al. Predictable Roles of Peripheral IgM Memory B Cells for the Responses to Anti-PD-1 Monotherapy Against Advanced Non-Small Cell Lung Cancer. Front Immunol 2021, 12, 759217, doi:10.3389/fimmu.2021.759217.
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561-565, doi:10.1038/s41586-019-1914-8.
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549-555, doi:10.1038/s41586-019-1922-8.
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556-560, doi:10.1038/s41586-019-1906-8.
- Stankovic, B.; Bjørhovde, H.A.K.; Skarshaug, R.; Aamodt, H.; Frafjord, A.; Müller, E.; Hammarström, C.; Beraki, K.; Bækkevold, E.S.; Woldbæk, P.R.; et al. Immune Cell Composition in Human Non-small Cell Lung Cancer. Front Immunol 2018, 9, 3101, doi:10.3389/fimmu.2018.03101.
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. American journal of respiratory and critical care medicine 2014, 189, 832-844, doi:10.1164/rccm.201309-1611OC.
- Germain, C.; Devi-Marulkar, P.; Knockaert, S.; Biton, J.; Kaplon, H.; Letaïef, L.; Goc, J.; Seguin-Givelet, A.; Gossot, D.; Girard, N.; et al. Tertiary Lymphoid Structure-B Cells Narrow Regulatory T Cells Impact in Lung Cancer Patients. Front Immunol 2021, 12, 626776, doi:10.3389/fimmu.2021.626776.
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer cell 2018, 33, 547-562, doi:10.1016/j.ccell.2018.03.012.
- Labanieh, L.; Majzner, R.G.; Mackall, C.L. Programming CAR-T cells to kill cancer. Nature biomedical engineering 2018, 2, 377-391, doi:10.1038/s41551-018-0235-9.
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. 'Off-the-shelf' allogeneic CAR T cells: development and challenges. Nature reviews. Drug discovery 2020, 19, 185-199, doi:10.1038/s41573-019-0051-2.
- Manfredi, F.; Cianciotti, B.C.; Potenza, A.; Tassi, E.; Noviello, M.; Biondi, A.; Ciceri, F.; Bonini, C.; Ruggiero, E. TCR Redirected T Cells for Cancer Treatment: Achievements, Hurdles, and Goals. Front Immunol 2020, 11, 1689, doi:10.3389/fimmu.2020.01689.
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nature reviews. Cancer 2020, 20, 218-232, doi:10.1038/s41568-019-0235-4.
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, doi:10.3390/cells9051305.
- Singh, A.K.; McGuirk, J.P. CAR T cells: continuation in a revolution of immunotherapy. The Lancet. Oncology 2020, 21, e168-e178, doi:10.1016/s1470-2045(19)30823-x.
- Wang, S.S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cellular & molecular immunology 2019, 16, 6-18, doi:10.1038/s41423-018-0027-x.
- Patel, A.J.; Richter, A.; Drayson, M.T.; Middleton, G.W. The role of B lymphocytes in the immuno-biology of non-small-cell lung cancer. Cancer immunology, immunotherapy : CII 2020, 69, 325-342, doi:10.1007/s00262-019-02461-2.
- Leong, T.L.; Bryant, V.L. B cells in lung cancer-not just a bystander cell: a literature review. Translational lung cancer research 2021, 10, 2830-2841, doi:10.21037/tlcr-20-788.
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: progress, challenges and recommendations. Nature reviews. Drug discovery 2019, 18, 41-58, doi:10.1038/nrd.2018.168.
- Guo, L.; Cukic, B.; Singh, H. Predicting Fault Prone Modules by the Dempster-Shafer Belief Networks. Proceedings of 18th IEEE International Conference on Automated Software Engineering (ASE'03) 2003, 249-252.
- Ye, Q.; Singh, S.; Qian, P.R.; Guo, N.L. Immune-Omics Networks of CD27, PD1, and PDL1 in Non-Small Cell Lung Cancer. Cancers (Basel) 2021, 13, doi:10.3390/cancers13174296.
- Ye, Q.; Falatovich, B.; Singh, S.; Ivanov, A.V.; Eubank, T.D.; Guo, N.L. A Multi-Omics Network of a Seven-Gene Prognostic Signature for Non-Small Cell Lung Cancer. International journal of molecular sciences 2021, 23, doi:10.3390/ijms23010219.
- Xu, J.Y.; Zhang, C.; Wang, X.; Zhai, L.; Ma, Y.; Mao, Y.; Qian, K.; Sun, C.; Liu, Z.; Jiang, S.; et al. Integrative Proteomic Characterization of Human Lung Adenocarcinoma. Cell 2020, 182, 245-261.e217, doi:10.1016/j.cell.2020.05.043.
- Petersone, L.; Edner, N.M.; Ovcinnikovs, V.; Heuts, F.; Ross, E.M.; Ntavli, E.; Wang, C.J.; Walker, L.S.K. T Cell/B Cell Collaboration and Autoimmunity: An Intimate Relationship. Frontiers in Immunology 2018, 9, doi:10.3389/fimmu.2018.01941.
- Chiou, S.H.; Tseng, D.; Reuben, A.; Mallajosyula, V.; Molina, I.S.; Conley, S.; Wilhelmy, J.; McSween, A.M.; Yang, X.; Nishimiya, D.; et al. Global analysis of shared T cell specificities in human non-small cell lung cancer enables HLA inference and antigen discovery. Immunity 2021, 54, 586-602 e588, doi:10.1016/j.immuni.2021.02.014.
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med 2018, 24, 978-985, doi:10.1038/s41591-018-0045-3.
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897-907, doi:10.1007/s00408-020-00407-5.
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunology 2020, 20, 651-668, doi:10.1038/s41577-020-0306-5.
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. Journal of hematology & oncology 2018, 11, 39, doi:10.1186/s13045-018-0582-8.
- Burris, H.A.; Infante, J.R.; Ansell, S.M.; Nemunaitis, J.J.; Weiss, G.R.; Villalobos, V.M.; Sikic, B.I.; Taylor, M.H.; Northfelt, D.W.; Carson, W.E., 3rd; et al. Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients With Advanced Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017, 35, 2028-2036, doi:10.1200/jco.2016.70.1508.
- Guo, N.L.; Dowlati, A.; Raese, R.A.; Dong, C.; Chen, G.; Beer, D.G.; Shaffer, J.; Singh, S.; Bokhary, U.; Liu, L.; et al. A Predictive 7-Gene Assay and Prognostic Protein Biomarkers for Non-small Cell Lung Cancer. EBioMedicine 2018, 32, 102-110, doi:10.1016/j.ebiom.2018.05.025.
- Lizotte, P.H.; Ivanova, E.V.; Awad, M.M.; Jones, R.E.; Keogh, L.; Liu, H.; Dries, R.; Almonte, C.; Herter-Sprie, G.S.; Santos, A.; et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight 2016, 1, e89014, doi:10.1172/jci.insight.89014.
