Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Debashis Dutta and Version 2 by Rita Xu.
The world is grappling with the coronavirus disease 2019 (COVID-19) pandemic, the causative agent of which is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 symptoms are similar to the common cold, including fever, sore throat, cough, muscle and chest pain, brain fog, dyspnoea, anosmia, ageusia, and headache.
- SARS-CoV-2
- COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19) is a highly contagious communicable disease of the present time caused by a novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. It is believed that this viral infection was initiated with a zoonotic transfer from a seafood market in Wuhan, China [2]. Initially, the viral outbreak was considered endemic in China but, within a few weeks, the SARS-CoV-2 infection causing COVID-19 was declared a global pandemic by the World Health Organization (WHO) on 11 March 2020 [3]. Till now, the virus has infected 535,863,950 individuals worldwide and is infecting new individuals consistently, developing new clusters of infection (https://covid19.who.int/, accessed on 16 June 2022). RWesearchers have lost 6,314,972 people and persons aged 65 and older with compromised immunity and with underlying medical conditions, such as chronic lung or liver disease, asthma, diabetes, severe heart problems, etc., are at significant risk of illness, morbidity, and mortality (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html, accessed on 16 June 2022). The detailed chronology and epidemiology of the virus are discussed elsewhere [4]. After the identification of SARS-CoV-2 as the etiological agent of the illness, a race against time was started to develop rapid and efficient diagnostic methods, opening a new avenue for diagnostic innovations [5]. With the availability of the viral genome sequence, quantitative polymerase chain reaction (qPCR) was rapidly adopted as a reliable test for the diagnosis of infection [6]. Although exponential new studies propose novel therapeutic interventions and vaccines, there is a knowledge gap for understanding COVID-19 pathogenesis thoroughly and devising effective strategies to combat the virus in an attempt to alleviate human suffering.
Despite efficient testing and tracing of the infected individuals being central to the countermeasures against the management of the COVID-19, inaccurate testing can undermine these measures against the spread of the infection [7]. Contrarily, a false-positive result can cause avoidable psychological distress, besides wasting resources to manage the nonpatient [8].
2. Molecular Biology of SARS-CoV-2
SARS-CoV-2 is enveloped in a positive-sense single-stranded RNA virus with a genome size of 29,903 nucleotides (Figure 1) [9][10][41,42]. The virion size of this virus varies from 80–120 nm in diameter [11][12][43,44]. The nucleotide sequence of SARS-CoV-2 is 79.5% identical to SARS-CoV and 51.8% identical to MERS-CoV [9][13][41,45]. This suggests SARS-CoV-2 is closer to SARS-CoV. SARS-CoV and SARS-CoV-2 have similar lengths for most of the proteins. SARS-CoV-2 encodes four structural genes: spike glycoprotein (S), membrane glycoprotein (M), envelope glycoprotein (E), and nucleocapsid (N). The amino acid sequences of these structural genes are ~90% identical with SARS-CoV except the S gene [4][9][4,41]. The S protein of SARS-CoV-2 plays a crucial role in the viral entry into the host cell by binding to the host cell-surface receptor angiotensin-converting enzyme-2 (ACE2), and modifications in this protein may lead to different mechanisms and differential intensity of entry into the host cells [14][15][16][46,47,48]. Most of the SARS-CoV-2 non-structural proteins have greater than 85% amino acid sequence identity with SARS-CoV [17][49]. SARS-CoV-2 possesses four structural proteins: spike glycoprotein (S, 1273 amino acids), envelope glycoprotein (E, 75 amino acids), membrane protein (M, 222 amino acids), and nucleocapsid (N, 419 amino acids) (Figure 1) [9][18][41,50]. The N protein is involved in the RNA binding and packaging [18][19][50,51]. The most abundant protein in the outer membrane is M-glycoprotein. M and E proteins play a role in viral packaging and the S proteins play a crucial role in host cell binding and infection.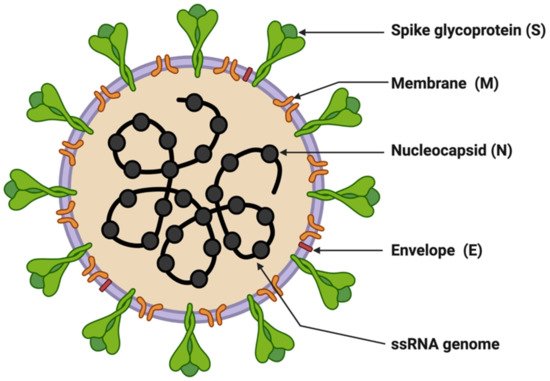
Figure 1. Structure of SARS-CoV-2. The figure was created with Biorender.com on 8 June 2022.
Table 1. List of SARS-CoV-2 variants.
| S.No. | Name of Variant | Lineage | Earliest Sample | First Outbreak | Designated | Reference |
|---|---|---|---|---|---|---|
| 1. | Epsilon | B.1.429, B.1.427 | March 2020 | United States | 5 March 2021 | [25][26][57,58] |
| 2. | Zeta | P.2 | April 2020 | Brazil | 17 March 2021 | |
| 3. | Beta | B.1.351 | May 2020 | South Africa | 18 December 2020 | [27][28][59,60] |
| 4. | Lambda | C.37 | August 2020 | Peru | 14 June 2021 | [29][30][31][61,62,63 |
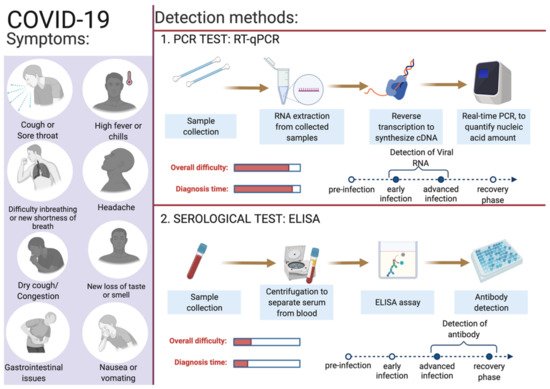
Figure 2.
Overview of COVID-19 symptoms and SARS-CoV-2 detection methods for COVID-19 diagnosis. This figure was created with
on 6 June 2022.
Table 2. List of different diagnostic methods in use.
| Test | Technique | Specimen | Advantages | Disadvantages | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viral test (Molecular genetics based) | ||||||||||||
| Antigen | Lateral flow immunoluminescent assay, single or double target | NPS and ANS | Rapid, point-of-care tests | Less sensitive, and chances of false positives | [67][98] | |||||||
| Nucleic acid | RT–qPCR | Saliva, NPS, nasal mid-turbinate and ANS | Sensitive, specific | Expensive, requires laboratory personnel, specialized lab equipment and reagents | [67][98] | |||||||
| ] | ||||||||||||
| Nucleic acid | Loop-mediated isothermal amplification (LAMP) | Saliva, urine, NPS, nasal mid-turbinate and ANS | Sensitive, specific, rapid | Complicated designing of assay, chances of false positives | [68][69][99,100] | 5. | Alpha | B.1.1.7 | September 2020 | United Kingdom | 18 December 2020 | [32][33][64,65] |
| Nucleic acid | Recombinase polymerase amplification (RPA) | NPS and ANS | Sensitive, specific, rapid | Complicated designing of assay, expensive | [70][101] | 6. | Delta | B.1.617.2 | October 2020 | India | 11 May 2021 | [34 |
| Nucleic acid | Nicking endonuclease amplification reaction (NEAR) | NPS and ANS | ] | Sensitive, rapid | Chances of false negatives | [69][70][100,101][35][66,67] | ||||||
| 7. | Gamma | P.1 | November 2020 | Brazil | 11 January 2021 | [36][ | ||||||
| Nucleic acid | Transcription mediated amplification (TMA) | 37 | NPS and ANS | ] | [ | 68,69] | ||||||
| Sensitive, specific | Expensive and less flexible | [ | 71 | ] | [102] | 8. | Lota | B.1.526 | November 2020 | United States | 24 March 2021 | [38][39][70,71] |
| Nucleic acid | Helicase-dependent amplification (HDA) | NPS and ANS | Sensitive, rapid | Chances of false positives | [69][100] | 9. | Eta | B.1.525 | December 2020 | Multiple Countries | 17 March 2021 | [40][72[41],73] |
| Nucleic acid | Clustered regularly interspaced short palindromic repeats (CRISPR) | AN, OPl, NP wash/aspirate and BAL |
Sensitive, specific, rapid, versatile | Target sequences of the Cas proteins are restricted; multiplexing can create interferences which may lead to cross-reactivities | [69][72][100,103] | 10. | Kappa | B.1.617.1 | December 2020 | India | 4 April 2021 | [42][43][74,75] |
| Nucleic acid | Strand displacement amplification (SDA) | NPS and ANS | Rapid, sensitive | Reverse transcription of virus RNA is required, shortcomings of chosen isothermal method. | [73][104] | 11. | Theta | P.3 | January 2021 | Philippines | 24 March 2021 | [44][76] |
| 12. | Mu | B.1.621 | January 2021 | Colombia | 30 August 2021 | [45][46][77,78] | ||||||
| Volatile organic compounds (VOCs) | Rapid gas chromatography-mass spectrometry (GC-MS) | Breath | Rapid | Presumptive | [67][98] | 13. | ||||||
| Radiological abnormalities caused by viral infection | Computed Tomography | Cross-sectional images of patient’s chest | Non-invasive, lesser expensive | Less specific because imaging features overlap with other viral pneumonia | [74][105] | B.1.1.318 | GR | January 2021 | Multiple Countries | 2 June 2021 | [47][79] | |
| 14. | ||||||||||||
| Serological/Immunological test | C.1.2 | GR | June 2021 | South Africa | 1 September 2021 | [48][80 | ||||||
| Antibody | ] | |||||||||||
| Enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (CIA) | Blood and tissue specimens | Rapid, point-of-care tests, can identify previous infection | Dependent on duration of infection, false-negative results | [ | 75 | 15. | B.1.640 | GH/490R | September 2021 | Multiple Countries | 22 November 2021 | [49][81] |
| ] | [ | 106 | 16. | Omicron | BA.1 | November 2021 | South Africa | 26 November 2021 | [50][51][82,83] | |||
| 17. | Omicron | BA.2 | November 2021 | South Africa | 26 November 2021 | [52][53][84,85] | ||||||
| 18. | Omicron | BA.3 | November 2021 | South Africa | 26 November 2021 | [54][86] | ||||||
| 19. | Omicron | BA.4 | January 2022 | South Africa | 12 May 2022 | [55][87] | ||||||
| 20. | XD | Omicron BA.1 and Delta | January 2022 | France | 9 Mar, 2021 | [56][88] | ||||||
| 21. | Omicron | BA.5 | February 2022 | South Africa | 12 May 2022 | [55][87] | ||||||
3. Diagnostics for COVID-19
Depending on an individual’s age, immune responses, and associated co-morbidities, infection by SARS-CoV-2 leads to highly amassed responses in different individuals ranging from asymptomatic to individuals exhibiting enormously diversified symptoms. Young and healthy people show no or mild symptoms, but they may act as silent carriers and can cause covert infections [60][92]. Severe COVID-19 cases can end in hospitalization, some necessitating assisted mechanical ventilation, and some cases may be fatal [61][93]. Identifying infected individuals and asymptomatic viral carriers with rapid and accurate testing has played a pivotal role in containing and mitigating the COVID-19 pandemic. Identification of individuals infected with SARS-CoV-2, either symptomatic or asymptomatic, has prevented further person-to-person disease transmission (https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html, accessed on 16 June 2022). A coalition of multiple methods is in use to diagnose the presence of viral infection in individuals [62][94]. The primary steps for COVID-19 diagnosis are examining the presence of classical signs and symptoms such as fever or chills, cough, shortness of breath, muscle or body aches, headache, fatigue, sore throat, the new loss of taste or smell, dyspnoea, congestion, or runny nose, nausea or vomiting, conjunctivitis, and gastrointestinal issues (Figure 2) [6]| ] | |||
| Antibody | |||
| Dried blood spot (DBS) | |||
| Dried blood samples pricked from fingers | |||
| Sensitive and rapid | |||
| Storage temperature sensitive | |||
| [ | |||
| 76 | |||
| ] | [ | 107 | ] |
3.1. Reverse-Transcriptase PCR (RT-qPCR)
The nucleic acid amplification test (NAAT) by RT-qPCR is a sensitive, accurate, and globally accepted gold standard diagnostic method for the SARS-CoV-2 detection [77][78][79][9,10,108]. PCR is being used as a diagnostic test to detect pathogens, novel infections, and antimicrobial resistance profiling [66][80][11,109]. PCR is a precise and sensitive method to detect nucleic acids and possesses the potential to generate billions of copies of target DNA from a single copy [80][109]. This technique relied on an enzyme-driven process for amplifying short regions of DNA in vitro. The requirement of this method is information on at least partial sequences of the target DNA for designing oligonucleotide primers that hybridize specifically to the target sequences [80][109]. In clinical settings, real-time RT-qPCR is a revolutionary advancement where detection and expression analysis of gene(s) can be carried out in real time, as PCR reaction progresses, and amplification and analysis are done simultaneously in a closed system. This closed system further helps to minimize false-positive results associated with the amplification product contamination [81][110]. In addition to this, RT-qPCR is fast, sensitive, and reproducible; with the use of automated instrumentation, these features are further enhanced. Recently, NAAT have included other techniques such as isothermal amplification platforms with nicking endonuclease amplification reaction (NEAR), loop-mediated isothermal amplification (LAMP), and transcription-mediated amplification (TMA) [82][111]. A detailed overview of the RT-qPCR method for SARS-CoV-2 detection is depicted in Figure 3.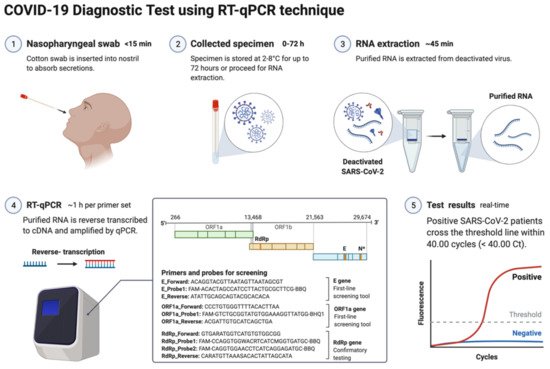
Figure 3. Schematic representation of COVID-19 diagnostic test using RT-PCR. This figure was created with Biorender.com on 18 May 2022.
3.2. Specimens for Detection of SARS-CoV-2
The genetic material of SARS-CoV-2 (RNA) is first converted into complementary DNA (cDNA) by the action of RNA-dependent DNA polymerase (reverse transcriptase) prior to the actual amplification. For this, viral RNA can be collected from diverse specimens such as ocular secretions, saliva, sputum, bronchoalveolar lavage (BAL), blood, and fecal material, but upper respiratory system samples such as oropharyngeal swabs (OPS) and nasopharyngeal swabs (NPSs) are widely in use [64][65][96,97]. In detecting SARS-CoV-2 in various samples, limit of detection (LoD) plays a crucial role [83][112]. Presently, the best-of-class assay has LoD of ~100 copies of viral RNA per milliliters of transport media; assays with higher LoDs may result in a false negative [83][112]. Though OPS and NPSs are primarily in use because of lower LoDs, there is a recommendation for the use of combined swabs for COVID-19 diagnosis to avoid false-negative results [84][113]. Saliva has also been used as a reliable, noninvasive approach for SARS-CoV-2 detection and disease progression [85][114]. The advantages of using saliva for diagnosis are self-collection, reduced transmission risk during the sample collection, and also a lesser requirement of PPE, trained healthcare professionals, transportation, and storage costs [86][115]. Importantly, viral load over the course of the infection is detrimental to the analytical sensitivity of assays. It was reported in several studies that the viral load of SARS-CoV-2 peaks during or even shortly before the onset of symptoms and decreases rapidly within the first seven days [86][87][115,116]. Furthermore, the virus can be detected in samples for longer periods from the onset of symptoms, usually for 20 days or longer in some patients [88][117]. There are specific guidelines for sample collection for different specimens by the CDC (https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html, accessed on 16 June 2022). For NPSs and OPS, collecting using only synthetic fiber swabs with thin plastic or wire shafts specifically designed for sampling nasopharyngeal mucosa is recommended. For this patient, the head needs to be tilted back 70 degrees and the swab needs to be inserted slowly into the nostril to contact the nasopharynx. Thereafter, gently rub and roll the swab and leave it for a few seconds to absorb secretions; remove it slowly and place it in the transport tube. These samples can be stored at 2–8 °C for up to 72 h; for longer duration, samples must be stored at −70 °C. Extracted nucleic acid samples must be stored at −70 °C or lower. The collected specimen must be transported to the laboratory while maintaining a cold chain of 2–4 °C throughout [89][118].3.3. Biomarkers/Genes Used for RT-qPCR
According to Centers for Disease Control and Prevention (CDC) and WHO guidelines, the RNA samples are reverse-transcribed into cDNA using different primers specific for the open reading frame 1ab (ORF1ab), ORF8, RNA-dependent RNA polymerase (RdRp), hemagglutinin-esterase (HE), and the nucleocapsid genes N1, N2, envelope genes (E), spike genes (S), and transmembrane gene (M), while human RNase P is used as control (Table S1). Some other controls in use for each reaction are no template control, 2019-nCoV positive control, and human specimen control (CDC 2020) [90][91][92][119,120,121]. Additionally, ORF1ab and RdRp are included in RT-qPCR reactions to rule out any potential cross-reactivity, which may occur with other coronaviruses, and to avoid chances of genetic drift in the SARS-CoV-2 genome [93][122]. As per the CDC recommendation, screening must be done targeting nucleocapsid genes (N1 and N2), but the WHO recommendations require targeting E genes, which must be followed by confirmation using the RdRp gene [93][122]. Though there is less impact on the detection of SARS-CoV-2 because of emergent variants as most mutations accumulated in the S gene and not in other genes, which are a common target for detection assays. Some VOCs of SARS-CoV-2 (Alfa and Omicron) provides negative results or weaker signals with S-gene RT-qPCR assays, while positive ones with other genes (https://www.ecdc.europa.eu/sites/default/files/documents/Methods-for-the-detection-and-characterisation-of-SARS-CoV-2-variants-first-update.pdf, accessed on 16 June 2022). This effect of no detection of the S gene or weaker signals is referred as S-gene target failure (SGTF) and is due to deletion at nt207–212 (Δ69–70) [94][123]. Alfa and the majority of Omicron variants of SARS-CoV-2 give negative RT-qPCR results using the S gene, but positive ones with ORF1 and the N gene [95][124]. The RT-qPCR reaction can be performed in either one or two steps [96][97][125,126]. In the conventional two-step RT-qPCR, the reactions for cDNA synthesis and amplification of DNA are conducted separately in two sequential steps, while in one-step RT-qPCR, both the above-mentioned cDNA synthesis and DNA amplification reactions are performed in a single step within one tube containing the requirements to accomplish the entire assay [96][125]. In detecting SARS-CoV-2 for COVID-19 diagnosis, this one-step RT-qPCR is preferred over the two-step method owing to it being fast and efficient and involving limited sample handling, minimal experimental errors, and a reduced bench time [65][96][97,125]. This is followed by cDNA being amplified using fluorescent-based quantitative PCR assays to allow sensitive detection and quantification of the viral RNA [65][97]. Figure 4 shows the mechanistic steps of DNA amplification and its detection. The qPCR reaction steps are similar to the PCR steps, with initial denaturation of the template at 95 °C for 5–10 min followed by cyclic steps including denaturation (95 °C, 15–20 s), primer/probe annealing (60 °C, 15–20 s), and primer extension (72 °C, 1 min) for gene amplification. Annealing temperature plays a critical role in efficient amplification of the gene of interest and requires optimization and varies from template to template. The annealing temperature determines the qPCR efficiency and depends on the melting temperature (Tm) and is well-established for SARS-CoV-2 detection using different regions of the RNA genome. The qPCR is thereafter continued for 35–45 cycles; during each cycle, the template DNA amount is doubled, resulting in an increase in fluorescent signals. In Figure 4, the sigmoidal curve represents a typical result of the qPCR results, and this helps reusearchers interpret the assay outcomes. This curve has three distinct phases: up to cycle 15 or so the curve is near the baseline, in the second phase there is a strong upswing of the cure, usually between 15–30 cycles, and in this phase the amplification signal crosses the threshold. In the third phase, generally after 30 cycles there is a plateau where amplification tapers off and ceases to grow. This curve helps in determining the cycle threshold (Ct) value; this is the point where the curve first clearly rises off the baseline to a statistically significant degree. Crossing this noise threshold is the basis for calling a sample positive in the qualitative assay and the Ct value is the basis for the generation of the standard curve used in the quantifying template in quantitative PCR.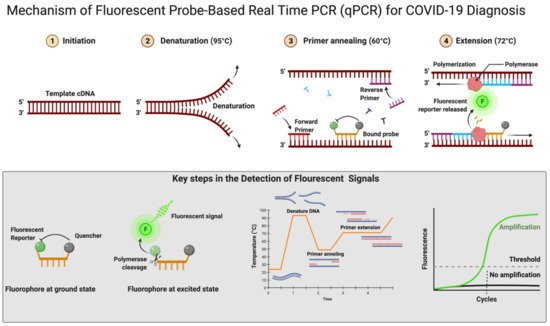
Figure 4. Mechanism of fluorescent probe-based real-time PCR (qPCR) for COVID-19 diagnosis. Figure was created with Biorender.com on 20 May 2022.
